Abstract
Early-life iron deficiency anemia (IDA) alters the expression of critical genes involved in neuronal dendritic structural plasticity of the hippocampus, thus contributing to delayed maturation of electrophysiology, and learning and memory behavior in rats. Structural maturity in multiple cortical regions is characterized by the appearance of parvalbumin-positive (PV+) GABAergic interneurons and perineuronal nets (PNNs). Appearance of PV+ interneurons and PNNs can serve as cellular markers for the beginning and end of a critical developmental period, respectively. During this period, the system progresses from an immature yet highly plastic condition, to a more mature and efficient state that is however less flexible and may exhibit poorer potential for recovery from injury. To test if fetal-neonatal IDA alters parvalbumin (PV) mRNA expression, protein levels, and the number of PV+ interneurons and PNNs in the male rat hippocampus, pregnant dams were given an iron deficient (ID) diet (3 mg iron/kg chow) from gestational day (G)2 to postnatal day (P)7 and then placed on an iron sufficient (IS) diet (198 mg/kg) for the remainder of the experiment. On this regimen, formerly-ID (FID) animals become fully iron-replete by P56. Minimal levels of PV (mRNA and protein), PV+ interneurons, and PNNs were found in IS and ID P7 rats. By P15, and continuing through P30 and P65, ID rats had reduced PV mRNA expression and protein levels compared to IS controls. While there were no differences in the number of PV+ neurons at either P30 or P65, the percentage of PV+ cells surrounded by PNNs was slightly greater in ID rats as compared to IS controls. The lower levels of these acknowledged critical period biomarkers in the ID group are consistent with studies that demonstrate later maturation of the acutely ID hippocampus and lower plasticity in the adult formerly ID hippocampus. The findings provide additional potential cellular bases for previously described electrophysiologic and behavioral abnormalities found during and following early life IDA.
Keywords: Parvalbumin, hippocampus, iron deficiency, anemia, perineuronal nets, interneurons, critical periods
Introduction
Iron availability is essential for neurodevelopment, especially during late fetal and early postnatal stages. Iron is distributed heterogeneously throughout the brain and is incorporated into heme moieties and iron clusters such as cytochromes, hydroxylases, and iron regulatory proteins. Iron deficiency is the world’s leading nutrient deficiency, affecting more than 2 billion people worldwide, and is evident in 30–50% of all pregnancies and as many as 80% of pregnancies in developing countries [1]. Nutrient deficiencies and malnutrition in the late fetal and the early neonatal period are thought to affect later adult brain function by causing neural systems to deviate from their developmental trajectory across the life span [2]. Gestational and early postnatal iron deficiency anemia (IDA) in humans results in concurrent and persistent learning and memory deficits in later childhood and adulthood in spite of early and prompt treatment upon diagnosis [3–5].
The hippocampus develops rapidly throughout the late fetal and early postnatal period and therefore is particularly susceptible to the disruption of iron delivery because it supports neuronal metabolism [6]. In rats, aspects of hippocampal development that progress between postnatal day (P)15 and P30 include rapid dendritogenesis and synaptogenesis [7], excitatory neuronal activity reaching adult levels [8], energy production and utilization [9], and brain-derived neurotrophic factor (BDNF) expression [10], all accompanied by increased hippocampal iron uptake and utilization [11,12]. Failure to provide iron in this timeframe alters each of these processes and likely contributes to the long-term learning and memory deficits seen in humans and animal models following early-life iron deficiency [3–5,13–15], defining it as a potential critical period [16].
The opening and closing of a sensitive or critical period is indexed by the appearance of parvalbumin-positive (PV+) interneurons and perineuronal nets (PNNs), respectively [16]. While serving as useful biomarkers for critical periods, these cellular events also play a physiologic role in regulating excitatory and inhibitory activity. For example, the mature hippocampus has several classes of GABAergic interneurons that are responsible for inhibitory control of principle excitatory cells [17,18]. A specific subset of interneurons that are required for synaptic plasticity express the calcium-binding protein parvalbumin (PV) [18]. Increased inhibitory interneuron activity and specifically the appearance of PV+ interneurons have been shown to mark the onset of experience-dependent plasticity and correspond closely to the opening of critical periods [16]. PNNs are a specialized form of the extracellular matrix that are composed largely of chondroitin sulfate proteoglycans and appear to be responsible for synaptic stabilization in the adult brain [19,20]. PNNs preferentially surround the soma and dendrites of mature, fast-spiking PV+ interneurons [21]. The appearance of PNNs in other brain regions is associated with reduced plasticity and limited potential for recovery from injury [22]. Digestion of these PNNs has been shown to allow plasticity to be re-established. Collectively, these data indicate a role for PNNs in the limitation of plasticity at the closure of critical periods [16,23].
The mechanisms by which gestational-lactational IDA causes long-term deficits in spite of iron repletion remain unclear. One possibility is that IDA delays the maturation of the neural circuitry [8,24]. Hippocampal GABAergic interneurons are generated prenatally in the rat between embryonic day (E)13 and E18, migrate to populate the hippocampal formation by approximately P0, and mature morphologically during the postnatal period [25–28]. Recently, our group showed that gestational IDA reduces whole-brain PV mRNA expression in rat at P12 [29]. The objectives of the current study were to establish the developmental trajectory of PV mRNA and protein levels, PV+ interneurons, and PNNs in the IS rat hippocampus between P7 and P65, and to determine if IDA during gestation and the early neonatal period disrupts this ontogeny.
Materials and Methods
Animals
All protocols were approved by the Institution Animal Care and Use Committee at the University of Minnesota. Pregnant Harlan Sprague-Dawley rat dams were fed an iron deficient (ID) diet (3 mg/kg iron) from gestational day (G)2 to P7, the approximate equivalent of human term birth with respect to hippocampal development [30], after which they were placed back onto a normal IS diet (198 mg/kg iron). This dietary IDA model induces global anemia and a 40% reduction in whole-brain iron concentration in P15 pups with complete brain iron sufficiency by P56 [6,8,10]. With this model of iron repletion beginning at P7, the P65 animals are iron sufficient and thus labeled as formerly iron-deficient (FID). Control dams were fed the IS diet throughout the full duration of the experiment. Litters were culled to 8 pups and weaned at P21. Pups were sacrificed with an overdose of sodium pentobarbital (390mg/mL, Fatal-Plus®, Vortech Pharmaceuticals, Dearborn, MI). Bilateral hippocampi were dissected for quantitative (q)PCR and Western blot, and frozen whole-brain sections were obtained for histochemical analysis at P7, P15, P30, and P65 as described previously [6,10,31]. The P7 time point precedes the beginning of rapid dendritogenesis, P15 marks the beginning of rapid dendritogenesis, and P30 is at the end of rapid dendritogenesis in the rat hippocampus, while P65 represents the young adult animal [7].
Quantitative Real-Time PCR
Hippocampal tissue used for quantitative RT-PCR (Taqman™) mRNA analysis was dissected following rapid decapitation and brain removal. Dissected hippocampi were flash frozen in liquid nitrogen and stored at −80°C. Isolation of total RNA and qPCR was performed as previously described [10]. Briefly, total RNA was isolated from hippocampal hemispheres using an RNA-isolation kit (Applied Biosystems, Foster City, CA) and concentrations were measured by absorbance at 260 nm (A260/280) using a NanoDrop ND-1000 (NanoDrop Technologies Inc., Wilmington, DE). Approximately 1 µg of total RNA and a high-capacity cDNA kit (Applied Biosystems) were used for cDNA generation. The resulting cDNA was diluted 10-fold to give a final volume of 200 µL. qPCR experiments were performed on sample triplicates using Taqman™ quantitative RT-PCR Universal Mix and Taqman™ gene expression assay probes for genes of interest. The eukaryotic ribosomal protein 18S was used as an endogenous control (Applied Biosystems, Assay # Rn01428915_g1). Taqman™ gene expression assay probes were used for parvalbumin, calbindin, and calretinin (Applied Biosystems, Pvalb – Assay # Rn00574541_m1; Calb1 – Assay # Rn00583140_m1; Calb2 – Assay # Rn00588816_m1). Thermocycling was carried out according to the manufacturer's protocol (Applied Biosystems) using a MX3000P instrument (Stratagene, La Jolla, CA).
Western Blot
Protein samples were prepared from individual dissected hippocampal hemispheres by sonication in RIPA lysis buffer. 30 µg of total protein was separated using NuPAGE 4–12% gradient Bis-Tris gels (Invitrogen, Carlsbad, CA). Protein was transferred onto nitrocellulose membrane (Thermo Fisher Scientific, Rockford, IL), blocked in Rockland Near-Fluorescence blocking solution (Rockland Immunochemicals, Gilbertsville, PA), and incubated overnight at 4°C with mouse anti-parvalbumin monoclonal antibody (1:1000, Millipore, Temecula, CA) and rabbit anti-β-actin monoclonal antibody (1:10,000, Cell Signaling Technologies, Danvers, MA) diluted in Rockland blocking buffer. Following primary incubation, the blots were washed 4 × 15 min using PBS + 0.1% Tween-20, and incubated for 45 minutes at room temperature in secondary antibody diluted in Rockland blocking solution plus 0.1% Tween-20 and 0.02% SDS. The blots were then washed again 4 × 15 min and imaged with Odyssey infrared scanning (LiCor Bioscience, Lincoln, NE).
Histochemistry
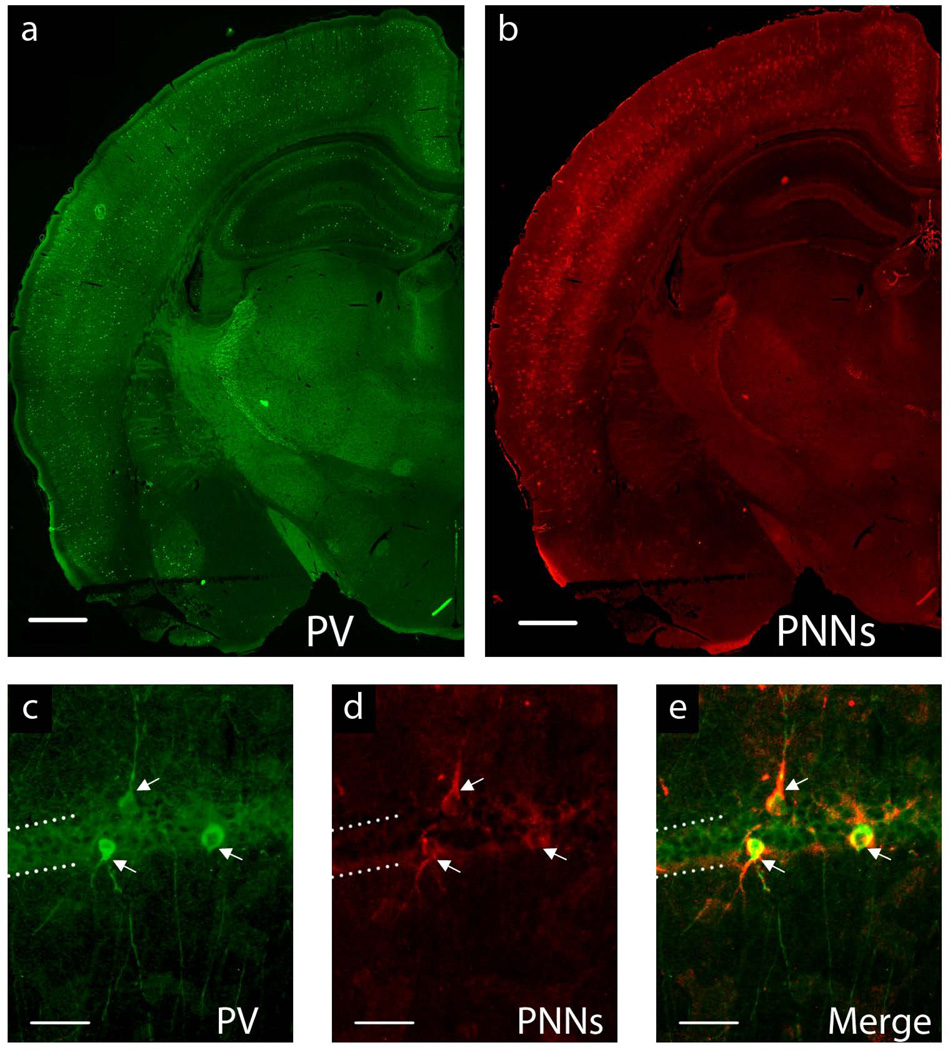
Histochemistry was performed on 20-µm frozen rat brain sections to count the number of PV+ interneurons and PNNs in all subregions of the hippocampus. Antigen unmasking was enhanced by pre-treating the sections with 90°C 50 mM Citric acid buffer for 30 minutes before washing sections twice in Tris-buffered solution (TBS) with 0.1% Triton X;-100. Sections were then blocked in 10% goat serum diluted in 0.1% Triton X-100 for 30 minutes and incubated overnight at 4°C in a mixture of goat anti-parvalbumin primary antibody (1:8000, Abcam, Cambridge, MA) and biotin-conjugated lectin from Wisteria floribunda (1:50, Sigma, St. Louis, MO) diluted in blocking solution. The sections were then washed twice in TBS with 0.1% Triton-X 100 and incubated with Alexa-488 rabbit anti-goat (1:200, Invitrogen, Grand Island, NY) and Alexa-555 Streptavidin (1:50 Invitrogen) in TBS with 0.2% Triton X-100 for 30 minutes at room temperature. Omission of the primary antibodies as a control treatment resulted in absence of staining. The sections were imaged using a Nikon microscope (Eclipse 600). Six positionally-similar sections per animal (n=7–8), representing anterior, middle and posterior aspects of the hippocampus were assessed blindly by two of the authors. Every PV+ interneuron and stained PNN was counted at 20× magnification in each slice by both investigators seperately, corroborated for inter-observer variability, and averaged for each animal. The total number of PV+ interneurons and PNNs surrounding PV+ interneurons were counted in the fasciola cinerea (FC), cornu ammonis 1 (CA1), CA2, CA3, and the dentate gyrus (DG) of the hippocampus according to the regions as defined by Allen Institute for Brain Science [32]. Only PNNs that evidently surrounded the soma and processes of PV+ interneurons were considered (See Figure 1).
FIGURE 1.
a–b. Representative image of P65 IS whole-brain hemisphere at 4× magnification showing a. parvalbumin-positive interneurons and b. perineuronal nets. Scale bar = 1mm. c–e. Representative hippocampal CA1 interneurons at 20× magnification: c. stained with a primary antibody for parvalbumin (PV+), d. perineuronal nets (PNNs) stained with biotin-conjugated lectin from Wisteria floribunda e. Merge of images c. and d. showing PV+ interenurons surrounded by PNNs. Dotted lines indicate the CA1 hippocampal pyramidal cell layer. Arrows denote PV+ GABAergic interneurons surrounded by PNNs. Scale bar = 50µm.
Statistical Analysis
In order to assess the relative maturity of PV+ interneurons and PNN expression, the percentage of PV+ cells surrounded by PNNs was compared between IS and ID samples across the P30 and P65 time points. For the developmental ontogenies of PV+ interneurons and PNNs, group differences across ages were assessed by ANOVA, and differences due to iron status were analyzed at each age by t-test. When data were not normally distributed, nonparametric tests (e.g. Mann-Whitney test) were utilized. The mRNA expression of PV was assessed using a 2-way ANOVA with a post-hoc Bonferroni comparison in order to distinguish group differences across ages and between dietary groups. Transformations were performed for each comparison to normalize gene expression changes as a fold change of the average value of the control. In ID vs. IS comparisons, the IS group was the control and given the value of 1. For developmental ontogeny, the P7 timepoint was given a value of 1.
Results
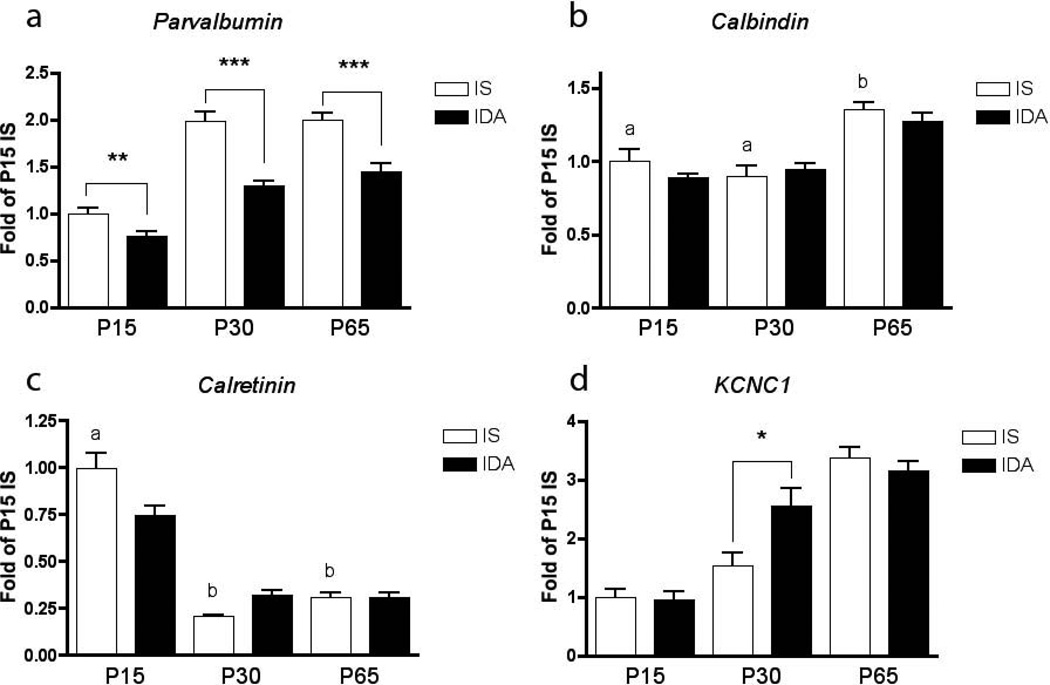
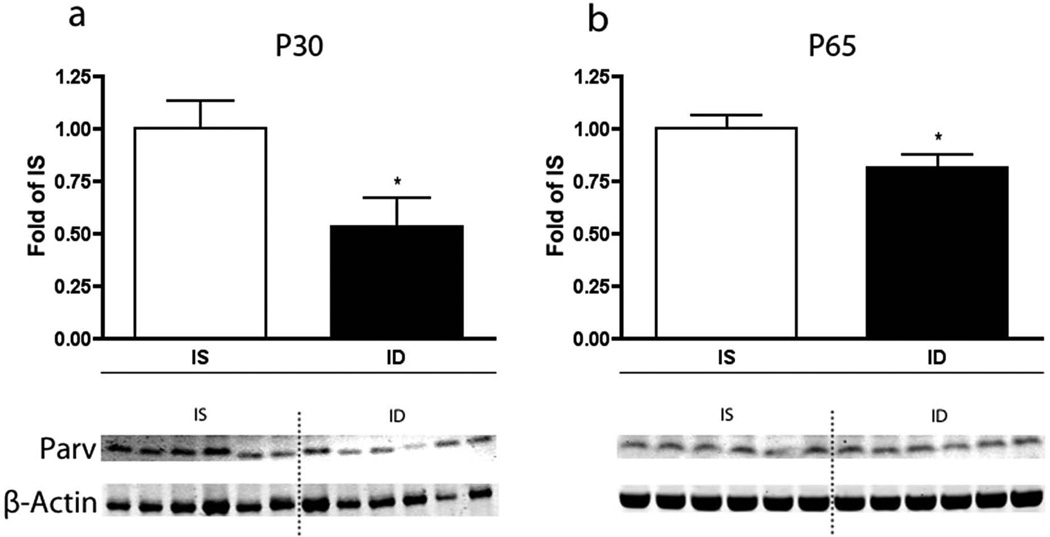
In the IS hippocampus, PV mRNA expression (n=8 for each age and condition) was detectable, but low at P7 (data not shown), increased through P15 and reached adult levels by P30 (Figure 2a). There was no difference in PV expression between P30 and P65. Fetal-neonatal IDA reduced PV mRNA expression at P15 by 24% (p<0.01), at P30 by 35% (p<0.001), and at P65 by 28% (p<0.001) compared to IS controls. No significant differences were seen between the diet groups at P7, likely because of the low baseline expression at this point of hippocampal development. Levels of PV protein (n=6 for each age and condition) corroborated the mRNA expression data (Figure 3). P30 ID rats had 47% less PV protein (p<0.05, Figure 3a). In the FID P65 rats, PV protein levels were still reduced by 20% (p<0.05, Figure 3b). The levels of mRNA expression for calbindin and calretinin were also determined at P15, P30, and P65 to assess whether the parvalbumin findings were specific and not due to a general effect on calcium-binding proteins. The mRNA levels of calbindin and calretinin (n=8) were not affected by IDA at any time point (Figure 2b–c). The mRNA levels for KCNC1 were measured at the three time points to determine whether its development was disrupted by IDA. KCNC1 transcript levels were greater in the IDA group at P30 (p<0.05, Figure 2d).
FIGURE 2.
Developmental ontogeny of a. parvalbumin b. calbindin c. calretinin and d. Kcnc1 mRNA expression (n=8 at each age and condition) in the rat hippocampus and the effect of fetal-neonatal IDA. For each time-curve, the P15 IS group is set at a relative expression of 1. a≠b≠c, * = p<0.05, ** = p< 0.01, *** = p< 0.001
FIGURE 3.
Effect of fetal-neonatal IDA on parvalbumin protein levels at a. P30 and b. P65 (n=6 for each age and condition). White bars denote IS animals, and black bars denote the ID group. The IS groups are set at a relative expression value of 1. * = p <0.05. Representative Western blots are shown indicating parvalbumin protein expression and β-actin as the control protein.
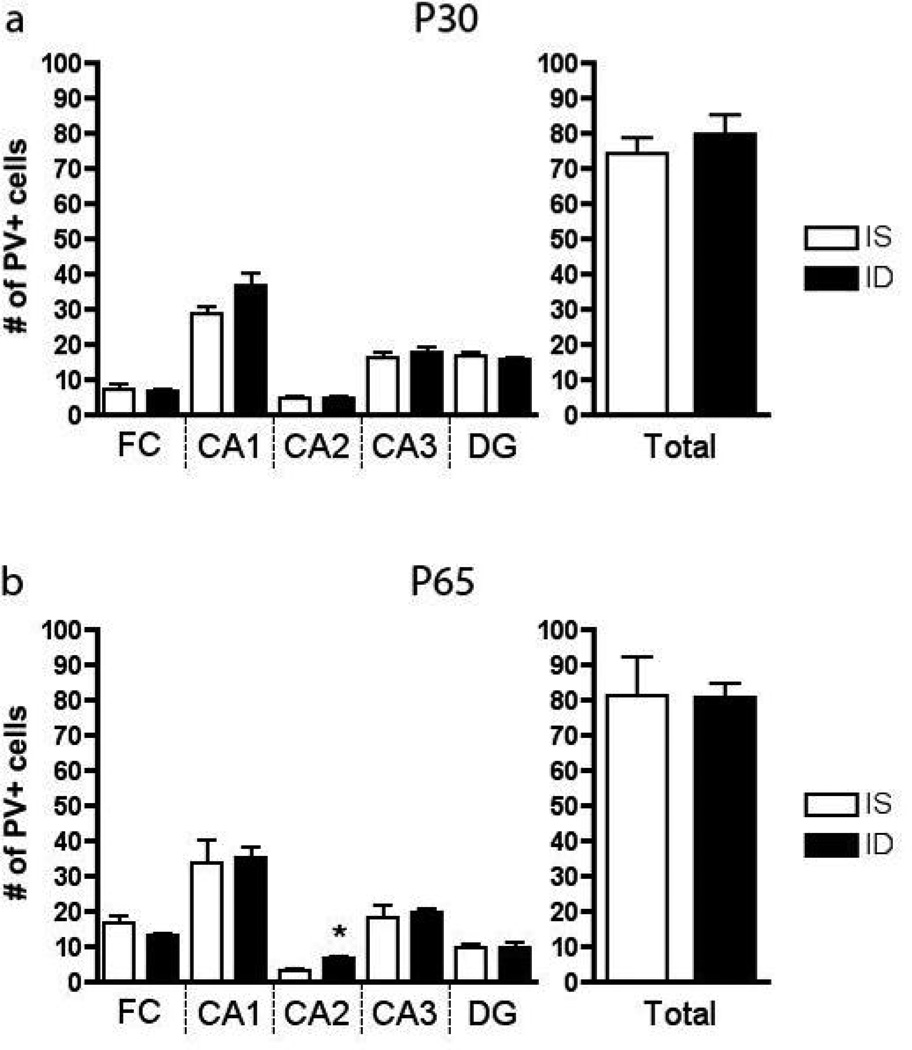
In the IS hippocampus, PV+ interneurons were barely distinguishable at P15, but were present at adult levels by P30. The number of PV+ cells was not significantly different between P30 and P65 IS rats (Figure 4, n=7–8). At both ages, area CA1 had the highest concentration of cells compared to FC, CA2, CA3 and DG. Fetal-neonatal IDA did not affect the overall number of PV+ cells in the hippocampus at P30 or P65 (Figure 4, n=7–8), but a difference was detected in CA2 where a 2-fold increase was seen in the ID group.
FIGURE 4.
The number of parvalbumin-positive cells by region of the hippocampus at a. P30 and b. P65 (n=7–8). * = p <0.05. Values represent means ± SEM
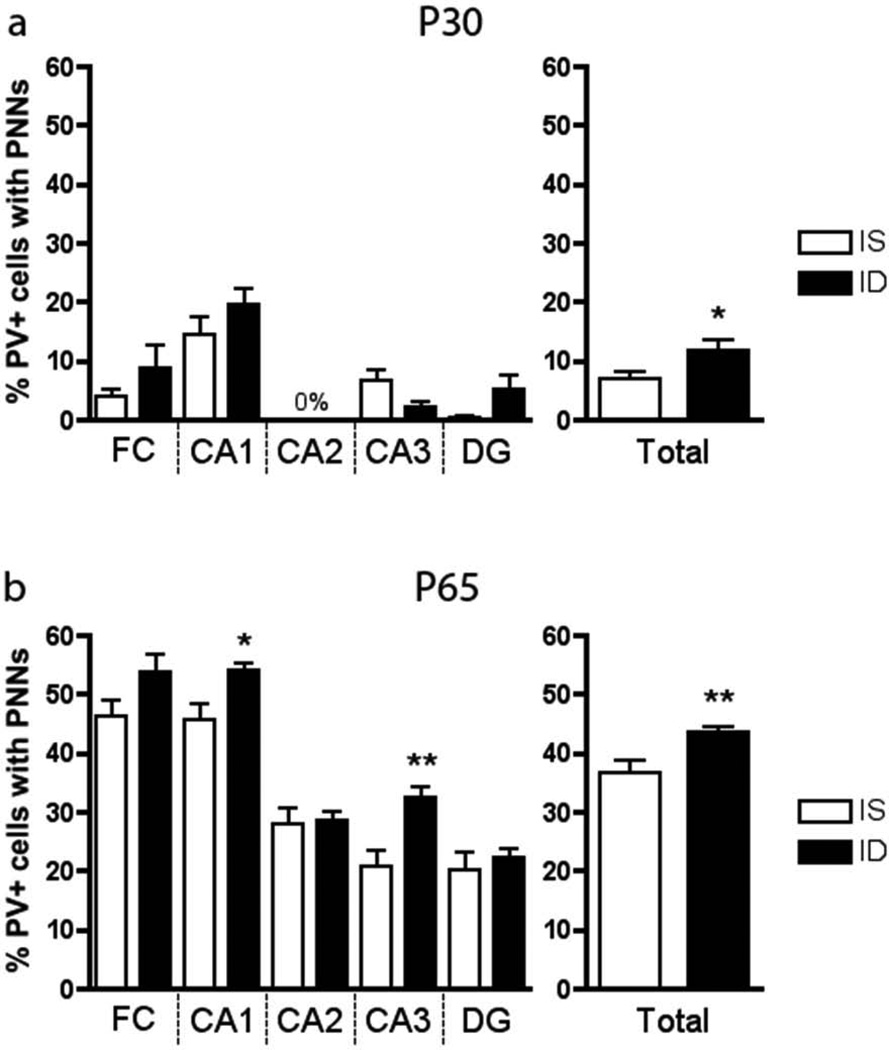
PNNs were not observed until P30 in the IS hippocampus (Figure 5, n=7–8). The total hippocampal percentage of PV+ interneurons surrounded by PNNs increased from 7% at P30 to 37% at P65 in IS rats (Figure 5). At both P30 and P65, the ID group had a higher percentage of total PV+ interneurons surrounded by PNNs compared to IS controls (Figure 5, n=7–8). Regionally, at P65 this difference was driven by increases in the CA1 and CA3 areas of the FID hippocampus (Figure 5b).
FIGURE 5.
The percentage (%) of parvalbumin-positive cells surrounded by perineuronal nets by region of the hippocampus at a. P30 and b. P65 (n=7–8). * = p <0.05; ** = p< 0.01, comparing IS to ID at same age. a<b with p< 0.001. Values are means ± SEM.
Discussion
A major conundrum in the field of neurodevelopment following early IDA is determining the mechanisms of the long-term deficits in learning and memory that persist in spite of early and prompt iron treatment. A recent study in formerly iron deficient anemic monkeys indicates that these long-term functional changes relate to developmental alterations in proteins that are important for establishing and maintaining normal neuronal connectivity [33].
Consistent with this observation, several processes of hippocampal development that relate to long-term functional connectivity undergo rapid maturation between P15 and P30 in rats and mice [7–12]. Furthermore, the hippocampus is highly vulnerable to insults during this period of development, suggesting that this early life period is a potential sensitive window of development. Thus, we sought to further pursue this question of developmentally-dependent plasticity by establishing whether the cellular biomarkers of both the opening and closing of critical periods are present in the hippocampus during this time period and whether they are affected by early-life IDA
The current study demonstrates that the appearance of critical period cellular markers in the IS rat hippocampus coincides with the period of rapid postnatal dendritogenesis. At P15, less PV mRNA was measurable compared to later time points, and virtually no PV+ interneurons were present in the hippocampal cell fields. Functionally, this may reflect a predominantly excitatory profile and is consistent with the observation of greater long-term potentiation (LTP) at this age compared to adulthood [8]. At P30, PV mRNA expression, PV protein levels, and PV+ cell counts in IS animals have peaked and reached their respective adult-level plateaus. These findings corroborate a handful of previous reports on the postnatal maturation of hippocampal interneurons. In those studies, PV immunoreactivity begins between P4 and P7, with PV mRNA expression and the number of GABAergic interneurons surrounding cell bodies of the pyramidal layer increasing during the first three postnatal weeks [26–28,34]. Functionally, these changes may reflect increasing inhibitory input and are consistent with the observed reduction in LTP to adult levels that occurs between P15 and P30 [8]. Our findings suggest that the process of hippocampal PV+ interneuron maturation is more or less complete by P30.
PNNs, which can serve as a biomarker of the closing of the critical period, developed along a later timeline than the appearance of PV+ cells in the IS hippocampus, with the percentage of PV+ interneurons being surrounded increasing between P30 and P65. The entire process is consistent with the maturation of the hippocampus from a state of lower efficiency, high plasticity, and high amenability to treatment, to a state of higher efficiency, lower plasticity, and less amenability to treatment [22]. Extrapolating from other brain areas (e.g. visual cortex), fewer PNNs are characteristic of an immature state of development, where there is greater plasticity, and a greater amenability to intervention [16]. Ultimately, as the percentage of PV+ cells surrounded by PNNs increases, a developing system becomes less malleable and the critical period draws to a close. Interestingly, the P65 IS hippocampus retains a relatively low percentage (37%) of PV+ interneurons surrounded by PNNs, consistent with the fact that the hippocampus exhibits ongoing plasticity in adulthood.
There was a marked difference in the staining between the hippocampus and the cortex in the adult IS brain. While low-magnification (4×) images of the whole-brain (Figure 1a–b) demonstrate that both PV+ cells and PNNs are expressed mostly in the cortex and hippocampus, PV+ cells and PNNs were much more numerous in the cortex than in the hippocampus, suggestive of a more developed and mature system. Furthermore, the intensity of PNN staining (regardless of iron status) was less in the hippocampus than in the overlying cortex, such that although the nets were clearly visible at 4× magnification in the cortex, a magnification of 20× is required to visualize hippocampal PNNs. Thus, in addition to a relatively low fraction of hippocampal PV+ cells being surrounded by PNNs, the overall density of those nets may not be complete. Functionally, this may be consistent with the hippocampus demonstrating increased plasticity across the lifespan as compared to cortex.
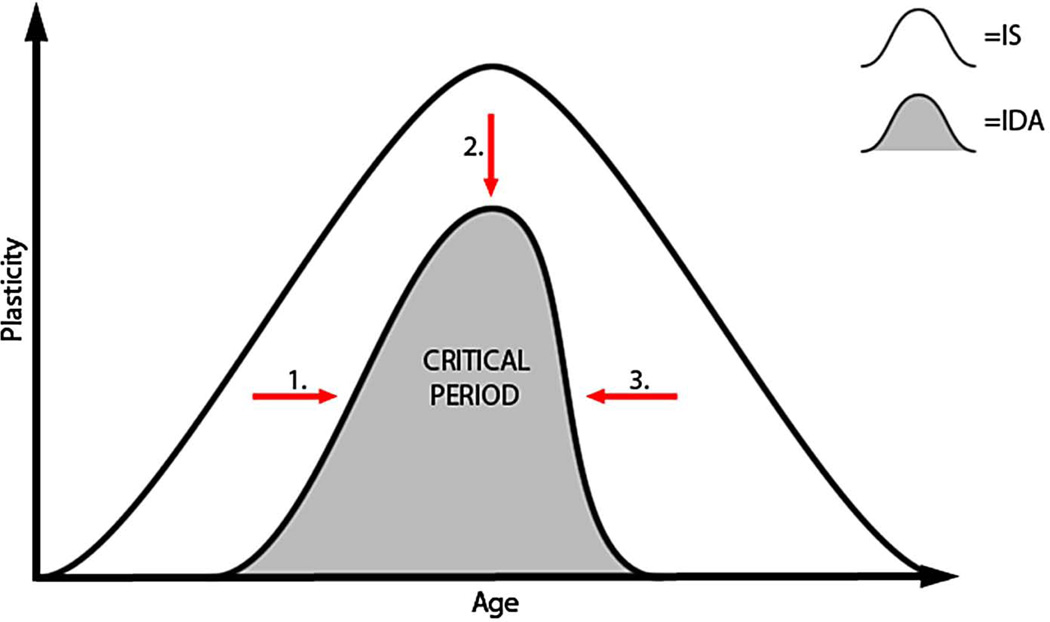
Early-life IDA reduced PV mRNA and protein expression throughout development and into adulthood in spite of iron treatment. Since there were no observed differences in the number of PV+ interneurons, we speculate based on the expression data that there is less parvalbumin per PV+ interneuron in both the currently ID hippocampus at P30 and in the FID hippocampus at P65. Reduced PV expression in the IDA group at P30 may be serving as a biomarker of a developmentally immature hippocampus as it emerges from its rapid period of differentiation. The maturational delay in PV expression without the presence of PNNs may be interpreted as a shift of the critical period to a later time point along the developmental axis [22] (Figure 6; arrow 1). We have previously demonstrated delays in maturation of LTP and hippocampally-dependent behavior at P30–35 in this model [8,24]. Not only is the appearance of this biomarker delayed (Figure 6; arrow 1), it does not reach the equivalent expression of the IS system during development (Figure 6; arrow 2). A lower level of this biomarker in the visual system has been interpreted as indexing lower overall plasticity potential [22, and Hensch, personal communication].
FIGURE 6.
Working model for the normal ontogeny of the hippocampal critical period and the effect of IDA on the upslope (arrow 1), peak (arrow 2), and downslope (arrow 3) of this critical period.
The greater percentage of PV+ cells surrounded by PNNs in the FID group compared to IS in adulthood may serve as a biomarker of earlier closure of the critical period (Figure 6; arrow 3); a finding that in other neural systems indexes a more rigid and less plastic system [16]. Electrophysiology and behavioral studies in FID rats demonstrate significant impairments in LTP and learning and memory; findings that are consistent with lower experience dependent plasticity [8,14,15,35]. These electrophysiological and behavioral findings are accompanied by short- and long-term reductions in brain-derived neurotrophic factor (BDNF) III and IV [10,36]. This is intriguing since PV expression in GABAergic interneurons is driven by the presence of BDNF [37,38]. ID-induced reductions in BDNF expression may play a role in the reduced amount of PV expression.
Whether altered hippocampal parvalbumin expression during development is directly detrimental to the function of the structure in adulthood, or is acting as a biomarker of developmental maturation, is unknown. Assessments of one potential functional marker, KCNC1 [21,39], shows that IDA alters its developmental ontogeny. While a reduction in parvalbumin expression can be accompanied by lower KCNC1 levels in other model systems [21,39], IDA has effects on multiple interacting neuronal cellular signaling systems including glutamatergic, GABA-ergic, dopaminergic, BDNF and mTOR pathways [Tran et al, under review, [36,40]. The 66% higher KCNC1 mRNA levels at P30 suggest complex regulation and point to IDA affecting post-synaptic processing of inhibitory information. These KCNC1 findings are consistent with IDA inducing higher intracellular GABA levels [41] and increased expression of the GABA synthesizing enzyme, GAD 65 [Tran et al, under review] in the hippocampus. Overall, the data suggest intact and responsive presynaptic, but potentially dysfunctional post-synaptic machinery; a hypothesis supported by lower GABAA and GABAB receptor protein levels [Tran et al, under review]. Clearly, further functional studies including inhibitory electrophysiologic experiments are indicated.
The findings of our study, and the concept of an appropriate level of excitatory/inhibitory balance during development may have clinical relevance to learning and memory deficits associated with early-life IDA in humans [42]. There is a large literature describing the negative consequences of early-life iron deficiency on both infant and adult learning and memory in humans [3–5,43]. Data from a non-human primate model suggests that abnormally patterned neural connectivity from early in life likely contributes to these abnormalities [33]. Our study may provide some of the specifically hippocampal cellular mechanisms for these observations.
The dietary rat model of iron deficiency anemia used in this study was selected due to its resemblance to the human condition and treatment of early-life IDA. The persistent changes in the adult animal seen in this study, and in others using the same model, appear to be due to a failure to replete iron in a timely manner, suggesting a sensitive window of development. However, this dietary model is limited in that it induces whole-body iron deficiency and takes a long time period before hippocampal iron repletion occurs. Future experiments using a hippocampal-specific iron deficient model will allow for prompt iron repletion and a closer dissection of the critical period for iron’s role in hippocampal development.
Acknowledgements
We would like to extend a special thank you to Dr. Teresa Nick for her advice and manuscript review, to Dr. Takao Hensch for his input and personal communication, and also to Thomas Bastian for his troubleshooting help on the Western blotting experiments.
Financial Support: NICHD R01 HD29421-17 to M.K.G., and NIH T32 NS048944 to T.J.E.
Abbreviations
- PV(+)
parvalbumin (positive)
- PNN(s)
perineuronal net(s)
- IDA
iron deficiency anemia
- IS
iron sufficient
- ID
iron deficient
- FID
formerly iron deficient
- E
embryonic day
- G
gestational day
- P
postnatal day
- FC
hippocampal region, fasciola cinerea
- CA1
hippocampal region, cornu ammonis 1
- CA2
hippocampal region, cornu ammonis 2
- CA3
hippocampal region, cornu ammonis 3
- DG
hippocampal region, dentate gyrus
- GABA
γ-aminobutyric acid
- BDNF
brain-derived neurotrophic factor
- LTP
long-term potentiation
Footnotes
The authors have no financial interest or potential conflict of interest to disclose.
References
- 1.Geneva: World Health Organization (WHO); 1998. Global database on child growth and malnutrition. [Google Scholar]
- 2.Fuglestad A, Rao R, Georgieff M. Handbook in developmental cognitive neuroscience. Cambridge MA: MIT Press; 2006. The role of nutrition in cognitive development. [Google Scholar]
- 3.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 6.deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (cytox) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal ca1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981;7:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- 8.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area ca1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 9.Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog Neurobiol. 2004;73:397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr. 2008;138:2495–2501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res. 1990;55:35–42. doi: 10.1016/0165-3806(90)90103-6. [DOI] [PubMed] [Google Scholar]
- 13.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 15.Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22:1691–1702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-d28k, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- 18.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 19.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: A different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 20.Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: Freeing the synapse. Neuron. 2004;44:905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, Reichenbach A, Bruckner G. Cortical neurons immunoreactive for the potassium channel kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- 22.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 23.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 24.Gewirtz JC, Hamilton KL, Babu MA, Wobken JD, Georgieff MK. Effects of gestational iron deficiency on fear conditioning in juvenile and adult rats. Brain Res. 2008;1237:195–203. doi: 10.1016/j.brainres.2008.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann I, Nitsch R, Frotscher M. Area-specific morphological and neurochemical maturation of non-pyramidal neurons in the rat hippocampus as revealed by parvalbumin immunocytochemistry. Anat Embryol (Berl) 1991;184:403–409. doi: 10.1007/BF00957901. [DOI] [PubMed] [Google Scholar]
- 27.Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal gabaergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 28.Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J Comp Neurol. 1990;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- 29.Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010;151:4055–4065. doi: 10.1210/en.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- 31.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of ca1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–248. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen mouse brain atlas [internet] Seattle: Allen institute for brain science; 2009. Available from: Http://mouse.Brain-map.Org. [Google Scholar]
- 33.Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using itraq in a primate model of iron deficiency anemia. Dev Neurosci. 2012;34:354–365. doi: 10.1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swann JW, Brady RJ, Martin DL. Postnatal development of gaba-mediated synaptic inhibition in rat hippocampus. Neuroscience. 1989;28:551–561. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 36.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–498. doi: 10.1203/PDR.0b013e31819d90a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berghuis P, Dobszay MB, Sousa KM, Schulte G, Mager PP, Hartig W, Gorcs TJ, Zilberter Y, Ernfors P, Harkany T. Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking gabaergic interneurons. Eur J Neurosci. 2004;20:1290–1306. doi: 10.1111/j.1460-9568.2004.03561.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. Bdnf regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 39.Du J, Zhang L, Weiser M, Rudy B, McBain CJ. Developmental expression and functional characterization of the potassium-channel subunit kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci. 1996;16:506–518. doi: 10.1523/JNEUROSCI.16-02-00506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fretham SJ, Carlson ES, Georgieff MK. Neuronal-specific iron deficiency dysregulates mammalian target of rapamycin signaling during hippocampal development in nonanemic genetic mouse models. J Nutr. 2013;143:260–266. doi: 10.3945/jn.112.168617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 42.Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: Gabaergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- 43.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]