Abstract
Objective:
To profile the reactivity of CSF-derived immunoglobulin from patients with multiple sclerosis (MS) against a large panel of antigens, to identify disease-specific reactivities.
Methods:
CSF from subjects with MS with elevated immunoglobulin G and CSF from control subjects presenting with other inflammatory neurologic disease were screened against a protein array consisting of 9,393 proteins. Reactivity to a candidate protein identified using these arrays was confirmed with ELISA and immunocytochemistry.
Results:
Autoantibodies against one protein on the array, recombination signal binding protein for immunoglobulin kappa J region (RBPJ), discriminated between patients with MS and controls (p = 0.0052). Using a large validation cohort, we found a higher prevalence of autoantibodies against RBPJ in the CSF of patients with MS (12.5%) compared with the CSF of patients with other neurologic diseases (1.6%; p = 0.02) by ELISA. This difference in reactivity was restricted to the CSF as serum reactivity against RBPJ did not differ between patients and controls. The presence of CSF autoantibodies against RBPJ was further confirmed by immunocytochemistry.
Conclusions:
These data indicate that RBPJ, a ubiquitous protein of the Notch signaling pathway that plays an important role in Epstein-Barr virus infection, is a novel MS autoantigen candidate that is recognized by CSF-derived immunoglobulin G in a subset of patients with MS.
The pathophysiology and immunopathology of multiple sclerosis (MS)1 are not completely understood. An affirmed role for B cells and autoantibodies in MS immunopathology is supported by the detection of CSF oligoclonal bands (OCB) in >90% of patients, the presence of clonally expanded B cells in the CNS, the response to B cell–targeted therapies, and genetic studies.2–5 We previously demonstrated that antigen-experienced B cells populate the parenchyma, meninges, and CSF, and that related B-cell clones are present in these distinct compartments.6 These clonal B cells participate in the production of immunoglobulin in the CSF and OCB.7–9 The antigen targets of these experienced B-cell clones and the immunoglobulin they produce remain unknown. We sought to explore the specificity of the MS CSF-derived immunoglobulin that can serve as a proxy for the B cells residing in the CNS. We focused this investigation by initiating our search with MS CSF that included both elevated immunoglobulin content and OCB. This set was compared to that of patients with other inflammatory neurologic diseases (OIND) that also included elevated CSF immunoglobulin. To screen a large number of candidates, we utilized a protein antigen array composed of 9,393 proteins that were expressed in a system that included physiologic posttranslational modifications and processing.
METHODS
Patients and controls.
CSF from 8 patients fulfilling McDonald revised diagnostic criteria10 for MS that included OCB and a CSF immunoglobulin G (IgG) index >1 were obtained from the Human Brain and Spinal Fluid Resource Center, Veteran's Administration, West Los Angeles Health Center, Los Angeles, California, and used for the protein array experiments. Control CSF cases from the same source included 7 subjects with OIND, all of whom had an IgG index >1. The clinical demographics of the patients are detailed in table e-1 on the Neurology® Web site at www.neurology.org. CSF was obtained from additional cases to serve as an independent validation cohort. These cases included CSF from 61 patients with relapsing-remitting MS (RRMS), 11 patients with secondary progressive MS (SPMS), 35 patients with OIND, and 27 patients with noninflammatory neurologic diseases (NIND). Cells were removed from the CSF; then, the material was aliquoted and stored at −80°C until use. The clinical demographics of this second cohort of patients are detailed in table e-2.
Protein microarray to screen for autoantibodies.
ProtoArray Human Protein Microarrays version 5.0 (Invitrogen, Carlsbad, CA), containing 9,393 unique human proteins, was used. The assay was performed according to the manufacturer's instructions. Briefly, protein microarray slides were blocked with the manufacturer's buffer before overnight incubation with CSF at 4°C. Prior to the assay, the IgG content of each CSF specimen was determined with an assay developed in our laboratory,11 so that application of each CSF to the array (20 μg/mL in the manufacturer's washing buffer) was normalized for total IgG content. Bound IgG was detected with an Alexa Fluor 647-conjugated goat antihuman IgG (Invitrogen) applied at 1 μg/mL in washing buffer for 1 hour at room temperature. The arrays were washed and dried, and then scanned using a GenePix 4200A (Molecular Devices) fluorescent microarray scanner. GenePix software was used to align the scanned image to the template and to determine the pixel intensities for each spot on the array. The reported pixel intensity was calculated as the average of duplicate signals after background subtraction.
Data analysis.
Two quality control steps were imposed to identify array experiments that were technically unsuccessful. First, individual arrays in which the histograms of the raw reactivity values were significantly shifted were removed. Second, proteins with a coefficient of variation greater than 0.5 between the individual probes in a given array were removed (there are 2 independent probes for every protein). A single reactivity measurement for each protein was then generated by averaging all the individual probe values corresponding to the same protein. An initial set of hits was defined for each array as proteins with a log-reactivity Z score larger than 2.324 (corresponding to the top 1% of a normal distribution). These hits were then further filtered for proteins with a defined minimum prevalence among the MS cases and a maximum prevalence among the control cases, where the specific thresholds are indicated in each analysis. Statistical significance was calculated by performing a Mann-Whitney-Wilcoxon test and correcting for multiple hypotheses using the false discovery rate (FDR) method.12 For the final reactivity measurements, the arrays of each group (control and MS) were quantile-normalized separately.
Protein expression and purification.
Recombination signal binding protein for immunoglobulin kappa J region (RBPJ) and the control antigen, myosin light chain 5 (MYL5), were both expressed in a recombinant system. Cells (293A HEK) were cultured in 100-mm plates and transiently transfected using polyethylenimine (PEI, Polysciences Inc., Warrington, PA) with the pCMV6-Entry (Myc-DDK tagged at C-terminus) plasmid (Origene, Rockville, MD) containing full-length RBPJ or MYL5 cDNA. Twenty-four hours later, transfection media was replaced with fresh culture media. Seventy-two hours after transfection, the cells were washed with PBS, then lysed in PBS containing 1% Triton X-100, 1 mM EDTA, and a protease inhibitor cocktail (Roche, Mannheim, Germany) for 30 minutes at 4°C (with gentle agitation). Cell debris was removed by centrifugation (13,200 rpm at 4°C for 5 minutes) before the transfected proteins were immunoprecipitated using M2-FLAG resin (Sigma-Aldrich, St. Louis, MO) according the manufacturer's instructions. Bound proteins were then eluted from the resin using FLAG peptide. Purity was confirmed with sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting.
ELISA.
ELISA plates were coated overnight at 4°C with RBPJ, MYL5, human histone H1 (Upstate Biotechnology, Lake Placid, NY), or human neutrophil-derived lysozyme (Sigma-Aldrich) at a concentration of 5 μg/mL in carbonate buffer (pH 8.3). The plates were then blocked with PBS-Tween (0.05%) containing 1% bovine serum albumin for 1 hour at room temperature. CSF (5 μg/mL) or serum samples (diluted 1:250) were diluted in blocking buffer, applied to wells, and incubated overnight at 4°C. Plates were then washed with PBS-Tween (0.05%) and incubated for 1 hour at room temperature with horseradish peroxidase–conjugated goat antihuman secondary antibody (Chemicon, Temecula, CA). Bound antibodies were detected with One-Step Ultra TMB reagent (Thermo Scientific, Waltham, MA). Optical density was measured at 455 nm. Nonspecific background binding was addressed by subtracting the binding signal observed with RBPJ to the binding observed with an irrelevant protein (MYL5). MYL5 was chosen because of its similarities to RBPJ: both are intracellular, both were expressed and purified under the identical conditions, both are present on the ProtoArray, and both include a DDK tag. Reactivity against RBPJ was considered positive when the signal exceeded the mean observed for the NIND cases by 4 SDs (99.9% CI).
Immunocytochemistry.
Cells (293A HEK) were cultured in 6-well plates containing poly-L-lysine–coated coverslips and transfected, as described for the RBPJ expression and purification (above), at 70%–80% confluence. After 24 hours, cells were washed with PBS, fixed with 4% paraformaldehyde in PBS, permeabilized with 0.3% Triton X-100 in PBS, and washed with PBS again. The coverslips were then blocked with 5% normal goat serum in PBS for 1 hour and frozen at −20°C until needed. Expression of RBPJ was evaluated by incubating the coverslips with a mouse anti-DDK monoclonal antibody (Origene). Detection of RBPJ-specific antibodies in the CSF specimens was performed by incubating the coverslips containing RBPJ-transfected or nontransfected HEK cells with CSF diluted in blocking buffer at an IgG concentration of 5 μg/mL. After 1 hour at room temperature, coverslips were washed with PBS and bound antibody was detected by incubating with Alexa Fluor 594 goat antihuman IgG or Alexa Fluor 488 antimouse IgG (Molecular Probes, Eugene, OR), diluted in blocking buffer at 1:500. Following washes with distilled water, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich), diluted 1:1,000, washed with water, and mounted with FluorSave (Calbiochem, San Diego, CA). Images were obtained in a Carl Zeiss (Oberkochen, Germany) Axioskop with Axiovision version 3.0 software.
Statistics.
Fisher exact test was used to compare proportions between groups. Student t test and Mann-Whitney-Wilcoxon tests were used to compare differences between groups in the ProtoArray analysis and in the ELISA experiments. Analyses were performed with GraphPad Prism v5.0 and the R Project for Statistical Computing.
Standard protocol approvals, registrations, and patient consents.
Specimens originating from patients were collected after informed written consent was obtained, under a protocol approved by the Human Research Protection Program at Yale School of Medicine or Washington University School of Medicine. Specimens that did not include personally identifiable private information, intervention, or interaction with an individual were collected under an exempt protocol approved by the Human Research Protection Program at the Yale School of Medicine.
RESULTS
ProtoArray discovery cohort.
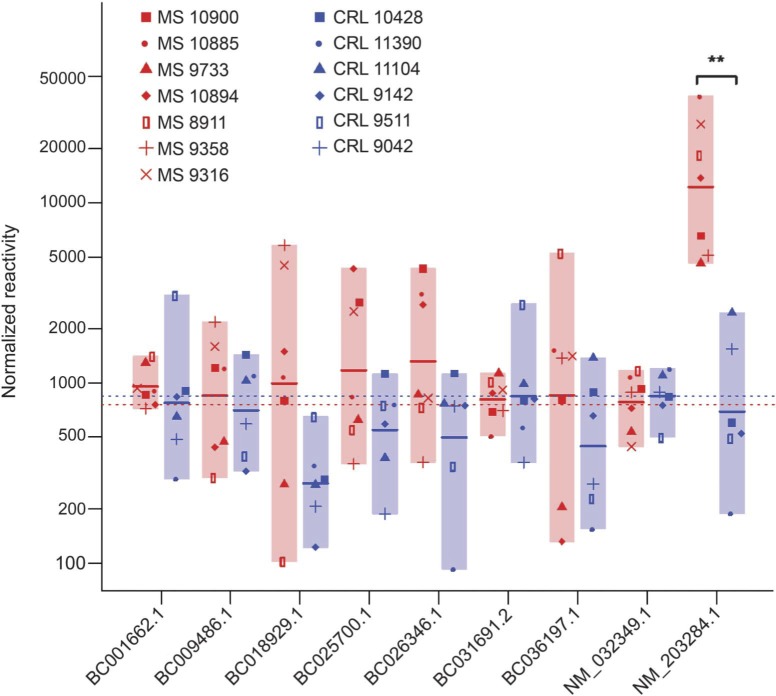
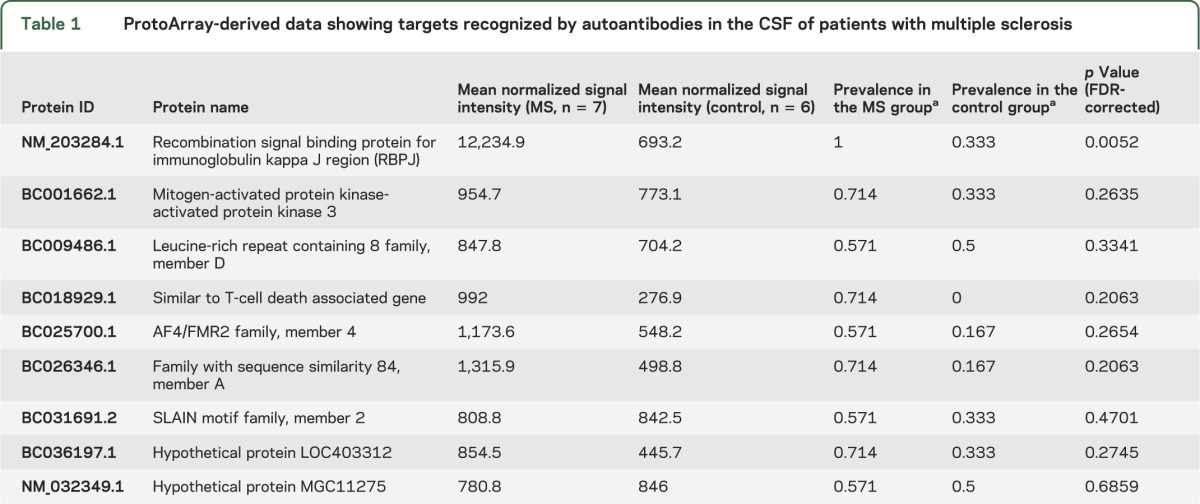
Eight patients with RRMS and 7 patients with OIND, all with a CSF IgG index >1, were used for antigen discovery with the ProtoArray. Quality control analysis identified 2 arrays (one in each group) where the reactivity histograms were shifted dramatically (the medians of these arrays were >10-fold larger than that of the others) (figure e-1). These 2 arrays were removed from further analysis. To identify antigens that discriminated between MS cases and controls, we filtered the protein hits to impose a minimum prevalence of 4/7 in the MS cases and a maximum prevalence of 3/6 in the controls. After filtering, only 9 proteins remained (figure 1 and table 1), but only one was statistically significantly different between the MS cases and controls (FDR <0.05, Mann-Whitney-Wilcoxon test). This protein, RBPJ, remained the only significant hit when different thresholds were used for the minimum and maximum prevalence (figure e-2).
Figure 1. ProtoArray-derived data show targets recognized by autoantibodies in the CSF of patients with multiple sclerosis.
Reactivity of CSF from patients with multiple sclerosis (red) was compared to that of CSF from controls (blue). Dotted horizontal lines represent the value above which a protein is considered a hit in each cohort. These thresholds are calculated as a log-reactivity Z score larger than 2.324 (corresponding to the top 1% of a normal distribution) after quantile normalization of each group. **p = 0.0052.
Table 1.
ProtoArray-derived data showing targets recognized by autoantibodies in the CSF of patients with multiple sclerosis
The arrays included antigens that had been previously described as targets of CSF-derived autoantibodies in MS. These included myelin oligodendrocyte glycoprotein (MOG),13 myelin-associated glycoprotein (MAG),14 myelin basic protein (MBP),15 and glyceraldehyde 3-phosphate dehydrogenase.16 Binding to these proteins was not statistically significantly different between the patients with MS and controls. Other candidates such as CNPase17 and contactin18 were not present on the array.
Validation experiments.
Considering the results of the array, we chose to focus on the candidate antigen RBPJ for our validation studies. First, to confirm that RBPJ was indeed a target of the autoantibodies present in the CSF of the MS cases tested on the array, an ELISA was performed. The RRMS CSF cases tested displayed significantly elevated ELISA signals relative to the control OIND CSF cases (RRMS array vs OIND array, p = 0.004; Mann-Whitney), confirming the findings attained with the array (figure 2A). Moreover, reactivity against RBPJ measured by ELISA correlated with the array signal of the same patients (r2 = 0.64, p = 0.000945), further supporting these findings (figure e-3). The presence of RBPJ autoantibodies did not correlate with CSF IgG levels, ruling out a bias due to CSF IgG levels (data not shown).
Figure 2. CSF and serum-derived immunoglobulin G reactivity to RBPJ.
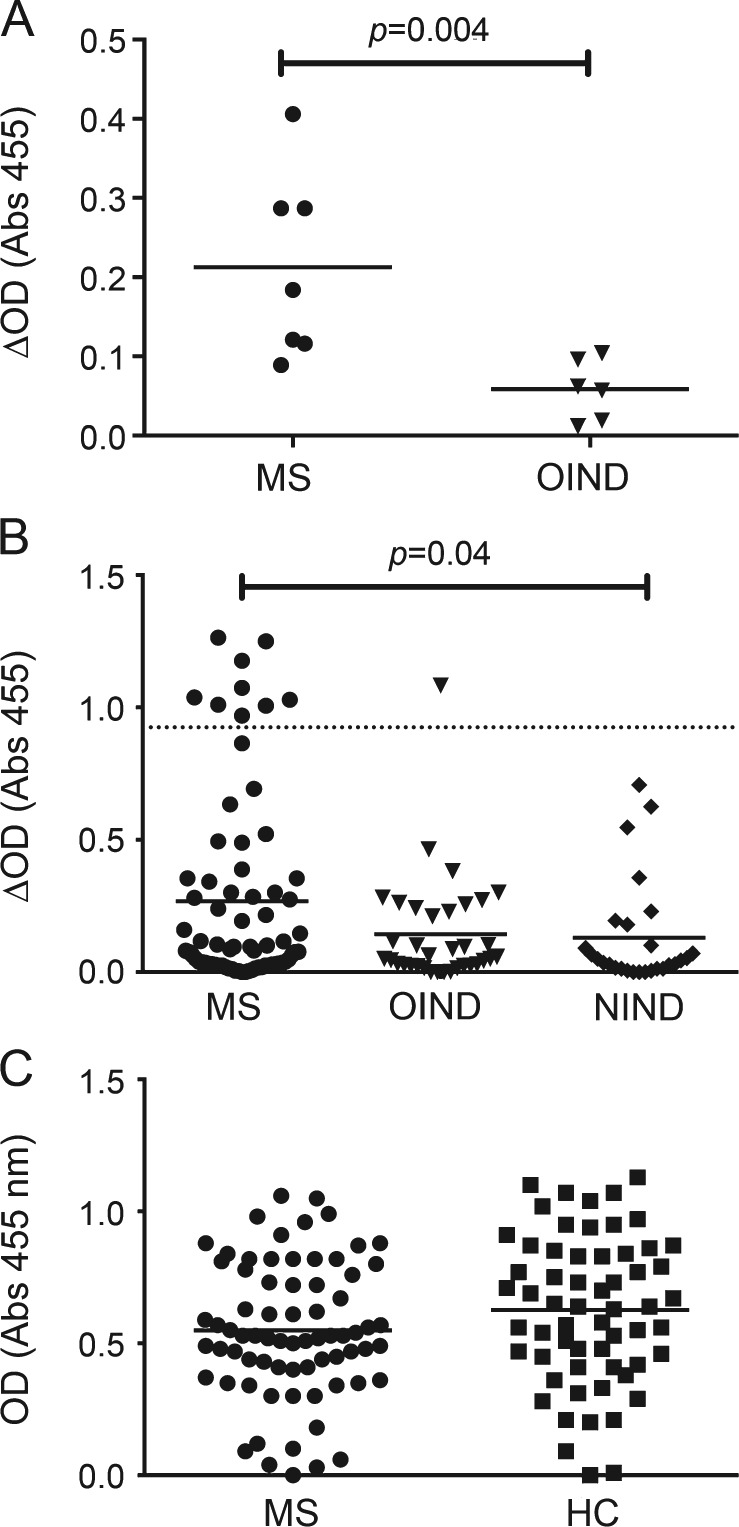
(A) ELISA was used to confirm that CSF-derived autoantibodies that recognized recombination signal binding protein for immunoglobulin kappa J region (RBPJ) in the array also recognized RBPJ in an independent assay platform. (B) ELISA demonstrates the presence of RBPJ autoantibodies in the CSF of patients with multiple sclerosis (MS) in a validation cohort. The dashed line marks the mean +4 SD of the noninflammatory neurologic diseases (NIND) cohort. Values above this line were determined to be positive. To correct for nonspecific binding in the CSF experiments, the reported ELISA signal (ΔOD) was calculated by subtracting the signal generated by binding to myosin light chain 5 from that of the RBPJ. (C) Serum reactivity to RBPJ in patients with MS did not differ from that of healthy controls (HC). Each point in the graphs represents an individual patient-derived specimen. Statistical differences are indicated when significant. OIND = other inflammatory neurologic diseases.
We next evaluated whether autoantibodies toward RBPJ were present in the CSF of a large independent cohort of MS cases that included progressive forms of the disease and CSF specimens without an elevated IgG index. As shown in figure 2B, the proportion of patients with MS testing positive for RBPJ reactivity (12.5%) was significantly higher than the controls without MS (1.6% vs 12.5%; p = 0.02, Fisher exact test). Reactivity against RBPJ was also significantly higher in the MS cases compared with NIND cases (p = 0.04; Mann-Whitney) but did not reach statistical significance between MS and OIND cases (p = 0.1). Eight (13.1%) patients with RRMS, 1 (9%) patient with SPMS, 1 (2.8%) patient with OIND, and 0 (0%) patients with NIND were classified as positive. Details regarding the CSF ELISA results are shown in table e-2. To further demonstrate the specificity of the RBPJ binding seen in a subset of MS cases, we examined binding to 2 irrelevant antigens, human histone H1 and lysozyme. All the CSF samples that gave a positive signal in the RBPJ ELISA showed no detectable binding to either antigen (figure e-4). We also evaluated whether serum from patients with MS harbored autoantibodies to RBPJ. Serum reactivity was assessed using ELISA comparing patients with MS (n = 72) and healthy donors (n = 60). No specific reactivity was observed in patients with MS relative to controls (figure 2C).
To further confirm the specificity for RBPJ, a second assay utilizing immunocytochemistry (ICC) was used to test the specimens. CSF, from a random selection of patients (30 RRMS, 10 SPMS) and controls (30 OIND, 17 NIND), was tested for RBPJ reactivity by ICC. Four RRMS samples (13.3%) contained autoantibodies that recognized RBPJ-transfected HEK cells, while none of the SPMS, OIND, or NIND-derived samples displayed detectable binding (figure 3). The 4 RRMS samples did not recognize nontransfected cells.
Figure 3. Immunocytochemistry demonstrates the presence of RBPJ autoantibodies in the CSF of patients with multiple sclerosis.
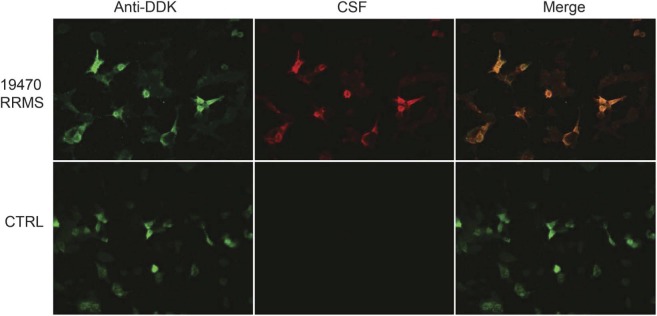
Images from testing a representative multiple sclerosis (MS) CSF specimen (19470) are shown on the top row. Recombination signal binding protein for immunoglobulin kappa J region (RBPJ) expression was confirmed through detection with an anti-DDK antibody (green). Strong reactivity to RBPJ by autoantibodies (red) in the CSF from this patient with MS is demonstrated by the colocalization (merge) of the signals. The bottom row shows lack of reactivity by a control CSF (other inflammatory neurologic diseases). RRMS = relapsing-remitting multiple sclerosis.
DISCUSSION
Our study describes the presence of CSF-derived IgG reactivity against RBPJ in a subset of patients with MS. Protein array–based screening of 9,393 proteins provided an unbiased approach for antigen discovery and identified RBPJ as a target of the CSF IgG from patients with MS. This reactivity was confirmed with a larger, more heterogeneous cohort using 2 independent assays (ELISA and ICC). Reactivity was restricted to the CSF as no binding was observed to RBPJ using a large cohort of serum samples. This suggests that the autoantibodies we measured in the CSF were of intrathecal origin, although serum-CSF pairs from patients testing positive were not available to address this.
The search for specific autoantibodies in MS has been an area of focus for decades but the antigens targeted by MS autoantibodies have remained elusive. A number of autoantibodies, mainly against myelin and neuronal antigens, have been described in MS. Several reports describe a higher frequency of antibodies against the myelin proteins MOG,11,19 MBP,20–22 and MAG14,23 in patients with MS than controls. In our study, however, reactivity against those proteins failed to discriminate between MS and OIND. A recent report identified autoantibodies against KIR4.1 in the serum of nearly half of all MS cases examined.24 KIR4.1 was not identified as a target in the protein array experiments described here. Perhaps this discrepancy is due to the fact that our discovery cohort was small (n = 7) and focused on CSF reactivity rather than serum. The manner in which an integral membrane protein, such as KIR4.1, is presented on the array must also be considered.
Antigen arrays provide a sensitive tool for high-throughput screening of autoantibodies. Several reports have described autoantibody profiles with lipid and protein arrays in MS.25−29 A recent report described an increased CSF reactivity against myelin and heat-shock proteins in patients with RRMS compared to controls.28 Another report, using a similar approach to ours, described the MS CSF reactivity profile in an array of more than 3,000 proteins.25 A common antigen was not found between these 2 studies and our own. The different antigen sets and presentation on the respective arrays could account for the disparity between the reactivity patterns. The arrays we used displayed 9,393 proteins expressed in a eukaryotic system to best preserve their native conformation and physiologic features, while others used peptide arrays28 and proteins expressed in Escherichia coli.25 Because of the substantial differences in which antigens are presented on these arrays, comparing results derived from different platforms is challenging. Antigens identified as targets in different array-based studies should be investigated with conventional techniques, such as ELISA, so that validation is possible.
As the array we used does not present an exhaustive representation of the human proteome, autoantigens may have been missed. Similar array-based studies performed with larger protein sets would likely deliver new and relevant candidate antigens. Importantly, manufacturers and investigators should focus on developing arrays that include targets of antibodies that are more likely to be biologically relevant and expressed in a manner that most closely emulates their physiologic state. Cell surface proteins that have domains present outside of the cell where they would be available to circulating antibodies should be a priority.
RBPJ is a transcriptional regulator that is central in the Notch signaling pathway. It is a nuclear protein involved in the development and differentiation of multiple cell types. These features and its ubiquitous expression make it unlikely that antibodies against it are pathogenic, although this does not exclude it as a biologically relevant candidate autoantibody target. Indeed, examples of autoantibodies against intracellular antigens that may be pathogenic have been identified.30 RBPJ plays a central role in Epstein-Barr virus (EBV) infection, a known environmental MS risk factor,31 by interacting with EBNA proteins.32,33 Some studies have suggested that cross-reactivity between EBV proteins and MS-derived immune cells can occur.34,35 It is not clear that these mechanisms play a role in the development of RBPJ antibodies observed in our study. Antibodies directed toward RBPJ have also been described, using the same protein array, in patients with breast cancer36 and using phage libraries in myeloid leukemia.37 A possible explanation for this interesting coincidence is that antigen release due to tissue damage can elicit an antibody response against intracellular proteins such as RBPJ. However, it would not explain why the same does not occur to the same extent in patients with OIND, which includes chronic diseases such as subacute sclerosing panencephalitis, neurosyphilis, or neurosarcoidosis, in which tissue damage is also prominent. Finally, it remains possible that the reactivity observed to RBPJ is due to mimicry between this protein and a true undefined antigenic target.
The proportion of patients with MS reacting against RBPJ was modest in our validation cohort, but consistent using both ELISA and ICC. The identification of patients harboring autoantibodies against RBPJ with these 2 additional techniques, within a more heterogeneous cohort, supports the findings we obtained with the arrays. Although RBPJ reactivity is present in a subset of patients with MS, the description of small proportions of patients defined by autoantibodies has proven clinically useful in other diseases such as autoimmune encephalitis,38 myasthenia gravis,39 and chronic inflammatory demyelinating polyradiculoneuropathy.40 Detecting specific clinical features in the patients harboring RBPJ autoantibodies would be of great interest. Detailed clinical and therapeutic data were not available for many of our specimens, precluding any clinical-immunologic correlation. Nevertheless, studies addressing pathogenic roles or clinical-immunologic correlation should be performed in future studies to assess the relevance of anti-RBPJ antibodies in MS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Human Brain and Spinal Fluid Resource Center (VA Greater Los Angeles Healthcare System, West Los Angeles Healthcare Center) for providing a number of the CSF samples used in this study and Jace Forbes-Cockell for assisting with the data analysis.
GLOSSARY
- EBV
Epstein-Barr virus
- FDR
false discovery rate
- ICC
immunocytochemistry
- IgG
immunoglobulin G
- MAG
myelin-associated glycoprotein
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MYL5
myosin light chain 5
- NIND
noninflammatory neurologic diseases
- OCB
oligoclonal bands
- OIND
other inflammatory neurologic diseases
- RBPJ
recombination signal binding protein for immunoglobulin kappa J region
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
Footnotes
Editorial, page 944
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: S.N.W., L.Q., D.A.H., K.C.O. Performed the experiments: S.N.W., P.L.C., L.Q., K.C.O. Analyzed the data: S.N.W., C.C., M.A.B., J.-Y.L., A.H.C., L.Q., G.Y., S.H.K., K.C.O. Wrote and edited the paper: S.N.W., C.C., A.H.C., L.Q., J.-Y.L., G.Y., S.H.K., K.C.O. Provided characterized specimens: A.H.C., M.A.B.
STUDY FUNDING
Supported by grant R01NS024247 to D.A.H. from National Institute of Neurological Disorders and Stroke/National Institutes of Health (NINDS/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NINDS or NIH. K.C.O. receives support from the Nancy Davis Foundation for Multiple Sclerosis. S.N.W. receives support from National Health and Medical Research Council of Australia (CJ Martin Biomedical Research Fellowship). L.Q. is supported by the FIS CM 09/00017 grant from Fondo de Investigaciones Sanitarias—Instituto de Salud Carlos III, Spain. A.H.C. was supported in part by the Manny and Rosalyn Rosenthal–Dr. John L. Trotter Chair in Neuroimmunology of the Barnes–Jewish Foundation.
DISCLOSURE
L. Querol is supported by the FIS CM 09/00017 grant from Fondo de Investigaciones Sanitarias–Instituto de Salud Carlos III, Spain. P. Clark, M. Bailey, and C. Cotsapas report no disclosures. A. Cross was supported in part by the Manny and Rosalyn Rosenthal–Dr. John L. Trotter Chair in Neuroimmunology of the Barnes-Jewish Foundation. J. Lee, G. Yaari, and S. Kleinstein report no disclosures. D. Hafler: the project was supported by grant R01NS024247 from National Institute of Neurological Disorders and Stroke/National Institutes of Health (NINDS/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NINDS or NIH. S. Willis receives support from National Health and Medical Research Council of Australia (CJ Martin Biomedical Research Fellowship). K. O'Connor receives support from the Nancy Davis Foundation for Multiple Sclerosis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007;17:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest 2012;122:1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin KA, Wucherpfennig KW. B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol 2008;98:121–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klawiter EC, Cross AH. B cells: no longer the nondominant arm of multiple sclerosis. Curr Neurol Neurosci Rep 2007;7:231–238 [DOI] [PubMed] [Google Scholar]
- 5.Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 2012;337:1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovato L, Willis SN, Rodig SJ, et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 2011;134:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obermeier B, Mentele R, Malotka J, et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med 2008;14:688–693 [DOI] [PubMed] [Google Scholar]
- 8.Obermeier B, Lovato L, Mentele R, et al. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J Neuroimmunol 2011;233:245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Gilden D, Schambers L, et al. Peptide reactivity between multiple sclerosis (MS) CSF IgG and recombinant antibodies generated from clonally expanded plasma cells in MS CSF. J Neuroimmunol 2011;233:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connor KC, Appel H, Bregoli L, et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J Immunol 2005;175:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser 1995;57:289–300 [Google Scholar]
- 13.Xiao BG, Linington C, Link H. Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol 1991;31:91–96 [DOI] [PubMed] [Google Scholar]
- 14.Moller JR, Johnson D, Brady RO, Tourtellotte WW, Quarles RH. Antibodies to myelin-associated glycoprotein (MAG) in the cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 1989;22:55–61 [DOI] [PubMed] [Google Scholar]
- 15.Catz I, Warren KG. Intrathecal synthesis of autoantibodies to myelin basic protein in multiple sclerosis. Can J Neurol Sci 1986;13:21–24 [DOI] [PubMed] [Google Scholar]
- 16.Kolln J, Ren HM, Da RR, et al. Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol 2006;177:5652–5658 [DOI] [PubMed] [Google Scholar]
- 17.Walsh MJ, Murray JM. Dual implication of 2', 3'-cyclic nucleotide 3' phosphodiesterase as major autoantigen and C3 complement-binding protein in the pathogenesis of multiple sclerosis. J Clin Invest 1998;101:1923–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derfuss T, Parikh K, Velhin S, et al. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci USA 2009;106:8302–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klawiter EC, Piccio L, Lyons JA, Mikesell R, O'Connor KC, Cross AH. Elevated intrathecal myelin oligodendrocyte glycoprotein antibodies in multiple sclerosis. Arch Neurol 2010;67:1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz M, Olsson T, Ernerudh J, Hojeberg B, Link H. Immunoblot detection of oligoclonal anti-myelin basic protein IgG antibodies in cerebrospinal fluid in multiple sclerosis. Neurology 1987;37:1515–1519 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Merino A, Persson MA, Ernerudh J, Diaz-Gil JJ, Olsson T. Serum and cerebrospinal fluid antibodies against myelin basic protein and their IgG subclass distribution in multiple sclerosis. J Neurol Neurosurg Psychiatry 1986;49:1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reindl M, Linington C, Brehm U, et al. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 1999;122:2047–2056 [DOI] [PubMed] [Google Scholar]
- 23.Nobile-Orazio E, Spagnol G, Scarlato G. Failure to detect anti-MAG antibodies by RIA in CSF of patients with multiple sclerosis. J Neuroimmunol 1986;11:165–169 [DOI] [PubMed] [Google Scholar]
- 24.Srivastava R, Aslam M, Kalluri SR, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. New Engl J Med 2012;367:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyer NH, Lueking A, Kowald A, Frederiksen JL, Heegaard NH. Investigation of autoantibody profiles for cerebrospinal fluid biomarker discovery in patients with relapsing-remitting multiple sclerosis. J Neuroimmunol 2012;242:26–32 [DOI] [PubMed] [Google Scholar]
- 26.Brennan KM, Galban-Horcajo F, Rinaldi S, et al. Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J Neuroimmunol 2011;238:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanter JL, Narayana S, Ho PP, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med 2006;12:138–143 [DOI] [PubMed] [Google Scholar]
- 28.Quintana FJ, Farez MF, Izquierdo G, Lucas M, Cohen IR, Weiner HL. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology 2012;78:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana FJ, Farez MF, Viglietta V, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA 2008;105:18889–18894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto I, Maccioni M, Lee DM, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol 2002;3:360–365 [DOI] [PubMed] [Google Scholar]
- 31.Ascherio A, Munger KL, Lennette ET, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 2001;286:3083–3088 [DOI] [PubMed] [Google Scholar]
- 32.Henkel T, Ling PD, Hayward SD, Peterson MG. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science 1994;265:92–95 [DOI] [PubMed] [Google Scholar]
- 33.Hsieh JJ, Hayward SD. Masking of the CBF1/RBPJ kappa transcriptional repression domain by Epstein-Barr virus EBNA2. Science 1995;268:560–563 [DOI] [PubMed] [Google Scholar]
- 34.Gabibov AG, Belogurov AA, Jr, Lomakin YA, et al. Combinatorial antibody library from multiple sclerosis patients reveals antibodies that cross-react with myelin basic protein and EBV antigen. FASEB J 2011;25:4211–4221 [DOI] [PubMed] [Google Scholar]
- 35.Lang HL, Jacobsen H, Ikemizu S, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol 2002;3:940–943 [DOI] [PubMed] [Google Scholar]
- 36.Mange A, Lacombe J, Bascoul-Mollevi C, et al. Serum autoantibody signature of ductal carcinoma in situ progression to invasive breast cancer. Clin Cancer Res 2012;18:1992–2000 [DOI] [PubMed] [Google Scholar]
- 37.Wu CJ, Yang XF, McLaughlin S, et al. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest 2000;106:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011;77:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365–368 [DOI] [PubMed] [Google Scholar]
- 40.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 2013;73:370–380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.