Abstract
Purpose: Little is known about mental health disorders (MHDs) and their associated health care expenditures for the dual eligible elders across long-term care (LTC) settings. We estimated the 12-month diagnosed prevalence of MHDs among dual eligible older adults in LTC and non-LTC settings and calculated the average incremental effect of MHDs on medical care, LTC, and prescription drug expenditures across LTC settings. Methods: Participants were fee-for-service dual eligible elderly beneficiaries from 7 states. We obtained their 2005 Medicare and Medicaid claims data and LTC program participation data from federal and state governments. We grouped beneficiaries into non-LTC, community LTC, and institutional LTC groups and identified enrollees with any of 5 MHDs (anxiety, bipolar, major depression, mild depression, and schizophrenia) using the International Classification of Diseases Ninth Revision codes associated with Medicare and Medicaid claims. We obtained medical care, LTC, and prescription drug expenditures from related claims. Results: Thirteen percent of all dual eligible elderly beneficiaries had at least 1 MHD diagnosis in 2005. Beneficiaries in non-LTC group had the lowest 12-month prevalence rates but highest percentage increase in health care expenditures associated with MHDs. Institutional LTC residents had the highest prevalence rates but lowest percentage increase in expenditures. LTC expenditures were less affected by MHDs than medical and prescription drug expenditures. Implications: MHDs are prevalent among dual eligible older persons and are costly to the health care system. Policy makers need to focus on better MHD diagnosis among community-living elders and better understanding in treatment of MHDs in LTC settings.
Mental health disorders (MHDs) in older persons are prevalent (Bagchi, Verdier, & Simon, 2009; Lin, Zhang, Leung, & Clark, 2011b) and are associated with poorer physical health conditions (Anderson, Freedland, Clouse, & Lustman, 2001; Bijl & Ravelli, 2000), as well as inadequate health care access and utilization (Thorpe, Thorpe, Kennelty, & Chewning, 2012). In addition, the presence of an MHD often complicates medical care of chronic diseases (Frayne et al., 2005; Jones, Clarke, & Carney, 2004) and increases costs (Kasper, O’Malley Watts, & Lyons, 2010). This is particularly true for older people who are dually eligible for Medicare and Medicaid (dual eligibles) as they are usually sicker and poorer than their nondual eligible counterparts (Kaiser Commission on Medicaid and the Uninsured, 2011). Compared with other Medicare enrollees, they are more likely to require LTC services; are members of racial/ethnic minority groups; and have poorer health, more functional limitations, and higher prevalence of chronic conditions. They represent an especially complex and expensive population to care (Kaiser Commission on Medicaid and the Uninsured, 2011; Kasper et al., 2010).
Although dual eligibles are more likely to use LTC services, the prevalence of MHDs and their associated health care expenditures across LTC settings are not well described. This study uses 2005 Medicare and Medicaid claims data from seven states to address two aims: (a) to estimate the 12-month diagnosed prevalence of MHDs among dual eligibles in LTC and non-LTC settings and (b) to estimate the incremental effect of MHDs on medical care, LTC, prescription drug, and total health care expenditures. Incremental effects or marginal effects are commonly used to describe the change in outcome associated with the change in a treatment or policy variable of interest (Basu & Rathouz, 2005) and have also been used quantify expenditures associated with specific diseases (Trasande & Chatterjee, 2009; Trogdon & Hylands, 2008). We estimated the 12-month diagnosed prevalence and incremental expenditures separately for dual eligibles living in community but not using LTC services (non-LTC group), living in community and using LTC services (community LTC group), and living in institutions (institutional LTC group).
Methods
Data
We used the 2005 Medicaid (the Medicaid Analytic eXtract [MAX] data files) and Medicare claims data files for all Medicaid beneficiaries in Arkansas, Florida, Minnesota, New Mexico, Texas, Vermont, and Washington. These states’ Medicaid officials provided us finder files where they identified beneficiaries receiving LTC through home- and community-based services and institutions. These seven states were originally selected to illustrate a range of state circumstances (e.g., demographic composition, size, geography, county government structure, state policy, and LTC service structure) for another study that examined how state governments rebalance their LTC systems (Kane, Priester, Kane, & Mollica, 2006).
The study sample included dual eligibles aged 65 years and older in those seven states during 2005 (N = 799,313). State Medicaid officials provided information about Medicaid-paid LTC services in each state and identifiers that allowed us to merge Medicaid claims with these program data. This allowed us to group the sample into three study groups: those who were Medicaid-paid residents of nursing homes were in the “institutional LTC” group (assisted living and continuing care facilities were not included); those who used Medicaid-paid LTC services while living in the community, including Medicaid-paid state plan services and wavier services were in the “community LTC” group; and those who did not use any Medicaid LTC services were in the “non-LTC” group. We included only those who were in fee-for-service Medicaid and Medicare plans because claims data from managed care plans were reported inconsistently in both Medicaid and Medicare expenditure files.
For each beneficiary, we created the annual medical care expenditures by adding all medical care–related claims from the Medicare and Medicaid systems within the 2005 calendar year. Similarly, we created the annual LTC expenditures for each beneficiary by adding all LTC-related claims from the Medicaid system and the annual prescription drug expenditures for each beneficiary by adding all prescription drug-related claims from the Medicare and Medicaid systems within the 2005 calendar year. We calculated the beneficiary-level total health care expenditures by adding medical care, LTC, and prescription drug expenditures.
Medicaid payments for medical care included payments for inpatient hospital, ambulatory care services, labs and x-rays, rehabilitation services, physical therapy/occupational therapy/speech/hearing services, hospice care, and Medicaid-paid Medicare premiums. Medicare payments for medical care included payments for inpatient hospital services, outpatient hospital care (including emergency department visits), physician services, hospice care, durable medical equipment, postacute care, inpatient LTC services, and home health services.
Medicaid payments for LTC included payments for nursing facility services, intermediate care facility services for the intellectually disabled, home health services, personal care services, targeted case management, and transportation. Medicare payments were not counted toward LTC expenditures.
The prescription drug expenditures were obtained from the Medicaid claims because our period of study was prior to the implementation of the Medicare prescription drug benefit program.
Disease Identification
We identified beneficiaries having MHDs using algorithms similar to those used by the Centers for Medicare and Medicaid Services (CMS) Chronic Condition Data Warehouse—Chronic Condition Categories (CCW; although the CCW algorithm uses differing identification rules for different conditions, we define a beneficiary with a condition if they had a diagnosis code corresponding to that condition on one inpatient [hospital, LTC, or Medicare skill nursing facility] or two outpatients or carrier claims for the year.), based on the International Classification of Diseases Ninth Revision (ICD-9) codes associated with the claims data. Beneficiaries were identified as having an MHD if they had ICD-9 codes associated with that MHD on one inpatient (hospital, LTC, or Medicare skill nursing facility) or two outpatients or carrier claims for the year 2005. We did not include dementia as one of the MHDs, instead, we treated it as a comorbidity in our analysis. Supplemetary Appendix 1 shows the ICD-9 codes used to identify the five MHDs.
Analyses
First, we produced the diagnosed prevalence of five MHDs based on the disease identification criteria discussed earlier and examined the variations in the diagnostic prevalence across LTC and non-LTC settings. Second, we used logistic regression to examine factors associated with the odds of having a specific MHD diagnosis. Third, we modeled the association between MHD and health care expenditures using a two-part model (Buntin & Zaslavsky, 2004; Manning & Mullahy, 2001). The two-part model is preferred over generalized linear model to model health care expenditures when a significant proportion of beneficiaries in the study sample have zero expenditure. In our sample, 18% of the beneficiaries had no medical care expenditures, 64% had no LTC expenditures, and 36% had no prescription drug expenditures for the year. The first part of the two-part model is a logit model that predicts the probability that the beneficiary has nonzero health care expenditures. The second part of model uses a generalized linear model to predict the level of expenditure if health care expenditure is positive. In the generalized linear model, we used a log-linked-dependent variable with a gamma distribution because the health care expenditures were positively skewed in our sample of beneficiaries (Buntin & Zaslavsky, 2004; Manning & Mullahy, 2001). In both parts of the model, we controlled for other beneficiary covariates, such as age, gender, race, health risk score (using CMS’s Hierarchical Conditions Category (HCC) Score [Pope et al., 2000 , 2004]), urban/rural-residence, and state of residence. We ran separate two-part models to estimate the incremental effect of MHD on medical care, LTC, prescription drug, and total health care expenditures for beneficiaries in each study group. The incremental expenditure associated with an MHD for a beneficiary is the difference in predicted expenditure with and without the MHD for that beneficiary, with other covariates and conditions unchanged at their observed values. (The incremental expenditure for the MHD of interest, d i, can be written as follows:
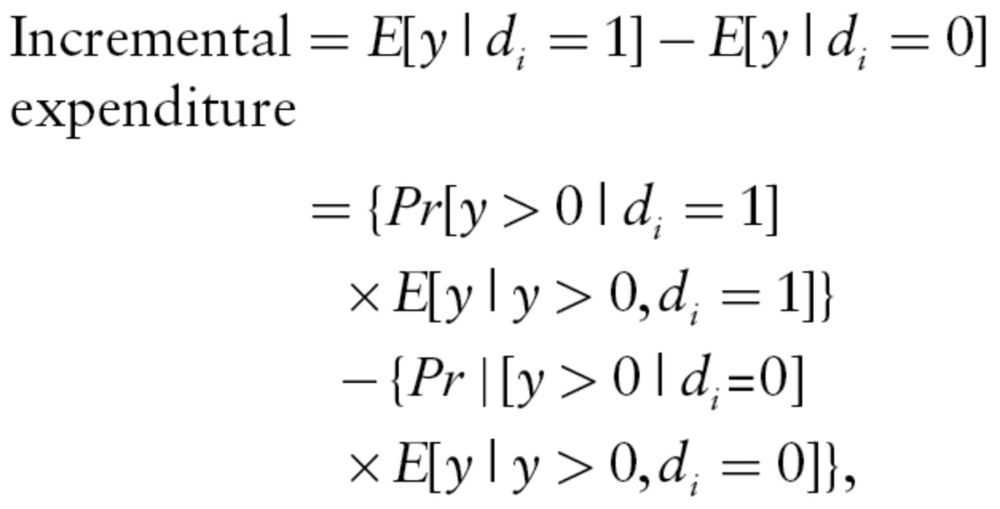 |
where y is the expenditure toward medical care, LTC, or prescription drugs.) We report the average incremental effect of each MHD on medical care, LTC, and prescription drug expenditures (and 95% confidence intervals [CI]) for beneficiaries by their LTC status.
Results
Table 1 shows the characteristics of the sample. Fifty percent of the beneficiaries in the study were white, 69% were female, and 66% were living in urban area. The average age was 77.4 years. Sixty-seven percent were in the non-LTC group, 16% were in the community LTC group, and 17% were in the institutional LTC group. The sample also distributed unevenly across the seven states, with about 40% and 36% living in Texas and Florida, respectively. Institutional LTC beneficiaries were older (average age = 83.9 years), more likely to be white (76%), and more likely to be living in an urban area (72%) than the other two groups.
Table 1.
Characteristics of Dual Eligible Elders by Long-Term Care (LTC) and Non-LTC Settings
| Total | Community LTC (%) | Institutional LTC (%) | Non-LTC | |
|---|---|---|---|---|
| Sample size | 799,313 (100%) | 130,729 (16%) | 133,443 (17%) | 535,141 (67%) |
| % White | 50% | 47% | 76% | 44% |
| % Female | 69% | 75% | 74% | 66% |
| States | ||||
| AR | 6.1% | 6.5% | 8.1% | 5.5% |
| FL | 36.1% | 10.9% | 33.8% | 42.9% |
| MN | 5.7% | 5.4% | 5.3% | 5.8% |
| NM | 2.9% | 2.1% | 2.2% | 3.3% |
| TX | 39.9% | 63.4% | 42.0% | 33.7% |
| VT | 1.9% | 0.1% | 0.7% | 2.7% |
| WA | 7.3% | 11.6% | 7.8% | 6.1% |
| % Metro | 66% | 67% | 72% | 65% |
| Average age (y) | 77.4 | 79.3 | 83.9 | 75.3 |
| 65–74 | 42% | 31% | 15% | 51% |
| 75–84 | 37% | 42% | 35% | 36% |
| 85 and older | 21% | 27% | 50% | 12% |
Notes. AR = Arkansas; FL = Florida; MN = Minnesota; NM = New Mexico; TX = Texas; VT = Vermont; WA = Washington.
“Community LTC” includes those who used Medicaid-paid LTC services while living in the community; “institutional LTC” includes Medicaid-paid residents of nursing homes and other institutional care facilities; those who did not use any Medicaid LTC services were in the “non-LTC” group.
All values are statistically significant at p < .001 level.
Table 2 shows the 12-month diagnosed prevalence of the five MHDs across the three settings. The prevalence varied by LTC settings. The non-LTC group had the lowest prevalence (7.5% had any MHD), followed by the community LTC group (13.0%) and the institutional LTC group (33.4%). Among the non-LTC group, the 12-month diagnosed prevalence ranged between 0.3% for bipolar and 4.3% for mild depression. Among the community LTC beneficiaries, the prevalence ranged from 0.6% for bipolar to 7.8% for mild depression. Among those who were in LTC facilities, the prevalence ranged from 1.9% for bipolar to 24.0% for mild depression. Across the three settings, mild depression was most prevalent, followed by anxiety and major depression.
Table 2.
Twelve-Month Diagnosed Prevalence of Mental Health Disorders by Long-Term Care (LTC) and Non-LTC Settings
| Total (%) | Community LTC (%) | Institutional LTC (%) | Non-LTC (%) | |
|---|---|---|---|---|
| Any mental disorder | 12.8 | 13.0 | 33.4 | 7.5 |
| Anxiety | 4.1 | 4.8 | 8.3 | 2.9 |
| Bipolar | 0.6 | 0.6 | 1.9 | 0.3 |
| Major depression | 1.3 | 1.2 | 4.0 | 0.7 |
| Mild depression | 8.2 | 7.8 | 24.0 | 4.3 |
| Schizophrenia | 1.3 | 1.4 | 4.0 | 0.7 |
Notes: Each mental illness is coded as a binary response.
All values are statistically significant at p < .001 level
Table 3 shows factors associated with the odds of each MHD diagnosis. The first column under each MHD reports the odds ratios (OR), whereas the second column reports the corresponding 95% CIs). Columns 2 and 3 reported the OR and 95% CIs of having any MHDs. Community LTC beneficiaries had a significantly higher odds of having an MHD diagnosis than the non-LTC beneficiaries (OR = 2.1; CI = 2.1–2.2). Furthermore, institutional LTC beneficiaries had a significantly higher odds of having an MHD diagnosis than the non-LTC beneficiaries (OR = 7.2; CI = 7.1–7.2). White beneficiaries had a higher odds of having an MHD diagnosis than non-White beneficiaries (OR = 1.4; CI = 1.4–1.5). Men had lower odds of having an MHD diagnosis (OR = 0.7; CI = 0.7–0.7) than women. Living outside of metropolitan areas was associated with a higher odds of having an MHD diagnosis (OR = 1.1; CI = 1.1–1.2) than those living in urban area. Older age was associated with lower odds of having an MHD diagnosis. There was significant state-by-state variation in the odds of having an MHD diagnosis. Table 3 also shows factors associated with the odds of having a specific MHD diagnosis, compared with not having such diagnosis.
Table 3.
Factors Associated With the Odds of Having Mental Health Disorder (MHD) Diagnoses Among Dual Eligible Elders
| Any MHD | Anxiety | Bipolar | Major Depression | Mild Depression | Schizophrenia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI | OR | CI | OR | CI | OR | CI | OR | CI | OR | CI | |
| Community LTC | 2.1 | 2.1–2.2 | 1.8 | 1.8–1.9 | 2.2 | 2.0–2.4 | 2.2 | 2.1–2.4 | 2.0 | 2.0–2.1 | 2.9 | 2.7–3.1 |
| Institutional LTC | 7.2 | 7.1–7.2 | 3.1 | 3.0–3.2 | 8.6 | 8.0–9.2 | 7.9 | 7.5–8.3 | 7.5 | 7.3–7.6 | 11.9 | 11.3–12.5 |
| No LTC | ||||||||||||
| Male | 0.7 | 0.7–0.7 | 0.6 | 0.6–0.6 | 0.7 | 0.6–0.7 | 0.9 | 0.8–0.9 | 0.7 | 0.7–0.7 | 0.8 | 0.8–0.9 |
| Female | ||||||||||||
| White | 1.4 | 1.4–1.5 | 1.5 | 1.5–1.6 | 2.3 | 2.2–2.5 | 1.1 | 1.0–1.1 | 1.4 | 1.4–1.5 | 1.1 | 1.0–1.1 |
| Non-white | ||||||||||||
| Non-metro | 1.1 | 1.2–1.1 | 1.2 | 1.2–1.2 | 0.8 | 0.8–0.9 | 1.4 | 1.3–1.5 | 1.0 | 0.9–1.0 | 1.2 | 1.2–1.3 |
| Metropolitan area | ||||||||||||
| FL | 1.0* | 1.0–1.0 | 1.0* | 0.9–1.0 | 1.1* | 1.0–1.3 | 2.2 | 2.0–2.4 | 0.9 | 0.9–0.9 | 1.0* | 1.0–1.1 |
| MN | 2.1 | 2.0–2.2 | 1.5 | 1.4–1.6 | 1.4 | 1.2–1.7 | 1.7 | 1.5–2.0 | 2.4 | 2.3–2.5 | 1.3 | 1.2–1.4 |
| NM | 1.2 | 1.2–1.3 | 1.1 | 1.1–1.2 | 1.7 | 1.4–2.1 | 1.1* | 0.9–1.3 | 1.3 | 1.2–1.4 | 0.8 | 0.7–0.9 |
| TX | 0.9 | 0.9–1.0 | 0.9 | 0.9–1.0 | 1.1 | 1.0–1.3 | 1.6 | 1.5–1.8 | 1.0 | 0.9–1.0 | 0.8 | 0.7–0.8 |
| VT | 1.4 | 1.3–1.4 | 1.0* | 0.9–1.1 | 1.5 | 1.2–1.9 | 1.2* | 0.9–1.4 | 1.6 | 1.5–1.7 | 1.0* | 0.9–1.2 |
| WA | 0.4 | 0.4–0.4 | 0.3 | 0.3–0.3 | 0.8 | 0.7–1.0 | 0.4 | 0.3–0.4 | 0.4 | 0.4–0.4 | 0.7 | 0.6–0.7 |
| AR | ||||||||||||
| Aged 75–84 y | 0.8 | 0.8–0.9 | 0.8 | 0.8–0.8 | 0.4 | 0.4–0.4 | 0.8 | 0.7–0.8 | 0.9 | 0.9–0.9 | 0.4 | 0.4–0.4 |
| Aged 85 and older | 0.5 | 0.5–0.6 | 0.6 | 0.6–0.7 | 0.1 | 0.1–0.2 | 0.5 | 0.5–0.6 | 0.7 | 0.7–0.7 | 0.1 | 0.1–0.1 |
| Aged 65–74 | ||||||||||||
| Wald χ2 (13) | 66,656.04 | 13,866.77 | 6,537.98 | 9,296.93 | 55,699.84 | 12,392.86 | ||||||
| Pseudo R 2 | 0.11 | 0.05 | 0.10 | 0.08 | 0.12 | 0.10 | ||||||
| Log likelihood | −271,803.86 | −130,898.79 | −28,039.452 | −51,465.102 | −199,758.86 | −51,918.77 | ||||||
Notes: LTC = long-term care; OR = odds ratio; CI = confidence intervals; OR; FL = Florida; MN = Minnesota; NM = New Mexico; TX = Texas; VT = Vermont; WA = Washington; AR = Arkansas.
Results calculated from logistic regressions. Each mental illness is coded as a binary response. All values are significant at p < .001 level, except *p > .05.
Table 4 shows the average incremental effect of MHDs on medical care expenditures, LTC expenditures, and prescription drug expenditures for beneficiaries in different LTC settings. The incremental effect of MHDs on expenditure was estimated from the two-part model (results shown in Supplementary Appendix 2) and represents the additional care expenditures associated with MHDs after controlling for all other beneficiary covariates. For each expenditure category, the first column shows the average incremental expenditure and the 95% CI associated with a specific MHD diagnosis. The second column (difference [%]) shows the corresponding percentage increase (or decrease) from baseline expenditure without the specific MHD. For instance, among community LTC beneficiaries, having any MDH diagnosis was associated with an incremental expenditure of $18,850 toward medical care. This corresponds to an increase of 30% from a baseline expenditure of $64,773 (not shown).
Table 4.
Average Incremental Effect (and 95% Confidence Interval [CI]) of Mental Health Disorders (MHDs) on Medical Care (MC), Long-Term Care (LTC), and Prescription Drug Expenditures by LTC Settings
| MC ($) | Difference in MC (%) | LTC ($) | Difference in LTC (%) | Rx ($) | Difference in Rx (%) | Total ($) | Difference in Total (%) | |
|---|---|---|---|---|---|---|---|---|
| Community LTC | ||||||||
| Any MHD | 18,850 (18,582–19,118) | 30 | 524 (523–525) | 9 | 756 (754–775) | 33 | 20,130 (19,861–20,398) | 27 |
| Anxiety | 14,709 (14,499–14,920) | 23 | (34) (−35 to −33) | (0) | 226 (225–227) | 10 | 14,901 (14,690–15,111) | 18 |
| Bipolar | 2,641* (2,604–2,677) | 5 | 900 (898–901) | 15 | 1,360 (1,357–1,363) | 55 | 4,900 (4,862–4,938) | 9 |
| Major depression | 4,827* (4,760–4,895) | 8 | 510 (509–511) | 8 | 434 (433–435) | 18 | 5,771 (5,704–5,839) | 9 |
| Mild depression | 19,589 (19,309–19,869) | 31 | 156* (155–156) | 3 | 545 (544–546) | 23 | 20,290 (20,010–20,571) | 26 |
| Schizophrenia | −632* (−663 to −602) | 0 | 2,741 (2,738–2,745) | 47 | 1,810 (1,807–1,813) | 78 | 3,919 (3,889–3,949) | 12 |
| Institutional LTC | ||||||||
| Any MHD | 13,395 (13,307–13,484) | 42 | 1,278 (1,277–1,280) | 5 | 1,014 (1,013–1,016) | 29 | 15,688 (15,599–15,777) | 23 |
| Anxiety | 11,528 (11,449–11,606) | 33 | 182* (181–182) | 1 | 564 (563–565) | 15 | 12,273 (12,194–12,352) | 16 |
| Bipolar | 4,071 (4,043–4,098) | 12 | 1,263 (1,262–1,265) | 5 | 1,505 (1,503–1,507) | 39 | 6,839 (6,811–6,867) | 10 |
| Major depression | 9,286 (9,223–9,349) | 26 | 1,093 (1,092–1,094) | 4 | 693 (692–694) | 18 | 11,072 (11,009–11,135) | 15 |
| Mild depression | 11,905 (11,825–11,984) | 36 | 653 (652–654) | 2 | 659 (658–660) | 18 | 13,217 (13,137–13,296) | 19 |
| Schizophrenia | 922* (914–929) | 3 | 2,246 (2,243–2,249) | 8 | 1,428 (1,425–1,430) | 38 | 4,595 (4,587–4,604) | 8 |
| Non-LTC | ||||||||
| Any MHD | 17,608 (16,779–18,437) | 82 | 579*(579–580) | 54 | 18,187 (17,358–19,016) | 75 | ||
| Anxiety | 15,386 (14,674–16,098) | 72 | 303 (303-303) | 29 | 15,689 (14,977–16,401) | 63 | ||
| Bipolar | 6,464 (6,136–6,792) | 34 | 692 (692–693) | 59 | 7,157 (6,828–7,485) | 37 | ||
| Major depression | 16,997 (16,202–17,792) | 79 | 609 (609–610) | 55 | 17,607 (16,811–18,402) | 73 | ||
| Mild depression | 14,058 (13,412–14,704) | 66 | 272 (272–273) | 24 | 14,330 (13,685–14,976) | 57 | ||
| Schizophrenia | 12,929 (12,329–13,529) | 61 | 1,427 (1,425–1,428) | 132 | 14,356 (13,755–14,956) | 72 | ||
Notes: Results are calculated form the two-part regression model. Results control for state, urban–rural difference, health risk score, age, race, and gender; all values are significant at p < .001 level, except *p > .05.
MC ($) is the average incremental effect of the MHD on beneficiary’s annual MC expenditures. Difference in MC is the percentage increase from baseline beneficiary MC expenditure as a result of the MHD. LTC ($) is the average incremental effect of the MHD on beneficiary’s annual LTC expenditures. Difference in LTC is the percentage increase from baseline beneficiary LTC expenditure as a result of the MHD. Rx ($) is the average incremental effect of the MHD on beneficiary’s annual prescription drug expenditures. Difference in Rx is the percentage increase from baseline beneficiary prescription drug expenditures as a result of the MHD.
Among the community LTC dual eligible elders, mild depression (31% or $19,589) and anxiety (23% or $14,709) had the largest affects on annual medical care expenditures; schizophrenia (47% or $2,741) had the largest affect on annual LTC expenditures; and bipolar (55% or $1,360) and schizophrenia (78% or $1,810) had the largest affects on annual prescription drug expenditures. Overall, anxiety (18% or $14,901) and mild depression (26% or $20,290) were associated with highest increase in annual total health care expenditures for dual eligible elders in community LTC settings.
Among institutional LTC dual eligible elders, diagnoses of anxiety (33% or $11,528) and mild depression (36% or 11,905) were associated with large increases in annual medical care expenditures—as observed among community LTC beneficiaries. However, having an MHD diagnosis had little incremental effect on annual LTC expenditures (<10%). Schizophrenia and bipolar diagnoses were associated with large increases (39% and 39%, respectively) in annual prescription drug expenditures. Anxiety and mild depression were associated with respective increases of $12,273 (16%) and $13,271 (19%) in annual total health care expenditures for beneficiaries in institutional LTC settings. Although the community LTC and institutional LTC effects are basically comparable on total health care expenditures, the patterns vary by type of expenditures (medical care vs. LTC vs. prescription drug) and by MHDs.
Although the non-LTC dual eligible elders had the lowest 12-month prevalence of all MHDs among the three groups, they had the largest percentage increase in medical care, prescription drug, and total health care expenditures associated with MHDs. For example, having any MHD diagnoses was associated with 75% increase in total health care expenditures among the non-LTC dual eligible elders, compared with a 27% increase for the community LTC group and a 23% increase for institutional LTC group. The effects also differ by MHD. Among the five MHDs, major depression and schizophrenia were associated with the highest percentage increase in total health care expenditures of 73% ($17,607) and 72% ($14,356), respectively, followed by anxiety (63% or $15,689). Major depression and anxiety were associated with 79% ($16,997) and 72% ($15,386) increase in annual medical care expenditures. Schizophrenia was associated with the highest increase in prescription drug expenditures (132% or $1,427), followed by bipolar disorder (59% or $692) and major depression (55% or $609).
Implications
This is the first study to document the diagnosed prevalence of several common MHDs among dual eligible elders, the effects of these MHDs on health care expenditures, and how their effects on expenditures varied across LTC settings. Nondementia MHDs are prevalent among dual eligible older people and are costly to the health care system. Even with a relatively conservative approach using 12-month diagnosed prevalence and the CMS Chronic Condition Data Warehouse—Chronic Condition Categories criteria, we found that 12.8% of all dual eligible older people and one third of those living in LTC institutions had at least one of the five MHDs. A study of prevalence of MHDs in Massachusetts (Lin et al., 2011b) with all elderly Medicare or Medicaid enrollees estimated the rates at about 20% but did not differentiate by LTC settings. Actual rates of MHDs are hard to determine. There is evidence that the prevalence varies with the data source (Bagchi et al., 2009).
The prevalence for any mental disorder in our study (12.8% of all duals, 13% in the community LTC group, 33.4% for those in nursing homes, and 7.5% for non-LTC) is based on five diagnosed conditions and focuses on dual eligible older adults only. Therefore, it differs from previous studies that have examined broader lists of conditions among different populations. Jeste and colleagues’ (1999) analysis of data from the epidemiologic catchment area study on older adults in general, included affective disorders, anxiety disorders, and schizophrenia, as well as alcohol/drug abuse and dependence and antisocial personality disorder found a 1-year prevalence of 13% among all older adults (Jeste et al., 1999). Lin and colleagues (2011b) examined Medicare and Medicaid enrollees in Massachusetts and identified any behavioral health disorder; they found 12-month prevalence of 19% among older adults (38.8% for duals; Lin et al., 2011b). In another article, they estimated that 39% of nursing home residents had serious mental illness; almost all of those LTC residents were dual eligible (Lin, Zhang, Leung, & Clark, 2011a). This rate was close to our finding. Bagchi and colleagues (2009) included a broad range of mental conditions such as stress and adjustment reactions, sexual disorders, and others; they found a range of up to 33%–46% of nursing home residents with any diagnosis of a mental illness. In sum, differences between previous studies and ours reflect differences in included diagnoses and populations under study but when broken down by dual status and residence, prevalence rates suggest some similarities.
Dual eligible elderly people are more likely to be users of LTC services than nondual eligible older adults. The 12-month diagnosed prevalence rates varied widely across three settings. Those in LTC groups had much higher prevalence rate than the non-LTC group. Between the two LTC groups, the 12-month prevalence rate of those in institutional LTC group was more than double of the rate of the community LTC group. The high diagnosed prevalence of MHDs among institutional dual eligible elders was likely due to the policy effect of the Preadmission Screening and Resident Review (PASRR) program, which requires states to screen all applicants to Medicaid-certified nursing facilities for mental disorders prior to admission and to provide appropriate specialized mental health services when needed. Therefore, figures on the 12-month prevalence of MHDs among institutional beneficiaries may be more accurate than the figures of noninstitutionalized beneficiaries because of the compulsory screening requirement under PASRR, although the quality of its implementation has been criticized (Linkins, Lucca, Housman, & Smith, 2006). The fact that Medicaid beneficiaries living in the community reported much lower diagnosed prevalence of MHDs may reflect a significant underdiagnosis among those populations.
Like prior studies (Kessler et al., 1994; Meeks & Murrell, 1997), we found a lower prevalence of MHDs among older age groups among this age-delimited analytic sample. Also similar to prior literature (Kessler et al., 1994), and again among this selectively older sample, men had lower rates of MHDs. These patterns suggest that age- and sex-related patterns in mental illness are relatively immutable across age and institutional barriers.
The presence of MHDs was usually associated with higher health care expenditures. The increase in health care expenditures associated with MHDs results from beneficiaries with MHDs using more care or MHDs complicating care for beneficiaries thereby making it more expensive. On average, having an MHD was associated with 27% higher health care expenditures for dual eligibles receiving community LTC services; 23% higher health care expenditures for dual eligibles living in LTC institutions; and 75% higher health care expenditures for those not receiving any LTC services.
The effect of MHDs on health care expenditures varied by diagnosis. Having a mental disorder diagnosis added between $3,919 (schizophrenia in the community-LTC group) and $20,290 (mild depression in the community-LTC group) to total health care expenditures. Overall, mild depression and anxiety were two most expensive MHDs in LTC settings, whereas schizophrenia and major depression were two most expensive MHDs in non-LTC setting. Bipolar and schizophrenia were consistently associated with the largest increase in prescription drug expenditures. Although the greater prescription drug expenditures for bipolar disorder and schizophrenia are to be expected because they have a relatively greater need for medication, the higher medical and LTC expenditures associated with anxiety and depression are notable because all four (anxiety, depression, bipolar, and schizophrenia) are usually associated with functional morbidity and poorer physical health (Colton & Manderscheid, 2006; U.S. Department of Health Human Services, 1999). These higher medical and LTC expenditures for anxiety and depression may represent the high (and even excessive) utilization patterns commonly associated with them (Simon, VonKorff, & Barlow, 1995). Alternatively, bipolar and schizophrenia may be better managed through pharmaceutical drugs, resulting in the relatively lower medical and LTC cost burdens observed here. Further attention is needed to address these issues. We did a separate analysis with unadjusted expenditures and the unadjusted patterns were similar to the adjusted patterns, indicating that the findings are not due to our statistical modeling.
The effect of MHDs on health care expenditures also varied by LTC settings. Non-LTC beneficiaries had the largest percentage increase in medical care, prescription drug, and total health care expenditures. In terms of actual dollar increases, non-LTC dual eligibles also had increases comparable to or even a little bit higher than that of the community-LTC dual eligibles. These figures suggest that even though community-LTC dual eligibles were more likely to have an MHD diagnosis, once diagnosed, both group received comparable treatment.
MHDs were associated with consistently higher percentage increase in medical care expenditures in the institutional LTC setting than in community LTC setting. Some studies suggest that MHD may complicate medical care by adversely affecting adherence with medical regimens (Martin, Williams, Haskard, & DiMatteo, 2005). The lower incremental effect of MHD on medical care expenditures in the community LTC sample was likely due to two factors: (a) MDH is associated with chronic disease comorbidity and complicates its care in both settings and (c) adherence to treatment regimens is likely lower in the community. The observed difference is likely due to the combined effect of these two.
There was considerable variation by state, mirroring other reports of variations in diagnoses (Song et al., 2010; Welch, Sharp, Gottlieb, Skinner, & Wennberg, 2011). These differences persist even after we controlled for other beneficiary characteristics. It is not clear if they reflect differences in enthusiasm for treatment or differences in coding.
This study has some acknowledged limitations. The diagnosis-based estimates from payment records are conservative and may introduce some selection bias (e.g., those with nondiagnosed mental disorders may differ from those analyzed here). This is especially true in light of evidence that access and utilization of mental health services among older adults remains inadequate (Charney et al., 2003). However, this conservative approach and focus on numerable expenditures helps focus the discussion on what can be done in existing systems for identified/identifiable cases. In addition, the study uses estimates across only seven states. Medicaid programs vary in terms of the state plan services offered and in terms of expenditures for different services within these programs. Yet, our approach selected these states to obtain a diversified and large-scale view of prevalence patterns and Medicare and Medicaid expenditures, which is a large step forward from prior one-state or one-location analyses. However, because these states were selected for a study that focused on states’ efforts to rebalance their LTC systems, mental health–specific factors, such as the supply of psychiatric professionals and the historical role of behavioral management care, were not considered in the selection of these states. We did control the effects of these factors on the health care expenditures by including state as our control variables in our regression analyses. Researchers in the future could focus on one state to examine how local practice patterns and the state policy affect expenditures. Furthermore, we focused on the main effect of MHDs on health care expenditures. As comorbidities between MHDs and other health problems are common, researchers in the future could examine how such comorbidities affect health care expenditures. Finally, we used the health-risk score (CMS–HCC score) to control for all comorbidities of the beneficiaries in our data analysis. This may underestimate the effect of MHDs that are in the CMS–HCC algorithm (schizophrenia, bipolar disorder, and major depression). We believed that the gains from using the CMS–HCC score to control for other conditions offset its limitations in underestimating the effects of schizophrenia and bipolar disorder/major depression.
Despite these limitations, the findings of this study offer broadly measured benchmark estimates of the reach and affect of these disorders among dual eligible elders. As such, this study’s findings can help identify the ways in which mental health–related expenditures are apportioned across the spectrum of care for dual eligible older adults. They also indicate areas in which the most progress can be made in reducing excess expenditures, as well as suggesting strategies for targeting services for dual eligible older adults with mental health diagnoses.
Supplementary Material
Supplementary material can be found at: http://gerontologist.oxfordjournals.org.
Funding
This work was supported by the Centers for Medicare and Medicaid Services under Contract No. MRAD HHSM-500-2005-000271 Task Order #1 “Monitoring Chronic Disease Care and Outcomes Among Elderly: Extending the Use of MAX to Examine Rebalancing.”
Supplementary Material
References
- Anderson R. J., Freedland K. E., Clouse R. E., Lustman P. J. 2001. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care, 24, 1069–1078 [DOI] [PubMed] [Google Scholar]
- Bagchi A. D., Verdier J. M., Simon S. E. 2009. How many nursing home residents live with a mental illness?. Psychiatric Services (Washington, D.C.), 60, 958–964 [DOI] [PubMed] [Google Scholar]
- Basu A., Rathouz P. J. 2005. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics (Oxford, England), 6, 93–109 [DOI] [PubMed] [Google Scholar]
- Bijl R. V., Ravelli A. 2000. Current and residual functional disability associated with psychopathology: Findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Psychological Medicine, 30, 657–668 [DOI] [PubMed] [Google Scholar]
- Buntin M. B., Zaslavsky A. M. 2004. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. Journal of Health Economics, 23, 525–542 [DOI] [PubMed] [Google Scholar]
- Charney D. S., Reynolds C. F., 3rd, Lewis L., Lebowitz B. D., Sunderland T., Alexopoulos G. S., et al. 2003. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Archives of General Psychiatry, 60, 664–672 [DOI] [PubMed] [Google Scholar]
- Colton C. W., Manderscheid R. W. 2006. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing Chronic Disease, 3, A42 [PMC free article] [PubMed] [Google Scholar]
- Frayne S. M., Halanych J. H., Miller D. R., Wang F., Lin H., Pogach L., et al. 2005. Disparities in diabetes care: Impact of mental illness. Archives of Internal Medicine, 165, 2631–2638 [DOI] [PubMed] [Google Scholar]
- Jeste D. V., Alexopoulos G. S., Bartels S. J., Cummings J. L., Gallo J. J., Gottlieb G. L., et al. 1999. Consensus statement on the upcoming crisis in geriatric mental health: Research agenda for the next 2 decades. Archives of General Psychiatry, 56, 848–853 doi:10.1001/archpsyc.56.9.848 [DOI] [PubMed] [Google Scholar]
- Jones L. E., Clarke W., Carney C. P. 2004. Receipt of diabetes services by insured adults with and without claims for mental disorders. Annals of Epidemiology, 14, 611 [DOI] [PubMed] [Google Scholar]
- Kaiser Commission on Medicaid and the Uninsured 2011. Dual Eligibles: Medicaid’s Role for Low-Income Medicare Beneficiaries. Washington, D.C.: Kaiser Family Foundation; [Google Scholar]
- Kane R. A., Priester R., Kane R. L., Mollica R. L. 2006. Management Approaches to Rebalancing Long-Term Care Systems: Experience in Eight States up to July 31, 2005. Minnesota, MN: University of Minnesota School of Public Health; [Google Scholar]
- Kasper J., O’Malley Watts M., Lyons B. 2010. Chronic Disease and Co-Morbidity Among Dual Eligibles: Implications for Patterns of Medicaid and Medicare Service Use and Spending. Washington, D.C.: Kaiser Family Foundation; [Google Scholar]
- Kessler R. C., McGonagle K. A., Zhao S., Nelson C. B., Hughes M., Eshleman S., et al. 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry, 51, 8–19 [DOI] [PubMed] [Google Scholar]
- Lin W. C., Zhang J., Leung G. Y., Clark R. E. (2011). Chronic physical conditions in older adults with mental illness and/ or substance use disorders. Journal of the American Geriatrics Society, 59, 1913–1921 doi:10.1001/archpsyc.1994.03950010008002 [DOI] [PubMed] [Google Scholar]
- Lin W. C., Zhang J., Leung G. Y., Clark R. E. (2011). Twelve-month diagnosed prevalence of behavioral health disorders among elderly Medicare and Medicaid members. American Journal of Geriatric Psychiatry, 19, 970–979 [DOI] [PubMed] [Google Scholar]
- Linkins K. W., Lucca A. M., Housman M., Smith S. A. 2006. Use of PASRR programs to assess serious mental illness and service access in nursing homes. Psychiatric Services (Washington, D.C.), 57, 325–332 [DOI] [PubMed] [Google Scholar]
- Manning W. G., Mullahy J. 2001. Estimating log models: To transform or not to transform?. Journal of Health Economics, 20, 461–494 [DOI] [PubMed] [Google Scholar]
- Martin L. R., Williams S. L., Haskard K. B., Dimatteo M. R. 2005. The challenge of patient adherence. Therapeutics and Clinical Risk Management, 1, 189–199 [PMC free article] [PubMed] [Google Scholar]
- Meeks S., Murrell S. A. 1997. Mental illness in late life: socioeconomic conditions, psychiatric symptoms, and adjustment of long-term sufferers. Psychology and Aging, 12, 296–308 [DOI] [PubMed] [Google Scholar]
- Pope G. C., Ellis R. P., Ash A. S., Ayanian J. Z., Bates D. W., Burstin H., et al. 2000. Diagnostic cost group hierarchical condition category models for Medicare risk adjustment. Health Economics Research, Waltham, MA. [PMC free article] [PubMed] [Google Scholar]
- Pope G. C., Kautter J., Ellis R. P., Ash A. S., Ayanian J. Z., Iezzoni L. I., et al. 2004. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Review, 25, 119–141 [PMC free article] [PubMed] [Google Scholar]
- Simon G. E., VonKorff M., Barlow W. 1995. Health care costs of primary care patients with recognized depression. Archives of General Psychiatry, 52, 850–856 [DOI] [PubMed] [Google Scholar]
- Song Y., Skinner J., Bynum J., Sutherland J., Wennberg J. E., Fisher E. S. 2010. Regional variations in diagnostic practices. New England Journal of Medicine, 363, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe J. M., Thorpe C. T., Kennelty K. A., Chewning B. A. 2012. Depressive symptoms and reduced preventive care use in older adults: The mediating role of perceived access. Medical Care, 50, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L., Chatterjee S. 2009. The impact of obesity on health service utilization and costs in childhood. Obesity, 17, 1749–1754 [DOI] [PubMed] [Google Scholar]
- Trogdon J. G., Hylands T. 2008. Nationally representative medical costs of diabetes by time since diagnosis. Diabetes Care, 31, 2307–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health Human Services. Mental Health: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health. 1999 [Google Scholar]
- Welch H. G., Sharp S. M., Gottlieb D. J., Skinner J. S., Wennberg J. E. 2011. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. Journal of the American Medical Association, 305, 1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


