Abstract
The capsaicin receptor (TRPV1) is a non-selective cation channel predominantly expressed in specialized sensory neurons that detect painful stimuli. Although its many functional roles continue to be revealed, it has been confirmed to play a critical role in the perception of peripheral inflammatory hyperalgesia and pain. TRPV1 not only is sensitized and/or activated under a wide range of conditions including inflammation and nerve injury but also undergoes changes in expressed levels in response to these same pathologic conditions. Just as our understanding of the structural requirements of TRPV1 activation has grown, there is evidence that TRPV1 forms heteromeric channel complexes. This review is focused on the structural and functional consequence of TRPV1 splice variants: VR.5’sv, TRPV1b/beta and TRPV1var. Through their co-expression and formation of heteromeric complexes with TRPV1, they have been shown to modulate TRPV1 activation. Moreover, TRPV1 splice variant subunits may also contribute unique properties of activation such as the detection of hypertonic conditions.
Keywords: Ankyrin Repeat, Capsaicin, Capsaicin Receptor, Dominant-Negative, DRG, Ion Channel, Nociceptor, Pain, Sensory, Splice Variant, Resiniferatoxin, TRPV1, TRPV1b, TRPV1var, VR5’sv, Review
2. Introduction
With the isolation of the capsaicin receptor, now known as TRPV1 (transient receptor potential vanilloid subtype -1 channel), our understanding of peripheral sensory transduction has undergone a revolution (1-2). However, despite the realization that TRPV1 plays a central role in nociceptive processing, our understanding of the impact of variations in TRPV1 channel structure and subunit assembly on physiology remains very limited. As detailed below, comparison of the structural and functional attributes of the TRPV1 splice variants individually and when co-expressed with the full length TRPV1, should provide a new perspective in which to navigate future investigations. Moreover, TRPV1 participates in a diverse array of signaling processes including central temperature regulation, insulin release, lipid metabolism and bladder function. Therefore, a greater understanding of TRPV1 subunit diversity is expected to impact not only our understanding of nociception but other integrated physiologic systems as well.
3. TRPV1: Structure and Function
3.1. TRPV1 Structure
Following the initial isolation of a cDNA encoding a functional capsaicin receptor (TRPV1) isolated from rat dorsal root ganglia (DRG) (1), sequence analysis predicted an ion channel subunit containing an N-terminal intracellular domain notable for three ankyrin repeats, six transmembrane (TM) spanning regions interdicted with a pore loop domain (between TM 5 & 6) and a large C-terminal intracellular domain (Figure 1) (1). Furthermore, TRPV1 was found to share sequence identity with the transient receptor potential superfamily of ion channel subunits initially described in the Drosophila phototransduction system. This linked, for the first time, the capsaicin receptor (TRPV1) to a superfamily of TRP channels involved in sensory and cellular signal transduction (3).
Figure 1.
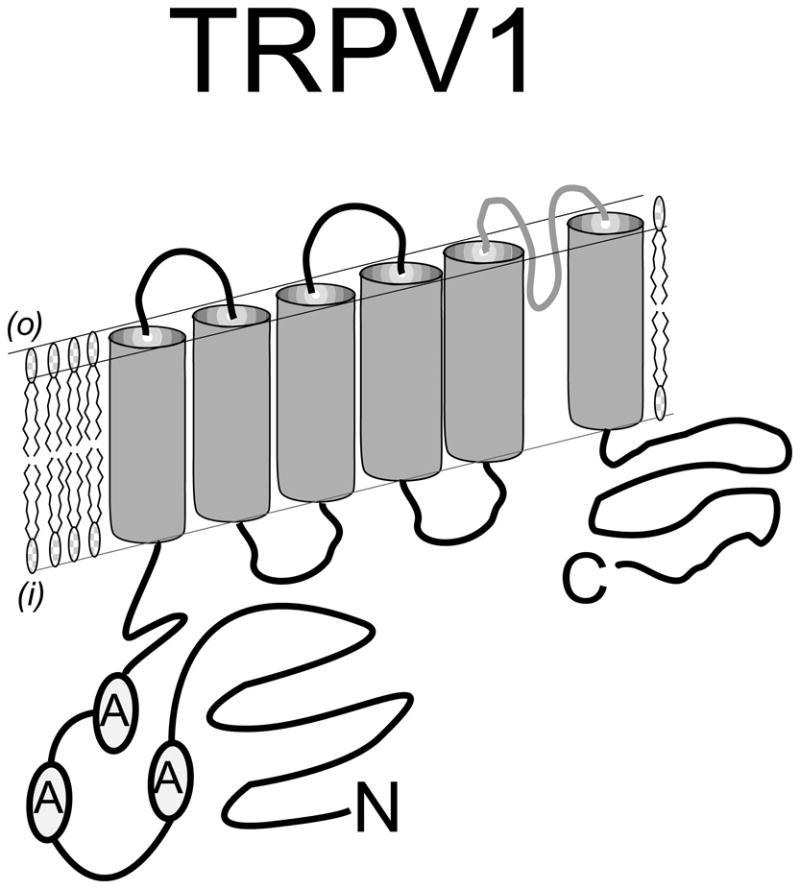
Predicted topology of a TRPV1 protein subunit spanning the plasma membrane. The N - terminal intracellular region is distinguished by three ankyrin repeat domains that participate in channel stability, activation and modulation. The C-terminal intracellular region also plays a critical role in channel activation – especially through interaction with second messenger pathways such as PKC. Six transmembrane spanning domains establish the channel structure with a pore – loop region interdicted between transmembrane domains five and six. The simplest model proposes that four of these subunits assemble to form a channel complex that is activated by the binding of capsaicin to the complex of intracellular domains.
Although the precise macro-molecular structure of the endogenous TRPV1 ion channel complex is unknown, important details have emerged following the study of TRPV1 subunit expression and comparative sequence analysis (4). From earlier work, the simplest model arising from non–denaturing protein gel analysis predicted a homo-tetrameric complex (5). Structurally, TRPV1 is homologous to members of the super-family of voltage-gated potassium channels providing useful data for structure function analysis (6). In fact, a tetrameric model has been supported through the analysis of cloned TRPV1 expressed on the cell surface using high-resolution electron microscopy and modeling data from the voltage gated potassium channel Kv1.2. In addition, there has been recent progress in structural analysis through the combination of various techniques including electron cryomicroscopy (7), although the full-length crystal structure of TRPV1 remains unknown. What has emerged thus far is an ion channel with a ‘basket’ like’ structure formed from each of four subunits contributing both an N-, and C-terminal intracellular domain (8). Importantly, the N-terminal intracellular domain appears to make a significant contribution to the intracellular activation of TRPV1. For example, activation of TRPV1 by pungent compounds ranging from onions to garlic can be profoundly altered by modification of a single N-terminal cysteine (9). The N-terminal intracellular domain also interacts with adjacent modulatory proteins and possibly the C-terminal intracellular domain. In the closed state of the channel, the N-terminal domain is likely exposed to the binding of ATP and the C-terminal to phosphoinositides that have been shown to facilitate channel activation. In contrast, a desensitized state may be promoted through the interaction of the N- and C-terminal domains which are brought closer together through a modulatory action involving calcium – calmodulin (10-11).
Another signature of TRPV channels is the identification of ankyrin repeat domains residing within the encoded N-terminal intracellular domain forming a region of three repeats spanning amino acids 101-364 in TRPV1 (1). These short 33 amino acid repeat units have previously been considered to participate in protein–protein (subunit) interactions (12). In addition to their importance for subunit interactions, recent data demonstrate the presence of concave binding surfaces for ATP within the ankyrin repeats of the N-terminal intracellular domain of TRPV1, modulating channel activation and function and preventing tachyphylaxis to various vanilloid agonists such as capsaicin (13). As we will discuss later, a functional consequence of splice-variants lacking one or more of their N-terminal ankyrin repeat domains is the loss of activation by capsaicin or other vanilloid– like agonists.
3.2. TRPV1 Function
TRPV1 expressed in peripheral sensory neurons functions to integrate a wide range of noxious stimuli into an inward current response resulting in depolarization of specialized small-diameter primary afferent nociceptive neurons - nociceptors. Following peripheral detection of a noxious stimulus, central afferent processes, entering at the superficial portion of the dorsal horn of the spinal cord, synapse with second order neurons to initiate signaling to the CNS. Subsequently, ascending tracks serve to transmit the nociceptive signals to higher centers where they are perceived as painful.
Agents capable of activating TRPV1 range from extracellular stimuli including plant derivatives (capsaicin) and other environmental irritants as well as by noxious heat (> 43-45 °C). Under certain conditions, thermal energy (physiologic temperatures of 34-37 °C) facilitates TRPV1 activation. This is probably best illustrated by proton-mediated reduction in the heat activation threshold of TRPV1 by binding to an extracellular domain (14-15). Other extracellular events such as inflammation may in turn trigger intracellular signaling leading to the activation or sensitization of TRPV1. Inflammatory compounds such as bradykinin can activate TRPV1 through distinct but associated receptor systems. Products of the lipoxygenases have also been implicated in mediating pain due to their increased production during inflammation and their ability to direct hyperalgesia when injected under the skin (16). In fact, bradykinin, a product of inflammation, results in the synthesis of membrane associated lipoxygenase products such as 12-HPETE and leukotriene B4 with concomitant activation of TRPV1 (17). Moreover, any process that leads to the intracellular synthesis of 12(S), 15(S)-HPETE (hydroperoxyeicosatetranoic acid) and/or leukotriene B4 in nociceptors could result in direct activation of TRPV1 (18). PKC-mediated sensitization can also reduce the thermal activation threshold into the physiologic temperature range (34-37 °C) (17, 19-20). In addition, phosphatidylinositol-4,5-bisphosphate (PIP2) binding to Pirt, a PIP2 regulatory subunit of TRPV1 has been proposed to mediate inflammatory pain (21).
Chief among the multiple inflammatory mediators is Nerve Growth Factor – NGF, a growth factor synthesized in abundance by target tissues (fibroblasts, peripheral blood mononuclear cells) and known to activate / sensitize TRPV1 through the TrkA receptor (22-24). Importantly, NGF mediated acute pain and thermal hyperalgesia act primarily through phosphoinositide-3-kinase (PI3K) and mitogen activated protein kinase signaling pathways (25-27). In addition to chemical and thermal stimuli, changes in physical properties such as osmolality / cell shape, have been shown to activate nociceptors and sensitize the response to capsaicin and other noxious stimuli. Notably, TRPV1 -/- mice have defects in systemic osmoregulation (28). In these studies, magnocellular neurons in the hypothalamus respond to application of hypertonic conditions in wild type mice but not in TRPV1 -/- mice. Interestingly, hypertonic responsive magnocellular cells do not express the full–length TRPV1 but rather a TRPV1 variant that contains a truncated N-terminal intracellular domain (28). Moreover, TRPV1- mediated diuresis and natriuresis by hypertonic saline in the kidney can be blocked by the TRPV1 antagonist, capsazepine (29). This suggests that hypertonic induced changes in cellular volume may trigger cellular responses through an expressed TRPV1 receptor splice variant.
Taken together, TRPV1 functions as an integrator for a variety of sensory inputs. Its activation requires thermal energy and the thermal activation threshold is modulated by a wide range of mechanisms. Our knowledge of the diverse structural and functional features of TRPV1 suggests that a modification of either the N-terminal and/or C-terminal intracellular domain will result in important changes in both intracellular and extracellular activation of TRPV1. Although most studies based on the functional expression of the cloned TRPV1 cDNA in mammalian cell lines support a homo-tetrameric structure (5, 30) the isolation of at least three divergent TRPV1 splice variant cDNAs suggests that a heteromeric TRPV1 receptor complex is plausible and is expressed in vivo. As described below, we will focus on the functional consequences of TRPV1 in combination with TRPV1 splice variants containing modifications of N-terminal intracellular domains – a common feature of three of the TRPV1 splice variants reported thus far.
4. TRPV1 Splice Variants
4.1. VR.5’sv
An important mechanism capable of regulating the activity of ion channels is a change in heteromeric subunit composition (31-32). In an effort to explain the divergent pharmacology, previously observed in early capsaicin receptor binding and electrophysiologic studies, the isolation of potential TRPV1 splice variants was undertaken. The first published TRPV1 splice variant cDNA was identified with the cloning and characterization of the rat vanilloid receptor 5’ splice variant (VR.5’sv) (33). VR.5’sv represents a combination of two variations when compared with the sequence of full length TRPV1. First: A TRPV1-like gene product that originates from an alternative initiation of translation resulting in expression of only a small portion of the predicted N-terminal intracellular domain (Figures 2 & 3) and Second: the skipping of an exon resulting in a loss of 60 amino acids encoding a portion of the N-terminal intracellular region including a portion of the third ankyrin repeat domain (Figure 2) (33). VR.5’sv is expressed in DRG at a ratio of 1:20 (TRPV1:VR.5’sv) but in brain, kidney and peripheral blood mononuclear cells at ratios are closer to 1:1 (33). VR.5’sv functions as a dominant negative subunit when co-expressed with TRPV1 in Xenopus oocytes. It is proposed that this results from the co-assembly of VR.5’sv as part of a heteromeric channel complex (34). This hypothesis is supported by evidence of overlapping TRPV1 and VR.5’sv protein expression in the plasma membrane of microinjected Xenopus oocytes. Other investigators using engineered constructs, have suggested that truncation of the TRPV1 N-terminus may result in a lack of transport to the cell membrane (35). However, in an oocyte expression model, TRPV1 protein expression appears unchanged at the plasma membrane surface with concomitant expression of VR.5’sv directing the dominant negative phenotype (34). The biologic relevance of VR.5’sv has also been advanced with the confirmation of a VR.5’sv – like protein expressed in rat DRG based on Western blot analysis (34).
Figure 2.
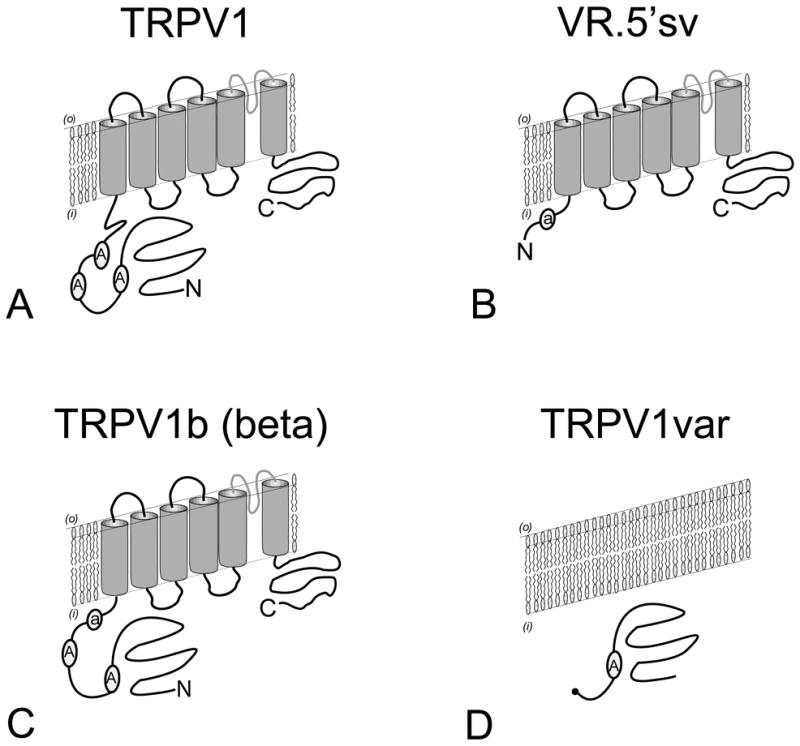
Comparison of predicted subunit topologies of TRPV1 (A) with TRPV1 splice variants (B-D). Splice variant VR.5’sv (B) is identical to TRPV1 except for a dramatically modified N-terminal intracellular region that retains only a small portion of the third ankyrin repeat domain. TRPV1b (C) is identical to TRPV1 except for the partial deletion of the third ankyrin repeat domain and adjoining polypeptide sequence. TRPV1var (D) is unlike TRPV1 or the TRPV1 splice variants as it completely lacks both transmembrane spanning and C-terminal intracellular regions. TRPV1var encodes a truncated N-terminal intracellular region retaining the first ankyrin repeat domain. Despite differences in their predicted N-terminal intracellular regions, there is evidence to support the formation of heteromeric complexes between TRPV1 and the TRPV1-splice variants.
Figure 3.
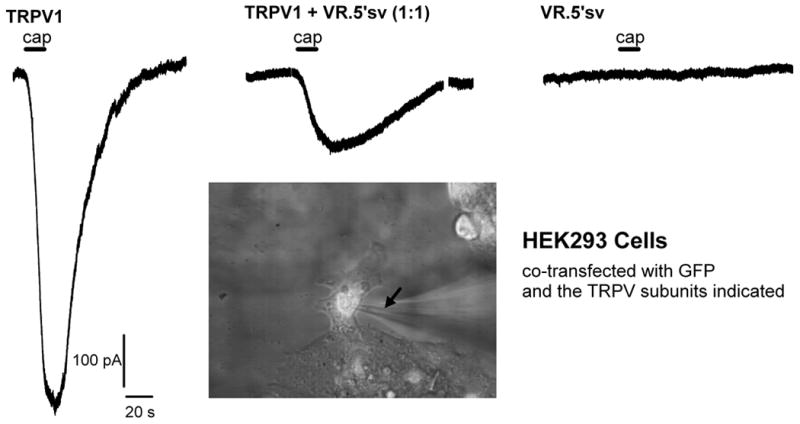
Whole cell inward current responses induced by capsaicin in HEK293 cells transfected with TRPV1 and VR.5’sv cDNAs or a combination of both cDNAs in equal amounts (0.8µg/coverslip/construct). From left to right the three traces are representative for recordings from cells expressing TRPV1 alone, TRPV1 and VR.5’sv (ratio 1:1), and VR.5’sv alone, respectively. Cells co-expressing TRPV1 and VR.5’sv show a greater than 70 % reduction in current response (1µmol/l capsaicin for 15 s). The application of capsaicin is indicated by bars above the traces (cap). The inset shows a microscopic image of a transfected cell during patch-clamp recording. The fluorescent signal from GFP expression can be appreciated in the cell indicating successful expression. The arrow points to the recording pipette on the cell. The recordings were obtained in voltage clamp mode in a whole cell patch clamp preparation. Solutions (in mmol/l: internal - KCl 140, MgCl2 1, EGTA 5, HEPES 5, and ATPNa2 5; external - NaCl 140, CaCl2 2, KCl 4, MgCl2, glucose 11, HEPES 5 and CsCl 3. The recording chamber was at room temperature (20-23°C) and continuously perfused with external solution (2 ml/min). Capsaicin was applied through a capillary, positioned 150 µm from cell. The holding potential was -60 mV.
To further test the hypothesis of a dominant-negative effect of VR.5’sv, we have examined its expressed function in a mammalian expression system. HEK293 cells were transiently transfected with cDNAs encoding TRPV1 or VR.5’sv or both in equivalent amounts. To allow for tracking of cells successfully expressing the desired messages, we performed co-transfection in the presence of a cDNA expressing green fluorescent protein (GFP). GFP positive cells were examined for their inward current response to noxious stimuli known to activate TRPV1 ion channels using a whole-cell patch clamp electrophysiology approach. As expected, cells expressing only VR.5’sv do not show any response to capsaicin exposure (Figure 3). In addition, HEK293 cells co-transfected with TRPV1 and VR.5’sv (in equal amounts) showed a greater than 70% reduction of the expected inward current response to capsaicin (1 µmol/l for 15 s) when compared to HEK293 cells expressing TRPV1 alone (Fig. 3). Interestingly, the magnitude of this effect also matches our previous findings in oocytes (34).
The genomic structure of the rat, mouse and human TRPV1 gene was also investigated to better understand the origins of VR.5’sv and determine if additional splice variants could be predicted. VR.5’sv is notable for its genomic origins that diverge from TRPV1 through the use of an additional transcriptional start site. The transcript that uses this site begins within intron IV, includes exon – intron 5 and has an alternative translation start site (Met 308 of TRPV1) within exon 6 that will encode a TRPV1–like channel subunit with a truncated N-terminal intracellular domain. VR.5’sv lacks exon 7 completely, resulting in an additional modification of the predicted third ankyrin repeat domain (36). This appears to be result of the use of a non-typical splice site between exon – intron 7 (Tgt rather than the more commonly found Ggt). Moreover, the 3’ splice site was agA rather than agG (36). We also proposed the existence of an additional TRPV1 splice-variant that was identical to TRPV1 except for the solitary loss of polypeptide sequence encoded by exon 7. This was initially identified as VR1L1 in reference to the Genbank No. AB041029. As discussed below, the independent isolation of such a splice variant was subsequently demonstrated with the isolation of a mouse TRPV1beta (37) and human splice variant TRPV1b (38). An additional TRPV1 variant was also reported and, at the time, termed SIC (39). However, following additional investigation, its genomic origin could not be confirmed and appears to be the result of an aberrant cDNA construction rather than representing an authentic mRNA cellular transcript (36).
Given that VR.5’sv encodes a variant of TRPV1 that is truncated at the N-terminus similar to that predicted by the RT-PCR results of the Bourque laboratory (28), we have become interested in determining if VR.5’sv or other TRPV1 splice variants participate in hypertonic-induced channel activation. Although VR.5’sv did not direct hypertonic induced current responses when expressed in oocytes (34), its response to osmotic stimuli in mammalian cells, either alone or when co-expressed with other variants (TRPV1var, TRPV1b, or TRPV4) has not been investigated nor has the relationship between hypertonic activated currents in DRG neurons and the presence of TRPV1 splice variant transcripts.
4.2. TRPV1b / TRPV1beta
Building on the isolation of VR.5’sv and the proposal that additional TRPV1 splice variants are expressed in DRG (33, 36), two TRPV1 splice variants were subsequently identified in mouse and human that contained a modification of the N-terminal intracellular domain encoded by exon 7. TRPV1beta was cloned from C57BL/6 mice and was found to be derived from an alternative intron signal within exon 7 resulting in a 30 nucleotide loss (10 amino acid loss) in the N-terminal intracellular domain disrupting the third ankyrin repeat domain (37). Expression of TRPV1beta was found to be abundant in DRG, skin stomach and tongue. However, TRPV1 beta expression in Xenopus oocyte and HEK293 cells did not direct functional channel activity and when co-expressed with the full length mouse TRPV1, directed a dominant – negative effect in response to noxious stimuli (37). Although it appeared that the TRPV1beta variant mRNA could be synthesized at a similar rate as that of the parent TRPV1, much lower amounts of the encoded protein were found in cells suggesting that the TRPV1beta mRNA and/or protein was unstable (37) and required an excess in TRPV1 beta subunit mRNA for adequate inhibition of TRPV1. Although evidence that a human variant existed was first presented based on RNAse protection experiments (40), a human cDNA with similar structural characteristics to TRPV1 beta was isolated and characterized (38). TRPV1b described a human clone that lacked exon 7, encoding a portion of the third ankyrin repeat domain. TRPV1b was resistant to capsaicin and protons but surprisingly could be activated by thermal stimuli at > 47°C (rather that ∼ 43 - 45 °C as previously reported for TRPV1 (1, 14).
An investigation of the human TRPV1b variant was subsequently undertaken with a detailed study of its co-expression with TRPV1 in HEK293 cells (41). In these studies, the resistance of TRPV1b to thermal stimuli (up to 50 °C) was convincingly demonstrated. When hTRPV1b was co-expressed with TRPV1 in HEK293 cells, it functioned as a dominant – negative subunit blocking activation of TRPV1 exposed to capsaicin, heat and low pH (41). Whereas previous reports found TRPV1b in rat trigeminal ganglion, it now was identified in DRG (41). In fact, when a panel of human RNAs was examined, DRG, fetal brain, and cerebellum contained the highest copy number of human TRPV1b mRNA. In comparison, TRPV1 was expressed at approximately 20 fold higher levels in these tissues. How does the loss of the third ankyrin repeat domain disrupt the functional integrity of TRPV1? Although in a related TRP channel, TRPV6, the third ankyrin repeat domain was required for the formation of channel complexes (42), this is not the case for homotetrameric complexes of TRPV1b or heteromeric complexes in combination with TRPV1 (41). Nevertheless, TRPV1b was still more difficult to express in HEK293 cells than TRPV1 and partially reduced the expression of TRPV1 when co-expressed. Therefore, loss of the third ankyrin domain in TRPV1 appears to decrease the stability of the channel subunit. In addition, TRPV1b may prefer to form homotetramers over heteromultimers with TRPV1 - possibly reducing the frequency of heteromeric channel formation (41). Nevertheless, the dominant – negative effects directed by TRPV1b are consistent with other studies that have examined sequential deletion of the N-terminal intracellular region of TRPV1 (43) as well as with our initial observations with VR.5’sv (33). Keratinocytes, a cell type known to express TRPV1 are surprisingly resistant to the cytotoxic effects of capsaicin. Interestingly, it has been observed that the splice variant TRPV1b is also expressed in keratinocytes, possibly explaining the relative resistance of these cells to capsaicin, especially if TRPV1b is indeed functioning as a dominant-negative subunit (44). An alternative explanation, is that expressed levels of TRPV1 are inadequate in keratinocytes to direct a classical response to capsaicin (44). Taken together, these findings may also explain the observation that certain tissues have TRPV1-like immunoreactivity but lack a robust response to capsaicin or other vanilloid compounds.
4.3. TRPV1var
Finally, a cDNA encoding TRPV1var was isolated from rat kidney papilla, a portion of the kidney that experiences high osmotic conditions secondary to sodium chloride and urea. Its genomic divergence from TRPV1 results from the failure to splice out intron 5 (36) and therefore retains an additional 101 bp of intron sequence. The resultant mRNA is slightly larger that the original TRPV1 mRNA but encodes a dramatically truncated N-terminal intracellular region (Figure 2). Therefore, TRPV1var encodes a portion of the N-terminal intracellular region that includes only the first ankyrin repeat domain, but is completely lacking in transmembrane spanning domains or a C-terminal intracellular region (45). This is the result of a translational read through into the retained intron 5 until an in-frame stop codon is reached. Although TRPV1 var was originally isolated in kidney, an mRNA based RT-PCR product was also reported in the original report (45). Our laboratory has identified both mRNA and a TRPV1var–like protein in rat DRG (unpublished observations). This suggests that TRPV1var, if co-expressed with TRPV1 could potentially regulate responses mediated by TRPV1 in nociceptors. In support of this hypothesis, we also have evidence (in collaboration with Dr. D. Cohen - Oregon Health & Science University) of TRPV1var directing a dominant-negative effect when co-expressed with TRPV in Xenopus oocytes (unpublished observations).
Beyond the commonly reported function of ion channel splice variants directing inhibitory (dominant-negative) action, TRPV1var may direct unique properties among the TRPV1 splice variants. For example, TRPV1var was reported to direct augmentation of TRPV1 activation in response to resiniferatoxin (RTX) in HEK293 cells but inhibition in COS cells (45). TRPV1var may also participate in mechanoreceptor function in specialized tissues such as the kidney or in the sensory terminal innervating structures specialized to detect changes in membrane stress or osmotic change (46).
5. TRPV1, TRPC and TRPM Splice Variants: Common Themes
TRPC channels are the mammalian homologues of TRP channels (trp1-7) initially identified in the drosophila photo-transduction pathway (3). In general, TRPC channels function in capacitative calcium entry under conditions of intracellular calcium store depletion resulting in prolonged influx of extracellular calcium (47). They can be activated by g-protein coupled receptors such as carbachol-induced activation of muscarinic receptors. In other cases, TRPC channels are coupled to IP3 receptor activation and/or diacylglycerol (DAG) (48). As compared with TRPV1, TRPC channels contain shortened N- terminal and C- terminal intracellular domains but retain the N-terminal ankyrin repeat domains observed in TRPV channels. Given their general role in the regulation of intracellular calcium, it has also been proposed that TRPC channels (TRPC 3 & 4) participate in the cellular response to oxidative stress that can lead to cellular injury. Although individual subfamily members (TRPC2) participate in specialized roles such as sex discrimination (49), TRPC members also form macromolecular complexes and in so doing will direct additional channel functions. For example, TRPC channels 1/4/5 and TRPC 3/6/7 preferentially form channel complexes and the TRPC 1/3 complex was shown to suppress carbachol – induced calcium entry (50).
Given the multiplicity of TRPC channel subunits (1-7) and their propensity to form heteromeric ion channel complexes, it may not be surprising that a number of TRPC splice variants have been identified. Importantly, the majority of the TRPC -variants diverge at their N-terminal intracellular domains – a phenomenon previously described for TRPV1 splice variants: VR5’sv, TRPV1b, and TRPV1var. Moreover, the co-expression of a TRPC channel member with a particular splice variant invariable leads to a blockade / inhibition of channel activation, as has been reported when TRPV1 splice variants are co-expressed with TRPV1. TRPC2 splice variants with progressively shorter N-terminal domains have been described that retain their six transmembrane spanning regions (51). However, the N-terminal splice variant of TRPC2 (smTRPC2) encodes a truncated N-terminal intracellular domain that lacks all 6 transmembrane spanning domains yet combines with the full length TRPC2 in the plasma membrane to block epo-induced calcium entry in primary erythroid cells (51). Therefore, the structure of smTRPC2 is nearly identical to that found for the TRPV1 splice variant TRPV1var (45). (Fig. 2) and both may function as dominant- negative subunits. Additional N-terminally modified splice variants of TRPC have been reported (47, 52) and include a TRPC-4 splice variant capable of binding the IP3 receptor, (53) and TRP7 splice variants 1 / 2 that demonstrate differential expression in smooth muscle cells (54). Beyond TRPC, other examples of TRP - related splice variants have been reported particularly amongst members of the TRPM channel family.
TRPM1 is expressed in melanocytes and its level of expression has been correlated with the aggressiveness of melanoma whereas other members participate in cellular proliferation (55). Functionally, the C-terminal portion of TRPM1 directs a wide range of functions in response to oxidative stress and cellular binding of TNF alpha. Curiously, TRPM2-delta N, includes a deletion of aa 538-557 (a small exon), resulting in an inactive channel as well as one that blocks wild type TRPM2 activation in a dominant-negative manner (56). Such a deletion is reminiscent of that found with TRPV1b and VR.5’sv (Figure 2). Other TRPM splice variants have been described without similarity to VR.5’sv, TRPV1b or TRPV1var. Notably, TRPM4 b, results from the skipping of a portion of exon 2 forming a channel subunit that is not active through the loss of integrity of its second transmembrane spanning domain (57) and TRPM-2 – S (short) containing a deletion of the entire C-terminal intracellular region (including 4 of the 6 transmembrane domains) (58). The result of co-expression of TRPM2 with TRPM-2 – S is suppression of cell death induced by oxidative stress mediated by the wild type version of TRPM2. Expression of such a dominant –negative subunit should provide an advantage under cellular stress – injury and promote survival (59). One might speculate whether selective expression of dominant-negative TRPV1 splice variants under conditions of inflammation / injury may play a similar protective role.
6. TRPV1 Splice Variants: Sensory Neuronal Expression
We (34, 36), and others (37-38, 45) have shown that TRPV1 splice variants are present in DRG. Is the functional consequence of TRPV1 splice variant expression in sensory neurons limited to blockade or reduction of TRPV1 activation? In addition to blockade of TRPV1 activation, TRPV1 splice variants may combine independently to direct physiologic functions that are not traditionally described for the parent TRPV1. Alternatively, are TRPV1 splice variants capable of independent function by association with other subunits of the TRPV sub family? Preliminary investigations in our laboratory now show that individual rat dorsal root ganglion neurons in culture contain a diverse pattern of full length versus splice variant TRPV1 mRNA expression. As shown in (Figure 4), using single cell RT-PCR techniques, a single sensory neuron was observed to express three isoforms of the nociceptive channel receptor: TRPV1, TRPV1b and TRPV1var. Whereas, another sensory neuron, expressed only VR.5’sv and yet in others, predominantly the full length TRPV1 was observed. In addition, Western blots from rat DRG protein probed with an antibody directed at the N-terminal of TRPV1 have revealed lower molecular weight bands in the size range as would be expected for TRPV1var. Although evidence for the presence of VR.5’sv, TRPV1b and TRPV1var mRNA and protein have been reported, data supporting a functional role regulating TRPV1 responsiveness in native tissues is only now emerging. An alternate hypothesis is that TRPV1 splice variants form receptor / channel complexes amongst themselves and/or with other TRP family members and direct a unique function such as depolarizing sensory terminals in response to hypertonic stimuli.
Figure 4.
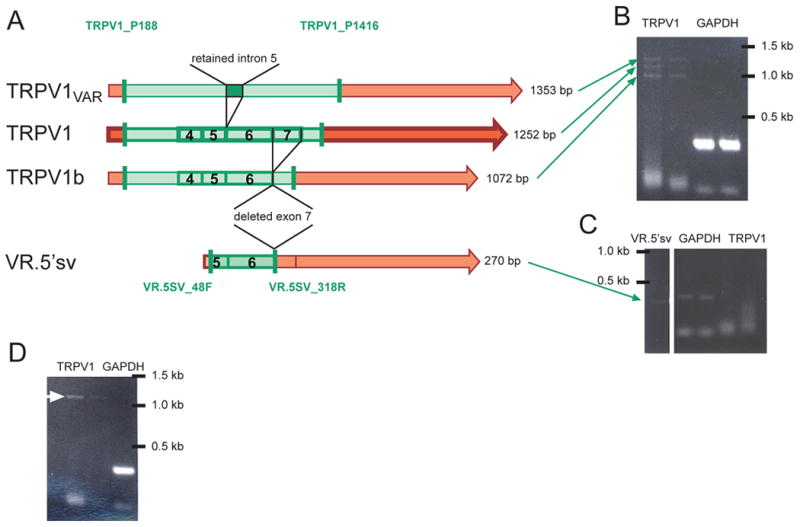
(A) Map illustrating the differences in organization of TRPV1 (center, darker color), TRPV1b and VR.5’sv (bottom), and TRPV1var (top) messages. TRPV1b skips exon 7 but is otherwise identical to TRPV1 whereas TRPV1var retains intron 5, as depicted above (exons 4-7 are indicated by boxes and numbers 4-7). VR.5’sv is missing the majority of the N-terminal intracellular region in addition to the polypeptide sequence associated with deletion of exon 7, also present in TRPV1b. It becomes clear that the four resulting PCR products (green shading) differ in size (length of product listed on right side). The three gel images (B-D) represent findings from single cell RT-PCR experiments and illustrate the presence of the different variants alone or in combination in different cells. The location of the primer pairs used to differentiate between the variants is indicated by the vertical green bars. (B) Three bands of the appropriate sizes were detected in the RT-PCR reaction from a single cell (left two lanes: TRPV1 primer pair, right lanes: GAPDH). (C) Another cell was negative for full length TRPV1 (right two lanes) but was positive for VR.5’sv (left lane, green arrow). The middle lanes show the GAPDH PCR product. (D) Single cell RT-PCR from this cell using the same primers as in (B) resulted in only one band, likely to be full length TRPV1 (white arrow). Double lanes represent different dilutions of product from first round PCR (left: 1:1000; right: 1:10,000) when added as template for second round PCR.
7. Perspective: TRPV1 Splice Variants-Inflammatory Pain to Diabetes
NGF applied to cultured DRG neurons or derived from increased production following tissue injury has been found to be a critical signal in increasing TRPV1 mRNA, protein and associated pain / hyperalgesia (60-62). In addition, increased NGF in tissue or in cultured DRG neurons has been shown to be a critical signal in increasing TRPV1 mRNA and protein (63-66). However, our understanding is now that there are at least three splice variants that potentially can be changed by the action of NGF. Moreover, TRPV1 may combine with these splice variant subunits, such as the VR.5’sv, TRPV1b, (36, 38, 41) or TRPV1var (45). This suggests a role for NGF in regulating the differential expression of TRPV1 versus TRPV1 variants and thereby the nociceptor phenotype / sensitivity. However, it remains to be shown what functional role these splice variants play in the modulation of TRPV1 activation in nociceptors under normal or inflammatory conditions. Interestingly, experimental (cyclophosphamide) cystitis is associated with TRPV1 b down regulation in DRG, and may representing an example where TRPV1 expression remains constant, while a variant splice variant subunit is altered (67).
Beyond the peripheral nervous system, TRPV1 has been localized in selective sub-regions of the brain such as the hippocampus (68), in peripheral blood mononuclear and dentate cells of the immune system (33, 69), cervical cancer cells (70), prostate cancer cells, normal prostate tissue (71), and insulin-secreting pancreatic islet beta cells (72). Our collaborative efforts have included helping to identify novel TRPV1 expression in skin keratinocytes and other subcutaneous structures of normal and pathologic skin (73). Moreover, recent developments have identified TRPV1 in such diverse physiologic processes as control of hair shaft elongation (74) and bladder (75-76) and gastrointestinal function (77-78). Perhaps even more intriguing is the link of TRPV1 to diabetes (79). Therefore, fundamental regulatory mechanisms controlling TRPV1 expression in nociceptors, may also apply to other systems or tissues under normal and pathophysiologic conditions. As the role of TRPV1 splice variants emerge, their particular pedigree in regulating these diverse physiologic functions will be revealed.
Acknowledgments
Supported by grants from the: NIH NS38737, UCSF School of Medicine / Springer H. Mem. Foundation, IARS Frontiers in Anesthesia Research Award.
Abbreviations
- CNS
central nervous system
- DRG
dorsal root ganglia
- NGF
nerve growth factor
- PKC
protein kinase C
- RTX
resiniferatoxin
- TM
transmembrane
- TRPV1
transient receptor potential vanilloid subtype -1
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaumer L, Yildirim E, Abramowitz J. A comparison of the genes coding for canonical TRP channels and their M, V and P relatives. Cell Calcium. 2003;33:419–432. doi: 10.1016/s0143-4160(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 5.Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 6.Blaine JT, Ribera AB. Heteromultimeric Potassium Channels Formed by Members of the Kv2 Subfamily. J Neurosci. 1998;18:9585–9593. doi: 10.1523/JNEUROSCI.18-23-09585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moiseenkova-Bell VY, Wensel TG. Hot on the trail of TRP channel structure. J Gen Physiol. 2009;133:239–244. doi: 10.1085/jgp.200810123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Ballester G, Ferrer-Montiel A. Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states. J Membr Biol. 2008;223:161–172. doi: 10.1007/s00232-008-9123-7. [DOI] [PubMed] [Google Scholar]
- 12.Bork P. Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Proteins. 1993;17:363–374. doi: 10.1002/prot.340170405. [DOI] [PubMed] [Google Scholar]
- 13.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 15.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine JD, Lau W, Kwiat G, Goetzl EJ. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science. 1984;225:743–745. doi: 10.1126/science.6087456. [DOI] [PubMed] [Google Scholar]
- 17.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim DJ, Haber NA, Reichling DB, Khasar SG, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 20.Summer GJ, Puntillo KA, Miaskowski C, Dina OA, Green PG, Levine JD. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–891. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang H, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 23.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–171. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. Epub 2005 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. Epub 2007 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. Epub 2005 Dec 4. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Xie C, Wang DH. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am J Nephrol. 2007;27:530–537. doi: 10.1159/000107665. [DOI] [PubMed] [Google Scholar]
- 30.Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J Neurosci. 2001;21:8697–8706. doi: 10.1523/JNEUROSCI.21-22-08697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. Epub 2006 Jul 31. [DOI] [PubMed] [Google Scholar]
- 32.Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86:941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher MA, Moff I, Sudanagunta SP, Levine JD. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J Biol Chem. 2000;275:2756–2762. doi: 10.1074/jbc.275.4.2756. [DOI] [PubMed] [Google Scholar]
- 34.Eilers H, Lee SY, Hau CW, Logvinova A, Schumacher MA. The rat vanilloid receptor splice variant VR.5'sv blocks TRPV1 activation. Neuroreport. 2007;18:969–973. doi: 10.1097/WNR.0b013e328165d1a2. [DOI] [PubMed] [Google Scholar]
- 35.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 36.Xue Q, Yu Y, Trilk SL, Jong BE, Schumacher MA. The genomic organization of the gene encoding the vanilloid receptor: evidence for multiple splice variants. Genomics. 2001;76:14–20. doi: 10.1006/geno.2001.6582. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004;279:37423–37430. doi: 10.1074/jbc.M407205200. Epub 2004 Jul 2. [DOI] [PubMed] [Google Scholar]
- 38.Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol. 2005;67:1119–1127. doi: 10.1124/mol.104.009852. Epub 2005 Jan 11. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M, Sato J, Kutsuwada K, Ooki G, Imai M. Cloning of a stretch-inhibitable nonselective cation channel. J Biol Chem. 1999;274:6330–6335. doi: 10.1074/jbc.274.10.6330. [DOI] [PubMed] [Google Scholar]
- 40.Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G. The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun. 2001;281:1183–1189. doi: 10.1006/bbrc.2001.4482. [DOI] [PubMed] [Google Scholar]
- 41.Vos MH, Neelands TR, McDonald HA, Choi W, Kroeger PE, Puttfarcken PS, Faltynek CR, Moreland RB, Han P. TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. J Neurochem. 2006;99:1088–1102. doi: 10.1111/j.1471-4159.2006.04145.x. [DOI] [PubMed] [Google Scholar]
- 42.Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem. 2004;279:34456–34463. doi: 10.1074/jbc.M404778200. [DOI] [PubMed] [Google Scholar]
- 43.Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;46:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- 44.Pecze L, Szabo K, Szell M, Josvay K, Kaszas K, Kusz E, Letoha T, Prorok J, Koncz I, Toth A, Kemeny L, Vizler C, Olah Z. Human keratinocytes are vanilloid resistant. PLoS ONE. 2008;3:e3419. doi: 10.1371/journal.pone.0003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian W, Fu Y, Wang DH, Cohen DM. Regulation of TRPV1 by a novel renally expressed rat TRPV1 splice variant. Am J Physiol Renal Physiol. 2006;290:F117–126. doi: 10.1152/ajprenal.00143.2005. [DOI] [PubMed] [Google Scholar]
- 46.Feng NH, Lee HH, Shiang JC, Ma MC. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors\ and cause substance P release and sensory activation in rat kidneys. Am J Physiol Renal Physiol. 2008;294:F316–325. doi: 10.1152/ajprenal.00308.2007. Epub 2007 Nov 21. [DOI] [PubMed] [Google Scholar]
- 47.Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store\ depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci U S A. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 49.Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci U S A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 51.Chu X, Tong Q, Wozney J, Zhang W, Cheung JY, Conrad K, Mazack V, Stahl R, Barber DL, Miller BA. Identification of an N-terminal TRPC2 splice variant which inhibits calcium\ influx. Cell Calcium. 2005;37:173–182. doi: 10.1016/j.ceca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Villereal ML. Mechanism and functional significance of TRPC channel multimerization. Semin Cell Dev Biol. 2006;17:618–629. doi: 10.1016/j.semcdb.2006.10.010. Epub 2006 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mery L, Magnino F, Schmidt K, Krause KH, Dufour JF. Alternative splice variants of hTrp4 differentially interact with the C-terminal \ portion of the inositol 1,4,5-trisphosphate receptors. FEBS Lett. 2001;487:377–383. doi: 10.1016/s0014-5793(00)02362-0. [DOI] [PubMed] [Google Scholar]
- 54.Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth\ muscles. Am J Physiol Cell Physiol. 2001;280:C1184–1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- 55.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. Epub 2006 Apr 17. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn FJ, Kuhn C, Naziroglu M, Luckhoff A. Role of an N-terminal splice segment in the activation of the cation channel\ TRPM2 by ADP-ribose and hydrogen peroxide. Neurochem Res. 2009;34:227–233. doi: 10.1007/s11064-008-9755-0. Epub 2008 Jun 3. [DOI] [PubMed] [Google Scholar]
- 57.Murakami M, Xu F, Miyoshi I, Sato E, Ono K, Iijima T. Identification and characterization of the murine TRPM4 channel. Biochem Biophys Res Commun. 2003;307:522–528. doi: 10.1016/s0006-291x(03)01186-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. Epub 2003 Feb 19. [DOI] [PubMed] [Google Scholar]
- 59.McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch. 2005;451:235–242. doi: 10.1007/s00424-005-1440-4. Epub 2005 Jul 16. [DOI] [PubMed] [Google Scholar]
- 60.Bennett G, al-Rashed S, Hoult JR, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–322. doi: 10.1016/S0304-3959(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 61.Herzberg U, Eliav E, Dorsey JM, Gracely RH, Kopin IJ. NGF involvement in pain induced by chronic constriction injury of the rat sciatic nerve. Neuroreport. 1997;8:1613–1618. doi: 10.1097/00001756-199705060-00012. [DOI] [PubMed] [Google Scholar]
- 62.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 63.Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 64.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 65.Eilers H, Trilk SL, Lee SY, Xue Q, Jong BE, Moff I, Levine JD, Schumacher MA. Isolation of an mRNA binding protein homologue that is expressed in nociceptors. Eur J Neurosci. 2004;20:2283–93. doi: 10.1111/j.1460-9568.2004.03703.x. [DOI] [PubMed] [Google Scholar]
- 66.Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 67.Charrua A, Reguenga C, Paule CC, Nagy I, Cruz F, Avelino A. Cystitis is associated with TRPV1b-downregulation in rat dorsal root ganglia. Neuroreport. 2008;19:1469–1472. doi: 10.1097/WNR.0b013e32830f1e73. [DOI] [PubMed] [Google Scholar]
- 68.Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. Epub 2005 Jan 22. [DOI] [PubMed] [Google Scholar]
- 69.Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. Epub 2005 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Contassot E, Tenan M, Schnuriger V, Pelte MF, Dietrich PY. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol Oncol. 2004;93:182–188. doi: 10.1016/j.ygyno.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, Prieto JC, Diaz-Laviadac I. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- 73.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 74.Bodo E, Biro T, Telek A, Czifra G, Griger Z, Toth BI, Mescalchin A, Ito T, Bettermann A, Kovacs L, Paus R. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 76.Lazzeri M, Vannucchi MG, Zardo C, Spinelli M, Beneforti P, Turini D, Faussone-Pellegrini MS. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol. 2004;46:792–798. doi: 10.1016/j.eururo.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Facer P, Knowles CH, Tam PK, Ford AP, Dyer N, Baecker PA, Anand P. Novel capsaicin (VR1) and purinergic (P2X3) receptors in Hirschsprung's intestine. J Pediatr Surg. 2001;36:1679–1684. doi: 10.1053/jpsu.2001.27959. [DOI] [PubMed] [Google Scholar]
- 78.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. Epub 2004 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]


