Abstract
Blood flow autoregulation results from the ability of resistance arteries to reduce or increase their diameters in response to changes in intravascular pressure. The mechanism by which arteries maintain a constant blood flow to organs over a range of pressures relies on this myogenic response, which defines the intrinsic property of the smooth muscle to contract in response to stretch. The resistance to flow created by myogenic tone (MT) prevents tissue damage and allows the maintenance of a constant perfusion, despite fluctuations in arterial pressure. Interventions targeting MT may provide a more rational therapeutic approach in vascular disorders, such as hypertension, vasospasm, chronic heart failure, or diabetes. Despite its early description by Bayliss in 1902, the cellular and molecular mechanisms underlying MT remain poorly understood. We now appreciate that MT requires a complex mechanotransduction converting a physical stimulus (pressure) into a biological response (change in vessel diameter). Although smooth muscle cell depolarization and a rise in intracellular calcium concentration are recognized as cornerstones of the myogenic response, the role of wall strain-induced formation of vasoactive mediators is less well established. The vascular system expresses a large variety of Class 1 G protein-coupled receptors (GPCR) activated by an eclectic range of chemical entities, including peptides, lipids, nucleotides, and amines. These messengers can function in blood vessels as vasoconstrictors. This review focuses on locally generated GPCR agonists and their proposed contributions to MT. Their interplay with pivotal Gq-11 and G12-13 protein signalling is also discussed.
Keywords: Myogenic tone, G protein-coupled receptors, Gq-11, G12-13, Rho, TRP channels
1. Introduction
The arterial microcirculation encompasses small arteries and arterioles of the vascular tree (10–100 µm in diameter) that are embedded within organs, as opposed to larger conduit arteries, which transport blood to organs. Arterioles carry blood to the capillaries and are responsible for the distribution of blood within tissues. The functions of microcirculation include the regulation of blood flow and tissue perfusion, blood pressure, fluid movement (swelling or oedema), oxygen and nutrient delivery, as well as the regulation of body temperature. According to Poiseuille's law, the laminar flow rate of an incompressible fluid along a tube is proportional to the fourth power of its radius. As a result, small changes in arterial radius strongly impact blood flow, peripheral vascular resistance, and mean arterial pressure. Microcirculatory tone is set by the sympathetic tone and neurohumoral vasoactive systems, but is largely dependent on its endogenous myogenic tone (MT), which relies on the intrinsic ability of small arteries to contract and reduce their diameter in response to increased internal pressure.1,2 Under physiological conditions, MT represents a rapid response of blood vessels to counteract pressure increases in capillaries and avoid fluid leakage and tissue damage. Importantly, MT represents an efficient mechanism allowing a vascular bed to maintain its nutritive blood flow constant despite wide changes in arterial pressure. Although taking place in most tissues, MT is particularly pronounced in the kidney, the brain, and the heart. Renal blood flow autoregulation is achieved by the myogenic response and by tubuloglomerular feedback.3,4 Cerebral blood flow autoregulation is critical in several disorders, including ischaemic disorders or Alzheimer's disease. Although cerebral blood flow can be measured using functional magnetic resonance imaging and positron emission tomography, the mechanisms involved in its autoregulation remain mainly puzzling. Vascular resistance in peripheral tissues is attributed to the resistance to flow offered by small arteries and arterioles, while in the cerebral circulation, large extra and intracranial arteries also contribute to vascular resistance.
Resetting MT to a normal level has been proposed as a valuable therapeutic target in several vascular pathologies.5 In hypertension, ‘resetting’ MT not only limits blood pressure increase but also prevents inward eutrophic remodelling, which is thought to be linked to excessive vasoconstriction.6 The mechanisms implicated in vasospasm following subarachnoid haemorrhage (SAH) also suggest that limiting MT could reduce the deleterious effects caused by blood flow cessation.7 Abnormally elevated MT may underlie the increased vascular resistance in chronic heart failure8 and diabetes.9 In the long term, chronic activation of resistance artery mechanotransduction initiates mitogenic signalling as well as transcriptional activation, thus stimulating smooth muscle growth that can account for both hypertrophy and inward eutrophic remodelling that occurs in hypertension.6 Therefore, targeting MT would limit not only exaggerated vascular constriction but also pathological arterial remodelling.10
The development of MT requires a complex mechanotransduction process in vascular smooth muscle cells (VSMCs). Although depolarization of the membrane potential and subsequent calcium entry is essential, the role of wall strain-induced formation of vasoactive mediators is less well established. We review here the cellular mechanisms of MT and we focus on locally generated mediators and their interplay with the pivotal Gq-11 G12-13 intracellular signalling pathways.
2. Mechanisms underlying vascular myogenic contraction
The understanding of the specific mechanisms underlying MT can be organized into a linear sequence including (i) detection of vascular wall strain/stretch by mechanosensors; (ii) parallel or sequential transduction of the signal initiated by depolarization, ion channel modulation, Ca2+ increase, and protein phosphorylation; (iii) activation of actin–myosin interaction; and (iv) vasoconstriction.2
Membrane depolarization of VSMCs is necessary for MT to take place.2 This depolarization depends on stretch-activated ion channels (SACs), which allow direct calcium entry and depolarization, inducing voltage-dependent calcium channel opening, and thus, a further increase in intracellular calcium concentrations. The molecular identity of SACs remains elusive. Transient receptor potential channels (TRP function will be detailed in Section 2.2.3) and the epithelial Na+ channel ENac have been proposed to play a permissive role in these currents.11,12 Membrane depolarization subsequently triggers a secondary Ca2+ entry through L-type voltage-gated calcium channels (VGCC).2 Concomitantly, a negative feedback occurs through the opening of Ca2+-activated potassium channels (BKCa), which leads to cell hyperpolarization.13 Adding to this classical scheme, arguments exist in favour of integrins as sensors/transducers initiating the myogenic process.14,15 More specifically, αvβ3 and α5β1 integrins were proposed to modulate cellular mechanotransduction,16 likely through phosphorylation of VGCCs and BKCa ion channels.17 Integrin activation could be the trigger for tyrosine phosphorylation occurring in the myogenic response.18
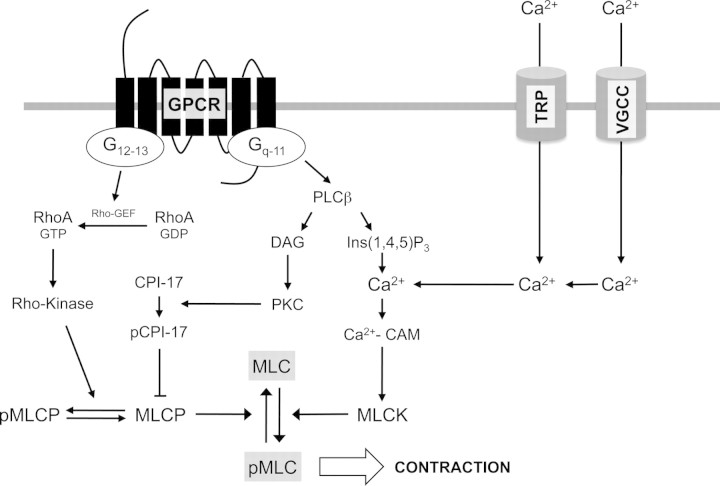
Vascular contraction correlates with the phosphorylation state of 20 kDa myosin light chain (MLC) that drives myosin–actin interaction. The extent of phosphorylation depends on the intracellular-free calcium concentration ([Ca2+]i) and on mechanisms modulating the sensitivity of the contractile apparatus to calcium.19 The degree of MLC phosphorylation is determined by myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP) activity. The activation of MLCK is regulated by the Ca2+-calmodulin complex formation, whereas MLCP activity, which constitutes the Ca2+-sensitization pathway, is controlled by both Rho-kinase and protein kinase C (PKC)-dependent mechanisms19 (Figure 1).
Figure 1.
Intracellular signalling underlying vascular smooth muscle cells contraction. Contraction is induced by the increased phosphorylation of 20 kDa myosin light chain (MLC). Activation of G protein-coupled receptors (GPCR) triggers both Ca2+-dependent phosphorylation of MLC and calcium facilitation inhibiting MLC dephosphorylation. Gq-11 protein activates phospholipase C (PLC) and induces intracellular Ca2+ rise through inositol (1,4,5) triphosphate (Ins(1,4,5)P3)-sensitive endoplasmic reticulum stores. This adds to extracellular Ca2+ entry through voltage-gated calcium channels (VGCCs) and transient receptor potential (TRP) channels. The Ca2+-Calmodulin complex (Ca2+-CAM) induces the phosphorylation of MLCK. The second product of PLC, diacylglycerol (DAG), activates protein kinase C (PKC), which, through its substrate CPI-17, inhibits MLCP, and thus, MLC dephosphorylation. G12-13 activates Rho guanine nucleotides exchanging factor (Rho-GEF) that activates RhoA protein through GDP exchange to GTP. GTP-bound Rho activates Rho-kinase which, through MLCP phosphorylation, inhibits MLC dephosphorylation.
Hence, [Ca2+]i is essential for the molecular mechanisms of vascular contraction. The regulation of [Ca2+]i entry in VSMCs encompasses: (i) Ca2+ entry via VGCC (especially the L-type Ca2+ channel) and ligand-gated Ca2+-channels; (ii) Ca2+ release from endoplasmic reticulum stores following phospholipase C (PLC) activation and inositol(1,4,5) triphosphate (Ins(1,4,5)P3) release; (iii) Ca2+ entry via transient receptor potential (TRP) channels; (iv) Ca2+-induced Ca2+ release (CICR) from endoplasmic reticulum stores; (v) Ca2+ entry through type 1 Na+/Ca2+ exchanger (NCX1); and (vi) other Ca2+ channels such as GPCR-operated Ca2+ channels and tyrosine-kinase-activated Ca2+ channels. Current knowledge of [Ca2+]i homeostasis in VSMCs has been reviewed recently.20
It was assumed for some time that transmembrane [Ca2+]i influx is essential for VSMC contraction in response to stretch.21,22 However, Meininger et al.,23 using real-time measurement of [Ca2+]i, pointed to the lack of a simple proportional relationship between calcium and contraction, and this was taken as evidence for alternative mechanisms regulating vascular contraction.
Calcium sensitization is clearly important for myogenic response. The Ca2+ requirement to sustain stretch-induced MT in the rabbit facial vein is much lower than that needed by equi-effective contractile agonists.24 Furthermore, direct PKC activation increases pressure-induced force without increasing Ca2+ influx,25 suggesting the existence of [Ca2+]i increase-independent and PKC-dependent MLC phosphorylation pathways. One arm of this Ca2+ sensitization has indeed been attributed to the PKC substrate CPI17,26 a 17 kDa peptide, whose phosphorylation enhances its ability to inhibit MLCP, and thus, MLC phosphorylation.19 The other arm is dependent on the Rho/Rho-kinase signalling pathway.27 In cerebral arteries, pharmacological inhibition of Rho-kinase reduces MT in vitro28 and in vivo.29 The contribution of Rho-dependent Ca2+ sensitization is enhanced during chronic hypertension.29 While the molecular events leading to Rho activation remains to be fully characterized, it seems that RhoA interaction with caveolin-1 is essential for Rho-kinase activation.30 This Ca2+ sensitization process probably regulates the interaction of MT with other constrictors, as initially described by the synergistic interaction between the sympathetic system and MT in cremaster muscle arteries.31
3. G protein-coupled receptors (GPCR) and MT
Vasoconstrictor hormones and autacoids acting through GPCRs exert their effect via activation of the Gq-11 and G12-13 subclasses.32 While Gq-11 protein leads to Ca2+ mobilization and PKC activation, G12-13 protein activates the Rho pathway. Both pathways work in synergy during the myogenic process.
3.1. Contribution of locally generated GPCR agonists to MT
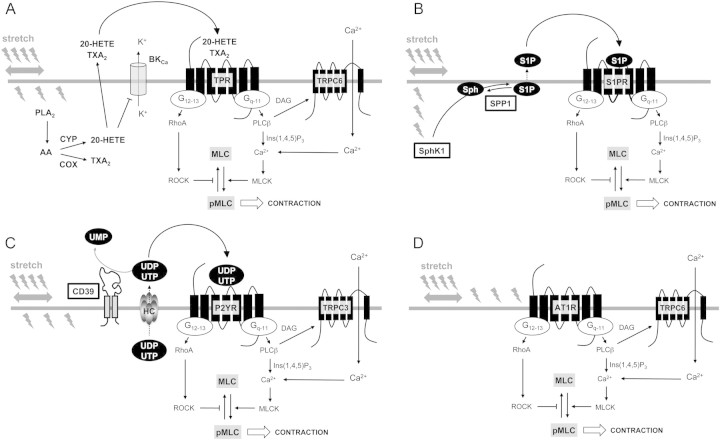
The mechanical stress imposed on the vessel wall following an increased intra-arterial pressure induces the local generation of an eclectic range of mediators including peptides, lipids, nucleotides, and amines. To date, evidence suggests that these mediators reinforce MT through interactions with intracellular second messenger and as autocrine/paracrine vasoconstrictors through Class1 GPCR activation. Proposed participation of locally generated GPCR agonists in MT is represented in Figure 2.
Figure 2.
Proposed mechanism of local GPCR agonists in response to smooth muscle stretch. VSMC stretch activates the generation and release of several autacoids. (A) Phospholipase A2 generates arachidonic acid (AA), which can be metabolized into thromboxane A2 (TXA2) and 20-hydroxyeicosatetraenoic acid (20-HETE) through the respective action of cyclooxygenase (COX) and cytochrome P450 (CYP) enzymes. 20-HETE inhibits calcium-activated potassium channels (BKCa), thus limiting VSMC hyperpolarization. Both TXA2 and 20-HETE may act locally on an autocrine mode on VSMC TP receptor (TPR). (B) Sphingosine kinase-1 (SphK1) translocates to plasma membrane in response to stretch and phosphorylates sphingosine (Sph) to generate sphingosine-1-phosphate (S1P). This reaction is functionally antagonized by the sphingosine-1-phosphate specific phosphatase (SPP1). S1P activates S1P2 or 3 receptors (S1PR) to reinforce contraction. (C) Nucleotides are released in response to cell stretch,72 most probably through membrane hemichannels (HC). Autocrine activation of purinergic receptor (P2YR) by uridine-5′-tri and/or diphosphate (UTP, UDP) reinforces contraction. UTP receptor-dependent activation of TRPC3 channel further increases Ca2+ rise. This effect of extracellular nucleotides is controlled by CD39 ectonucleotidase.70 (D) AngiotensinII type 1 receptor (AT1R) has been proposed to be directly activated by stretch and to subsequently activate TRPC6 channels.78
3.1.1. Endothelin-1
Endothelin-1 (ET-1) is released by endothelial cells in response to various stresses and its effect is mediated via the activation of two GPCR subtypes: ETA and ETB.33 It is commonly accepted that in human vessels, ETA receptors are mainly located on VSMCs, with ETB receptors being present on endothelial cells. ETB receptors, on the other hand, may play a role in the release of NO and PGI2.34
In spontaneously hypertensive rats (SHRs), the enhanced MT of skeletal muscle arterioles is due to the production of endothelium-derived constrictor factors such as Prostaglandin H2 (PGH2)/thromboxane A2 (TXA2) and ET-1.35 Both agents increase Ca2+ sensitivity of the contractile apparatus in arteriolar smooth muscle.36 Similarly, ETA antagonists abolish the exacerbated MT in tumour arterioles. This selectivity is associated with a large increase in ET-1 abundance in tumours and a higher ETA receptor density in tumour vessels.37 Indeed, oxidative stress increases ET-1 production in isolated and pressurized rat gracilis skeletal muscle arterioles. In these arteries, H2O2 increases MT through the production of ET-1.38 Basal ET-1 release has also been reported to mediate MT in coronary arteries, and it has been suggested that the contribution of ET-1 in coronary artery MT would lead to a reduction in coronary vasodilatory reserve, and thus, increase susceptibility to ischaemia and arrhythmia.39
3.1.2. Arachidonic acid-derived metabolites
Arachidonic acid (AA) released after membrane phospholipid hydrolysis by phospholipases is the precursor of a number of bioactive metabolites such as leukotrienes, prostaglandins, TxA2, epoxyeicosatrienoic acids (EETs), and hydroxyeicosatetraenoic acids (HETE). Many of these molecules are vasoactive, for instance, endothelial-derived prostacyclin induces vasodilation while TXA2 and PGH2 are potent vasoconstrictors.40
3.1.2.1. 20-Hydroxyeicosatetraenoic acid
20-Hydroxyeicosatetraenoic acid (20-HETE) is synthesized by ω-hydroxylation of AA via cytochrome P-450 (CP450) 4A. The contribution of 20-HETE to MT has been described in renal,41 coronary,42 skeletal,43 and mesenteric44 arteries. Inhibition of 20-HETE synthesis reduces infarct size after cerebral ischaemia reperfusion injury (IRI),45 whereas inhibition of 20-HETE activity decreases cerebral damage following stroke,46 suggesting that targeting the 20-HETE pathway could have therapeutic benefits in IRI and vasospasm following SAH.
Evidence supporting a key role of 20-HETE in MT is the following: First, 20-HETE inhibits the opening of the large conductance BKCa channel, causing depolarization and increasing Ca2+ influx through L-type Ca2+ channels.47,48 Second, the phosphorylation of myristoylated, alanine-rich PKC substrate (MARCKS), and the inhibition produced by PKC pseudosubstrate suggest that 20-HETE-induced inhibition of K+ currents largely depends on PKC activation.49 Third, 20-HETE activates the Rho pathway, leading to the phosphorylation of MLC and the sensitization of the contractile apparatus in coronary arteries.50 Finally, 20-HETE interacts with TRPC6,51 which appears to be a cornerstone of the myogenic process (Section 2.3.3.). Interestingly, 20-HETE-dependent vasoconstriction of cerebral arteries was reported to occur through G protein-coupled thromboxane prostanoid (TP) receptors.52 Together with 20-HETE-dependent Rho activation, this observation raises the question of the contribution of this mediator as a TP agonist in addition to its well-known role as an intracellular second messenger.
3.1.2.2. TXA2 and PGH2
Stimulation of TXA2/PGH2 (TP) receptors elicits contraction of VSMCs.53 In rat descending coronary artery, inhibition of prostaglandin synthesis or TP receptors blockade reduces MT.54 In SHRs, increased MT is thought to be due to an enhanced production of endothelium-derived constrictor factors, primarily PGH2.55 Interestingly, this seems to be specific to hypertension because endothelial-derived vasorelaxant prostanoids oppose MT in normotensive animals.56 An increased arteriolar MT accompanies microvascular growth in young rats through mechanisms that are sensitive to TP receptor blockade.57 The role of TXA2 or PGH2 in reinforcing MT during rapid juvenile growth also persists in hypertension. A proposed mechanism of action of TXA2 in MT is presented in Figure 2A.
3.1.3. Sphingosine-1-phosphate (S1P)
S1P is a bioactive lipid with pleiotropic cellular effects such as proliferation, differentiation, and cell migration.58 S1P exerts its effect through five GPCRs [S1P receptors 1–5] (previously endothelial differentiation gene-identified).59 Vascular myocytes express S1P2 and/or S1P3 receptors that are involved in S1P-mediated constriction and in vitro SMC proliferation through the Rho pathway and p42/p44 mitogen activated protein kinases (MAPK), respectively.60 Interestingly, some small-diameter arteries that develop MT (e.g. renal, mesenteric, and basilar arteries) are more responsive to exogenous S1P-mediated vasoconstriction compared with large-diameter conduit arteries.61,62 Cellular synthesis of S1P is limited under unstimulated conditions through the spatial separation of the sphingosine kinase 1 (Sphk1) enzyme in the cytosolic compartment from its membrane substrate, sphingosine. Bolz et al.63 proposed that arterial myocytes generate S1P in the vascular wall, which can then participate in MT. This mechanism implicates stretch-dependent relocation of Sphk1 and sphingosine phosphorylation. Locally generated S1P enhances vascular contraction through S1P2/Rho-kinase activation in an autocrine/paracrine mode (Figure 2B). Accordingly, the S1P2 receptor antagonist JTE-013 potently inhibits MT of cremaster arterioles.64 A second enzyme, the S1P-specific phosphatase (SPP1), functionally antagonizes S1P effects. By dephosphorylating S1P, SPP1 opposes the effect of Sphk1 and, consequently, constitutes a negative regulator of myogenic and resting microvascular tone.65 These molecular mechanisms suggest that a finely tuned mechanism of S1P receptor agonist formation and elimination may constitute regulatory steps of the myogenic response.
3.1.4. Extracellular nucleotides
Beside their well-characterized role in cellular metabolism, nucleotides can be released extracellularly following non-lytic mechanisms.66 Extracellular nucleotides exert both vasodilator and vasoconstrictor effects through EC and VSMC membrane-bound P2-type receptors. P2 receptors are divided into ligand-gated P2X ion channels (P2X1–7) and G protein-coupled P2Y receptors (P2Y1,2,4,6,11–14).67 ATP activates all P2X receptors, whereas P2Y receptors are differentially activated by ATP, ADP, UTP, UDP, or UDP-glucose.68 Ectonucleotidases from the ENTPDase family actively hydrolyse juxtacellular nucleotides and terminate activation of P2 receptors.69 Interestingly, invalidation of the dominant vascular NTPDase (CD39) results in a facilitated contractile response to exogenous nucleotides (prolonged bioavailability) but also in enhanced MT in resistance arteries.70 Nucleotides exert a P2 receptor-dependent potent vasoconstriction in mouse mesenteric arteries70,71 and the difference in MT may be attributable to the release of nucleotides upon cell stretching following intraluminal pressure increases. Accordingly, cellular mechanical stretch is a well-known nucleotide release process72 (Figure 2C). VSMCs themselves may be a source of uracil nucleotides as vascular smooth muscles have a low intracellular ATP/UTP ratio. It has also been reported that pyrimidines are released by perfused rat hind limbs in response to constrictor agents (noradrenaline, vasopressin, AngII).73 Finally, P2 receptor antagonists inhibit MT in mouse mesenteric arteries.70,74 Preliminary observations suggest that P2Y6 receptors may underlie this amplification process.75 Interestingly, another study reported a similar autocrine loop involving P2Y6 receptor activation in cardiomyocytes which, in this case, participates in the development of cardiac fibrosis.76 It is noteworthy that the proposed mode of action of nucleotides is quite similar to the mechanisms suggested for the action of S1P (Figure 2B). Further investigations are required to characterize the role of nucleotide release and the activation of specific P2 receptors in MT. Noteworthy is the suggestion that the ionotropic P2X1 ATP receptor participates in pressure-dependent autoregulation in kidney afferent arterioles,77 suggesting that locally released nucleotides can act on both ionotropic and metabotropic P2 receptors to modulate MT.
3.2. Agonist-independent activation
The angiotensin II type 1 (AT1) receptor was the first GPCR implicated to be mechanosensitive.78 Using angiotensinogen-deficient mice and a neutralizing antibody directed against angiotensin II, Zou et al. reported that mechanical activation of AT1 receptor in cardiac myocytes is agonist-independent.79,80 Direct mechanosensitivity of AT1 receptors has been shown more recently in rat aortic A7r5 cells. These authors have also shown that MT of cerebral and renal resistance arteries was strongly reduced by an inverse AT1 receptor agonist independently of AngII secretion79,80 (Figure 2D). This hypothesis is different from autocrine activation by locally generated GPCR agonists that have been discussed above such as S1P, 20-HETE, and nucleotides. However, both indirect and direct evidence exists for the local generation of AngII.81–83 Importantly, functionally relevant changes in local AngI to AngII conversion are not necessarily reflected by detectable changes in circulating AngII.84 Thus, although activation of AT1 receptors is involved in MT, the question related to the requirement for local AngII generation for AT1 receptor activation remains a matter of debate. It is noteworthy that direct mechanical activation of AT1 receptor leading to TRP channels opening was observed in transfected cells, and it remains to be determined whether these observations are physiologically relevant.
Recently, additional vasoactive GPCR agonists, including apelin, motilin, neuromedin U, and urotensin-II, have emerged.85 To date, no data have shown that they had a role in MT. However, considering the central role of GPCRs to trigger and/or amplify MT, it would be interesting to evaluate the contribution of these agents and their receptors in MT.
3.3. GPCR activation acts in synergy with myogenic contraction signal transduction pathways
Vasoconstrictor hormones and autacoids acting through GPCRs exert their effects through Gq-11 and G12-13 proteins activation,32 and these G protein subclasses are thought to modulate MT.
3.3.1. Gq-11- PLCβ pathway
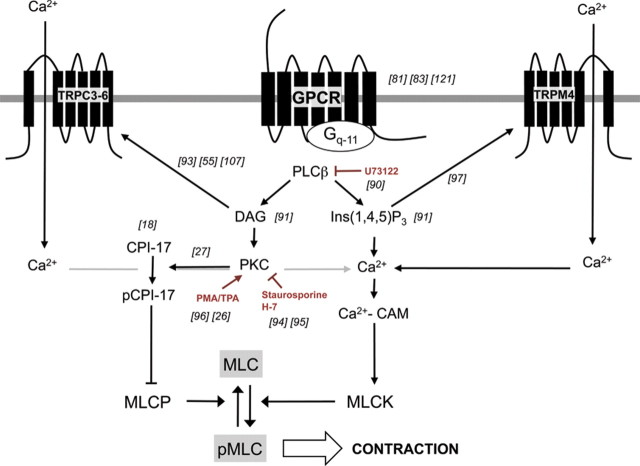
A large body of evidence suggests that the different elements of the Gq-11 transduction pathway act as MT enhancers, including G protein-coupled receptor itself, PLCβ, PKC, and Ins(1,4,5)P3-dependent Ca2+ mobilization (Figure 3).
Figure 3.
Implication of the Gq-11 transduction pathway in the myogenic process. There is a strong rationale regarding the participation of the different elements of the Gq-11 pathway in MT. Pharmacological, genetic, and electrophysiological approaches allowed to highlight that both intracellular Ca2+ stores mobilization, Ca2+ entry through TRP channels, and calcium facilitation pathway can be triggered through Gq-11 pathway activation. Abbreviations: CPI-17 indicates 17 kDa protein kinase C substrate phosphatase inhibitor; DAG, diacylglycerol; MLC, 20 kDa myosin light chain; MLCK, 20 kDa myosin light chain kinase; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; Staurosporine: [9S-(9α,10β,11β,13α)]-2,3,10,11,12,13-H exahydro-10-methoxy-9-methyl-11-(methylamino)-9,13 -epoxy-1H,9H-diindolo[1,2,3-gh:3′,2′,1′-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, protein Kinase C inhibitor. TPA, 12-O-tetradecanoylphorbol-13-acetate; TRP, transient receptor potential channel. U7122: 1-[6-[[(17β)-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione, phospholipase C inhibitor.
It has been almost 20 years since the first report showing that MT is sensitive to pharmacological inhibition of PLC,86 implicating G protein modulation of mechanotransduction through pathways superimposed on basal MT. This report was followed by a study by Narayanan et al.87 showing pressure-dependent Ins(1,4,5)P3 and DAG accumulation in dog renal arteries. Following Gq-11-coupled GPCR activation, Ca2+ mobilization resulting from Ins(1,4,5)P3 binding to its endoplasmic reticulum receptors adds to the overall [Ca2+]i increase, thus reinforcing VSMC contraction. A recent study suggests that Ins(1,4,5)P3 can increase [Ca2+]i independently of intracellular Ca2+ stores.88 This alternative mechanism requires a co-coordinated interaction of Ins(1,4,5)P3 receptors, TRPC3 channels, and voltage-dependent Ca2+ channel activation (Figure 3). Several TRP channels, potential components of the SAC involved in MT (i.e. TRPC3 and 6), are directly activated by DAG89 (Figure 2A, C, and D), suggesting that SAC-dependent non-selective cation currents may be partly dependent on Gq-11 through DAG formation. Moreover, MT is sensitive to pharmacological inhibition of PKC,90,91 whereas PKC activators, such as TPA or PMA, potentiate MT.92 PKC exerts its influence at different levels in MT. First, it increases sensitivity of the contractile apparatus to calcium,25 possibly through the intermediate C protein kinase inhibitor of phosphatase (CPI17),26 from which the phosphorylated form inhibits MLCP (Figure 3). Second, PKC phosphorylates and activates the TRPM4 channel, which is thought to be a key component of the SAC current.93 Based on studies using purified G proteins reconstituted into phospholipids vesicles, Gudi et al.94 proposed that G proteins are directly sensitive to shear stress through changes in membrane bilayer physical properties. So far, such direct mechanical activation of a G protein was not reported in MT.
The role of Gq-11-coupled receptors as direct mechanosensors has recently been reviewed.95 Membrane stretch induces agonist-independent activation of AT1 receptors, which subsequently signals TRPC channels in a Gq-11 and PLC-dependent manner.78 Antagonists and inverse agonists of AT1 receptors prevent this activation. Consistent with a contribution of this mechanism to MT, contraction of cerebral and renal arteries in response to increasing intraluminal pressure is reduced by AT1 receptor inverse agonists. According to this hypothesis, membrane stretch sensing by GPCRs is not only independent of the presence of agonist activation but can also be substituted by other (any) Gq-11-coupled receptors.78
Kinases from the mitogen-activated protein kinases (MAPK), whose activity depends on GPCR signalling, also play a role in MT. Massett et al.96 reported that whereas PKC and p42/44 MAP kinases are involved in both MT and agonist-induced contraction, p38 MAP kinase appears to be specifically involved in MT in rat gracilis muscle arterioles. Nevertheless, using a model that allows the application of stretch with or without the development of MT, we have shown that the MAPK p42/44 are activated by stretch but are not involved in MT.97 Similarly, using the same model, we have shown that p38 is only minimally involved in MT, although activated by wall stretch.98 Nevertheless, the latter study shows that the RhoA and Rho-kinase play a key role in MT.
3.3.2. G12-13-Rho pathway
Although the role of the Rho pathway in MT has been clearly shown, the involvement of GPCRs in Rho activation in response to pressure increases is not known. In VSMCs, GPCRs coupled to G12-13 proteins activate the RhoA pathway and cause MLC phosphorylation99 through indirect inhibition of its phosphatase (Figure 1). Interestingly, locally generated GPCR agonists are proposed to participate in MT (Section 2.1) and the corresponding GPCRs seem to couple preferentially to G12-13 proteins in arterial SMCs. This was shown for TXA2, endothelin-1,32 S1P,63 and P2Y uracyl nucleotides agonists,100 suggesting that locally generated G12-13-coupling GPCR agonists can trigger Rho activation.
Adding to the complexity of the scheme are the RhoA guanine nucleotide exchange factors (GEFs), which act as molecular switches to activate Rho. Specific GPCRs agonists may couple to distinct GEFs. Indeed, a recent work identified p115RhoGEF as specifically activated by AngII after binding to the AT1 receptor.101 The p63RhoGEF is directly coupled to and is activated by Gq protein establishing a link between Gq-11 and the Rho pathway.102 p63RhoGEF has been recently shown to mediate AT1 receptor-dependent RhoA activation in VSMCs, including the subsequent cellular proliferation and contraction.103 Considering the central role of RhoA in vascular contraction, the contribution of GEFs and other modulators of Rho activity, such as guanine dissociation inhibitors (GDIs) and GTPase-activating proteins (GAPs), in MT needs to be further investigated.
3.3.3. GPCRs act in synergy with TRP activation
As TRP are polymodal sensory ion channels involved in a number of mechanosensory systems,104 they are primary candidates for a role as SACs involved in Ca2+ entry in MT105,106 and possibly in MT.107 First, TRPC6 antisense oligonucleotides reduce pressure-induced depolarization, cationic currents, and MT in intact cerebral arteries,108 suggesting their participation in stretch-activated currents. Surprisingly, TRPC6-deficient mice display an exacerbated MT and vascular contraction to adrenergic agonists, likely due to compensatory TRPC3 over-expression and is reversible by TRPC3 silencing.109 This result suggests a possible redundancy between TRPC channels.110 Nevertheless, down-regulation of TRPC3 did not affect MT,111 leaving open the question of the direct contribution of TRPC3 and 6 in MT.
TRPM4 have properties very similar to that of the native SACs identified in isolated cerebral artery myocytes. Myocytes depolarization and MT were both attenuated in cerebral arteries treated with TRPM4 antisense oligonucleotides.112 Although the activation mode of TRPM4 channels remains unknown, intracellular ATP, PKC-dependent phosphorylation, and calmodulin binding are required.113 TRPM4 is a voltage-dependent channel,114 making it likely that its activation results from TRPC6-dependent Ca2+ influx and depolarization.
Finally, a role for TRPP1 and TRPP2 in pressure sensing was recently reported. TRPP2 inhibits SACs through a specific interaction with TRPP1.115 Hence, the TRPP1/TRPP2 ratio is critical, through filamin A coupled to the actin cytoskeleton, to convert intraluminal pressure to local bilayer tension into an increase in SAC mechanosensitivity. A specific decrease in the TRPP1 level in mouse SMCs induces a large decrease in MT, suggesting a critical role for the TRPP1/TRPP2 balance.
Several studies suggest that GPCRs interact with TRPs. Table 1 summarizes similarities between GPCR and TRP activation. GPCR-dependent TRP activation is well documented for DAG-sensitive TRPC3-6-7 channels.89 Mechanosensitive cation channels and TRPCs are activated by DAG and attenuated by PLC inhibitors.116 Knockdown of TRPC3 with specific small-interfering RNA significantly reduces AngII-dependent calcium influx.117 Similarly, the down-regulation of arterial TRPC3 expression with antisense oligodeoxynucleotides decreased cerebral arteries depolarization and vasoconstriction in response to the P2Y receptor agonist UTP.111 ATP and UTP, probably acting through P2Y2 receptors, induce TRPC3/7 channel opening. Uracyl nucleotides activation of neuronal PC12 cells increases TRPC5 currents, suggesting a general coupling of P2Y receptors to TRPC channel opening.118
Table 1.
Synergy between TRP and GPCR signalling
| TRP | GPCR ligand activation | PLCβ activation | DAG sensitivity | PKC activation | PtdIns(3,4,5)P3 sensitivity | [Ca2+]i sensitivity | Knockout phenotype |
|---|---|---|---|---|---|---|---|
| TRPC1 | S1P123; ET-1 | +123 | NI | NI | +124 | +125 | Normal126 |
| TRPC3 | UTP/P2Y111 | +127 | +89 | NI | NI | +127 | NI |
| TRPC6 | AT1R80 | +80; −128 | +89 | NI | NI | +105 | Increased vascular contraction, MT; elevated blood pressure109 |
| TRPM4 | NI | NI | – | Increase Ca2+ sensitivity129 | NI | +114 | Normal (Vennekens and Nilius, unpublished results) |
References reporting the interaction between GPCR signalling and TRPs proposed to be involved in MT are listed (NI, not investigated).
4. Conclusion/discussion
Reduced levels of MT occur in depressed cardiovascular conditions such as shock, resulting in organ perfusion failure, whereas exaggerated MT contributes to increased peripheral resistance in diseases, such as hypertension, type-2 diabetes, and SAH. Pharmacological control of MT would allow resetting of inappropriate vascular resistance, and consequently, alter the actions of other neurohumoral control mechanisms.5 Identification of the cellular and molecular determinants of MT is essential to enable such interventions. MT integrates a complex set of cellular and molecular process acting in synergy to produce vascular contraction. Parallel (amplifier) or in series (initiator) positioning of GPCRs in MT is not clearly known. There are numerous data to support the hypothesis that GPCRs could initiate the myogenic process: first, the generation of GPCR agonists in response to stretch; second, their ability to trigger TRP channels opening through DAG formation and PKC activation; third, the intrinsic property of some Gq-11-coupled GPCR exhibiting mechanosensitive properties. On the other hand, a recent study shows that GPCRs modulate TRP currents without affecting their mechanosensitivity nor MT,119 suggesting that these GPCRs are not involved in the triggering of MT but rather act as amplifiers. Such parallel and synergistic interaction was proposed for adrenergic receptor stimulation that complement MT.31 Also opposing this hypothesis is the short time of the myogenic response in cerebral arteries (in the millisecond range) that barely fits with agonist generation and GPCR activation (that takes several seconds). Noteworthy, as previously proposed, is that the determinants of MT may vary along the arteriolar tree and this may be true for the global contribution of GPCRs. In addition, the contribution of specific GPCRs/mediators in the myogenic process may depend on both the expression level of receptors and the local generation of the appropriate agonist. A good knowledge of the pharmacology of territory-specific arterial constriction may give insights concerning the potential contribution of specific GPCR in MT. Parallel contribution of GPCRs in the MT would act as backup in case other pathways are compromised.
A fundamental question concerns the means of GPCR activation during the myogenic response. Both ligand-dependent and -independent receptor activation have been proposed. Mechanical activation initiated by a conformational change of the receptor is discernible from agonist-bound activation.80 Indeed, AT1 receptors could be sensitive to mechanical stimuli.78 In apparent contradiction with these data is that membrane stretch releases autacoids such as nucleotides,70 S1P,63 and 20-HETE.120 Quantification of these molecules, particularly AngII, is hampered by the absence of sensitive detection methods suitable for use in resistance arteries in situ. However, some local generation of AngII in the microvasculature has been reported,81–83 making it difficult to fully rule out an agonist-dependent activation of AT1 receptor. Another possibility is that mechanoperception is a property shared by many GPCRs expressed in VSMCs and may depend on their expression level. It would be interesting to evaluate G12-13 protein and eventually other G protein (Gi/o, Gs, or Gz) susceptibility to mechanical stimulation and Rho pathway activation. Finally, it is conceivable that both ligand-dependent and -independent receptor activation may coexist.
The RhoA-Rho-kinase pathway appears to be a major component of the calcium facilitation pathway in VSMC, and thus, could be the focal point of different GPCRs involved in MT. Its greater contribution in hypertensive pathology has prompted many groups to propose specific RhoA pathway targeting as a possible antihypertensive approach.121 It seems reasonable to propose that an increase in pressure activates a sequence of events starting with the opening of ionic channels, the ‘stretch-activated channels’, together with the release of vasoactive autacoids, which are most likely sensitive to stretch. Both processes are fast responding, thus allowing the initial depolarization needed to rapidly increase wall force. In parallel, or with a small shift in time, mechanosensitive GPCRs or activated by stretch-induced agonists released (i.e. 20HETE, S1P, nucleotides) activate the RhoA-Rho-kinase pathway and, consequently, increase calcium sensitivity of the contractile apparatus. This latter would allow maintaining a new level of force over time. In this regard, the study performed by Cipolla et al.122 showing that the ratio of F to G actin rises when pressure increases in resistance arteries, leading to higher MT, is important, especially since this ratio is determined by RhoA-Rho-kinase activation.121 The large variety of GPCRs described as potentially activated by stretch, directly or not, certainly reflects some degree of tissue and/or species specificity of MT. In addition, in most, if not all, diseases associated with disturbed MT, GPCR expression level, and sensitivity to vasoconstrictor (i.e. endothelin, TXA2) change. Knowing more precisely which GPCRs are involved in each tissue would allow the design of more selective treatments or the prevention of some side effects. Thus, a more in-depth knowledge of the precise sequence of events involved in MT in each tissue or organ is probably the key for more selectivity and less side effects in cardiovascular diseases and other disorders affecting blood flow perfusion.
Conflict of interest: none declared.
Funding
G.K. was a recipient of fellowships from Angers Loire Metropole and the Lefoulon Delalande Foundation (Institut de France).
References
- 1.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 3.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 5.Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacological Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–2099. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 8.Gschwend S, Henning RH, Pinto YM, de Zeeuw D, van Gilst WH, Buikema H. Myogenic constriction is increased in mesenteric resistance arteries from rats with chronic heart failure: instantaneous counteraction by acute AT1 receptor blockade. Br J Pharmacol. 2003;139:1317–1325. doi: 10.1038/sj.bjp.0705367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1213–R1219. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Akker J, Schoorl MJ, Bakker EN, Vanbavel E. Small artery remodeling: current concepts and questions. J Vasc Res. 2010;47:183–202. doi: 10.1159/000255962. [DOI] [PubMed] [Google Scholar]
- 11.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension. 2004;44:643–648. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 13.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 14.D'Angelo G, Mogford JE, Davis GE, Davis MJ, Meininger GA. Integrin-mediated reduction in vascular smooth muscle [Ca2+]i induced by RGD-containing peptide. Am J Physiol. 1997;272:H2065–H2070. doi: 10.1152/ajpheart.1997.272.4.H2065. [DOI] [PubMed] [Google Scholar]
- 15.Mogford JE, Davis GE, Platts SH, Meininger GA. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res. 1996;79:821–826. doi: 10.1161/01.res.79.4.821. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, et al. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol. 2008;586:1699–1713. doi: 10.1113/jphysiol.2007.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TV, Spurrell BE, Hill MA. Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol. 2002;29:612–619. doi: 10.1046/j.1440-1681.2002.03698.x. [DOI] [PubMed] [Google Scholar]
- 19.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 20.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama K. Calcium-dependent contractile activation of cerebral artery produced by quick stretch. Am J Physiol. 1982;242:H760–H768. doi: 10.1152/ajpheart.1982.242.5.H760. [DOI] [PubMed] [Google Scholar]
- 22.Laher I, Bevan JA. Stretch of vascular smooth muscle activates tone and 45Ca2+ influx. J Hypertens Suppl. 1989;7:S17–20. [PubMed] [Google Scholar]
- 23.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol. 1991;261:H950–H959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 24.Henrion D, Laher I, Bevan JA. Intraluminal flow increases vascular tone and 45Ca2+ influx in the rabbit facial vein. Circ Res. 1992;71:339–345. doi: 10.1161/01.res.71.2.339. [DOI] [PubMed] [Google Scholar]
- 25.Laporte R, Haeberle JR, Laher I. Phorbol ester-induced potentiation of myogenic tone is not associated with increases in Ca2+ influx, myoplasmic free Ca2+ concentration, or 20-kDa myosin light chain phosphorylation. J Mol Cell Cardiol. 1994;26:297–302. doi: 10.1006/jmcc.1994.1038. [DOI] [PubMed] [Google Scholar]
- 26.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- 27.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 28.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. Am J Physiol Heart Circ Physiol. 2002;283:H2288–H2295. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 29.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ Res. 2001;88:774–779. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- 30.Dubroca C, Loyer X, Retailleau K, Loirand G, Pacaud P, Feron O, et al. RhoA activation and interaction with Caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res. 2007;73:190–197. doi: 10.1016/j.cardiores.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Meininger GA, Faber JE. Adrenergic facilitation of myogenic response in skeletal muscle arterioles. Am J Physiol. 1991;260:H1424–H1432. doi: 10.1152/ajpheart.1991.260.5.H1424. [DOI] [PubMed] [Google Scholar]
- 32.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 33.Davenport AP, D'Orléans-Juste P, Godfraind T, Maguire JJ, Ohlstein EH, Ruffolo RR. 2008. Endothelin receptors introductory chapter IUPHAR database http://wwwiuphar-dborg/DATABASE/FamilyIntroductionForward?familyId=21 .
- 34.Warner TD, de Nucci G, Vane JR. Rat endothelin is a vasodilator in the isolated perfused mesentery of the rat. Eur J Pharmacol. 1989;159:325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- 35.Huang A, Koller A. Endothelin and prostaglandin H2 enhance arteriolar myogenic tone in hypertension. Hypertension. 1997;30:1210–1215. doi: 10.1161/01.hyp.30.5.1210. [DOI] [PubMed] [Google Scholar]
- 36.Ungvari Z, Koller A. Endothelin and prostaglandin H(2)/thromboxane A(2) enhance myogenic constriction in hypertension by increasing Ca(2+) sensitivity of arteriolar smooth muscle. Hypertension. 2000;36:856–861. doi: 10.1161/01.hyp.36.5.856. [DOI] [PubMed] [Google Scholar]
- 37.Sonveaux P, Dessy C, Martinive P, Havaux X, Jordan BF, Gallez B, et al. Endothelin-1 is a critical mediator of myogenic tone in tumor arterioles: implications for cancer treatment. Cancer Res. 2004;64:3209–3214. doi: 10.1158/0008-5472.can-03-1291. [DOI] [PubMed] [Google Scholar]
- 38.Cseko C, Bagi Z, Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol. 2004;97:1130–1137. doi: 10.1152/japplphysiol.00106.2004. [DOI] [PubMed] [Google Scholar]
- 39.Petersen HH, Choy J, Stauffer B, Moien-Afshari F, Aalkjaer C, Leinwand L, et al. Coronary artery myogenic response in a genetic model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2002;283:H2244–H2249. doi: 10.1152/ajpheart.00606.2002. [DOI] [PubMed] [Google Scholar]
- 40.Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010;12:267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGiff JC, Quilley J. 20-HETE and the kidney: resolution of old problems and new beginnings. Am J Physiol. 1999;277:R607–R623. doi: 10.1152/ajpregu.1999.277.3.R607. [DOI] [PubMed] [Google Scholar]
- 42.Huang A, Sun D, Yan C, Falck JR, Kaley G. Contribution of 20-HETE to augmented myogenic constriction in coronary arteries of endothelial NO synthase knockout mice. Hypertension. 2005;46:607–613. doi: 10.1161/01.HYP.0000176745.04393.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol. 2001;280:H1066–H1074. doi: 10.1152/ajpheart.2001.280.3.H1066. [DOI] [PubMed] [Google Scholar]
- 44.Chu ZM, Croft KD, Kingsbury DA, Falck JR, Reddy KM, Beilin LJ. Cytochrome P450 metabolites of arachidonic acid may be important mediators in angiotensin II-induced vasoconstriction in the rat mesentery in vivo. Clin Sci (Lond) 2000;98:277–282. [PubMed] [Google Scholar]
- 45.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37:1307–1313. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- 46.Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507(Pt 3):771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 49.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 50.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 51.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 52.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feletou M, Vanhoutte PM, Verbeuren TJ. The thromboxane/endoperoxide receptor (TP): the common villain. J Cardiovasc Pharmacol. 2010;55:317–332. doi: 10.1097/fjc.0b013e3181d8bc8a. [DOI] [PubMed] [Google Scholar]
- 54.Szekeres M, Nadasy GL, Kaley G, Koller A. Nitric oxide and prostaglandins modulate pressure-induced myogenic responses of intramural coronary arterioles. J Cardiovasc Pharmacol. 2004;43:242–249. doi: 10.1097/00005344-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension. 1993;22:913–921. doi: 10.1161/01.hyp.22.6.913. [DOI] [PubMed] [Google Scholar]
- 56.Hill MA, Davis MJ, Meininger GA. Cyclooxygenase inhibition potentiates myogenic activity in skeletal muscle arterioles. Am J Physiol. 1990;258:H127–H133. doi: 10.1152/ajpheart.1990.258.1.H127. [DOI] [PubMed] [Google Scholar]
- 57.Samora JB, Frisbee JC, Boegehold MA. Increased myogenic responsiveness of skeletal muscle arterioles with juvenile growth. Am J Physiol Heart Circ Physiol. 2008;294:H2344–H2351. doi: 10.1152/ajpheart.00053.2008. [DOI] [PubMed] [Google Scholar]
- 58.Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 59.Kostenis E. Novel clusters of receptors for sphingosine-1-phosphate, sphingosylphosphorylcholine, and (lyso)-phosphatidic acid: new receptors for ‘old’ ligands. J Cell Biochem. 2004;92:923–936. doi: 10.1002/jcb.20092. [DOI] [PubMed] [Google Scholar]
- 60.Coussin F, Scott RH, Nixon GF. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- 61.Tosaka M, Okajima F, Hashiba Y, Saito N, Nagano T, Watanabe T, et al. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: possible role in pathogenesis of cerebral vasospasm. Stroke. 2001;32:2913–2919. doi: 10.1161/hs1201.099525. [DOI] [PubMed] [Google Scholar]
- 62.Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, et al. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 64.Hoefer J, Azam MA, Kroetsch JT, Leong-Poi H, Momen MA, Voigtlaender-Bolz J, et al. Sphingosine-1-phosphate-dependent activation of p38 MAPK maintains elevated peripheral resistance in heart failure through increased myogenic vasoconstriction. Circ Res. 2010;107:923–933. doi: 10.1161/CIRCRESAHA.110.226464. [DOI] [PubMed] [Google Scholar]
- 65.Peter BF, Lidington D, Harada A, Bolz HJ, Vogel L, Heximer S, et al. Role of sphingosine-1-phosphate phosphohydrolase 1 in the regulation of resistance artery tone. Circ Res. 2008;103:315–324. doi: 10.1161/CIRCRESAHA.108.173575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vial C, Evans RJ. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- 72.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 73.Clark MG, Richards SM, Hettiarachchi M, Ye JM, Appleby GJ, Rattigan S, et al. Release of purine and pyrimidine nucleosides and their catabolites from the perfused rat hindlimb in response to noradrenaline, vasopressin, angiotensin II and sciatic-nerve stimulation. Biochem J. 1990;266:765–770. doi: 10.1042/bj2660765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koltsova SV, Maximov GV, Kotelevtsev SV, Lavoie JL, Tremblay J, Grygorczyk R, et al. Myogenic tone in mouse mesenteric arteries: evidence for P2Y receptor-mediated, Na(+), K (+), 2Cl (−) cotransport-dependent signaling. Purinergic Signal. 2009;5:343–349. doi: 10.1007/s11302-009-9160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kauffenstein G, Drouin A, Loufrani L, Thorin E, Robaye B, Sevigny J, et al. Role of nucleotides, ectonucleotidases and P2 receptors in vascular contraction and myogenic tone. Hypertension. 2010;56:1169. [Google Scholar]
- 76.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, et al. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27:3104–3115. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mederos y Schnitzler M, Storch U, Gudermann T. AT1 receptors as mechanosensors. Curr Opin Pharmacol. 2011;11:112–116. doi: 10.1016/j.coph.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 80.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matrougui K, Loufrani L, Heymes C, Levy BI, Henrion D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- 82.Henrion D, Benessiano J, Levy BI. In vitro modulation of a resistance artery diameter by the tissue renin-angiotensin system of a large donor artery. Circ Res. 1997;80:189–195. doi: 10.1161/01.res.80.2.189. [DOI] [PubMed] [Google Scholar]
- 83.Muller DN, Fischli W, Clozel JP, Hilgers KF, Bohlender J, Menard J, et al. Local angiotensin II generation in the rat heart: role of renin uptake. Circ Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- 84.MaassenVanDenBrink A, de Vries R, Saxena PR, Schalekamp MA, Danser AH. Vasoconstriction by in situ formed angiotensin II: role of ACE and chymase. Cardiovasc Res. 1999;44:407–415. doi: 10.1016/s0008-6363(99)00249-7. [DOI] [PubMed] [Google Scholar]
- 85.Maguire JJ, Davenport AP. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol Sci. 2005;26:448–454. doi: 10.1016/j.tips.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol. 1993;265:H415–H420. doi: 10.1152/ajpheart.1993.265.1.H415. [DOI] [PubMed] [Google Scholar]
- 87.Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol. 1994;266:H1840–H1845. doi: 10.1152/ajpheart.1994.266.5.H1840. [DOI] [PubMed] [Google Scholar]
- 88.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, et al. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 90.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol. 1990;259:H1586–H1594. doi: 10.1152/ajpheart.1990.259.5.H1586. [DOI] [PubMed] [Google Scholar]
- 91.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 92.Laher I, Bevan JA. Protein kinase C activation selectively augments a stretch-induced, calcium-dependent tone in vascular smooth muscle. J Pharmacol Exp Ther. 1987;242:566–572. [PubMed] [Google Scholar]
- 93.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol. 2007;292:H2613–H2622. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- 94.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, et al. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2009;48:83–89. doi: 10.1016/j.yjmcc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 96.Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol Heart Circ Physiol. 2002;283:H2282–H2287. doi: 10.1152/ajpheart.00544.2002. [DOI] [PubMed] [Google Scholar]
- 97.Loufrani L, Lehoux S, Tedgui A, Levy BI, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arterioscler Thromb Vasc Biol. 1999;19:2878–2883. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- 98.Dubroca C, You D, Levy BI, Loufrani L, Henrion D. Involvement of RhoA/Rho kinase pathway in myogenic tone in the rabbit facial vein. Hypertension. 2005;45:974–979. doi: 10.1161/01.HYP.0000164582.63421.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 100.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, et al. P2Y(1), P2Y(2), P2Y(4), and P2Y(6) receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1751–H1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- 101.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 102.Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, et al. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280:11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 103.Wuertz CM, Lorincz A, Vettel C, Thomas MA, Wieland T, Lutz S. p63RhoGEF–a key mediator of angiotensin II-dependent signaling and processes in vascular smooth muscle cells. FASEB J. 2010;24:4865–4876. doi: 10.1096/fj.10-155499. [DOI] [PubMed] [Google Scholar]
- 104.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 105.Schubert R, Brayden JE. Stretch-activated cation channels and the myogenic response of small arteries. In: Kamkin A, Kiseleva I, eds. Mechanosensitivity in Cells and Tissues. Moscow: Academia, 2005. [PubMed] [Google Scholar]
- 106.Sharif-Naeini R, Dedman A, Folgering JH, Duprat F, Patel A, Nilius B, et al. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflugers Arch. 2008;456:529–540. doi: 10.1007/s00424-007-0432-y. [DOI] [PubMed] [Google Scholar]
- 107.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010;119:19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- 108.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 109.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 111.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- 112.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 113.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, et al. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 114.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 115.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 116.Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res. 2003;93:557–564. doi: 10.1161/01.RES.0000093204.25499.83. [DOI] [PubMed] [Google Scholar]
- 117.Liu D, Yang D, He H, Chen X, Cao T, Feng X, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- 118.Ohta T, Morishita M, Mori Y, Ito S. Ca2+ store-independent augmentation of [Ca2+]i responses to G-protein coupled receptor activation in recombinantly TRPC5-expressed rat pheochromocytoma (PC12) cells. Neurosci Lett. 2004;358:161–164. doi: 10.1016/j.neulet.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 119.Anfinogenova Y, Brett SE, Walsh MP, Harraz OF, Welsh DG. Do TRPC-like currents and G protein-coupled receptors interact to facilitate myogenic tone development? Am J Physiol Heart Circ Physiol. 2011;301:H1378–H1388. doi: 10.1152/ajpheart.00460.2011. [DOI] [PubMed] [Google Scholar]
- 120.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, et al. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 121.Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol. 2010;7:637–647. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- 122.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 123.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca(2+) channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- 126.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 127.Alvarez J, Coulombe A, Cazorla O, Ugur M, Rauzier JM, Magyar J, et al. ATP/UTP activate cation-permeable channels with TRPC3/7 properties in rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H21–H28. doi: 10.1152/ajpheart.00135.2008. [DOI] [PubMed] [Google Scholar]
- 128.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Earley S, Reading S, Brayden JE. Functional Significance of Transient Receptor Potential Channels in Vascular Function. Frontiers in Bioscience CRC Press; 2007. Liedtke WB, Heller S ch. 26. [PubMed] [Google Scholar]