Tumor necrosis factor alpha (TNF-α) inhibitors can increase the risk of infections. This systematic literature review describes the epidemiology, microbiology, and types of infections reported in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with TNF-α inhibitors.
Keywords: infliximab, adalimumab, etanercept, juvenile idiopathic arthritis, inflammatory bowel disease
Abstract
Tumor necrosis factor alpha (TNF-α) inhibitors are increasingly administered to children and adolescents with juvenile idiopathic arthritis (JIA) and pediatric inflammatory bowel disease (pIBD). Adult studies indicate that TNF-α inhibitors lead to an increased risk of serious infections compared to other disease-modifying antirheumatic drugs. We report herein a systematic literature review detailing the epidemiology and types of infections reported in children with JIA and pIBD treated with TNF-α inhibitors. The most frequently reported infections were mild and characterized as viral in etiology. Severe bacterial and fungal infections also occurred, but were less common and possibly associated with intrinsic risk factors and concurrent immunosuppressive therapy. Few pediatric patients developed Mycobacterium tuberculosis, likely due to effective screening. There were 8 infectious fatalities in children treated with TNF-α inhibitors. Overall, although rare, serious infections occur in immunocompromised children and adolescents with JIA and pIBD receiving TNF-α inhibitors.
Tumor necrosis factor alpha (TNF-α) inhibitors are increasingly being administered to children and adolescents with juvenile idiopathic arthritis (JIA) or pediatric inflammatory bowel disease (pIBD). Adult studies have, for the most part, shown that TNF-α inhibitors lead to an increased risk of opportunistic infections, serious infections, and hospitalizations, when compared to other disease-modifying antirheumatic drugs (DMARDs) [1–4]. Despite the extensive use of biologics in pediatrics, it remains unclear whether there is a similarly increased risk of infections and what types of infections occur. To date, studies in children with JIA or pIBD treated with TNF-α antagonists have consisted of small numbers of subjects, with outcomes mostly focused on drug efficacy and serious adverse events. Furthermore, strategies to prevent infections and safely monitor pediatric patients treated with TNF-α inhibitors are largely extrapolated from the adult literature. This systematic literature review describes the 3 main TNF-α inhibitors used in JIA or pIBD, summarizes the microbiology and types of infections pediatric patients treated with TNF-α inhibitors may be susceptible to, and provides an overview of strategies that may be useful for the prevention of infectious complications in this population. Characterizing the infections that occur in JIA and pIBD patients treated with TNF-α inhibitors may help prevent unnecessary treatment interruptions and unwarranted hospitalizations.
ROLE OF TNF-α IN PATHOGENESIS OF JIA AND pIBD
TNF-α is a cytokine with an important role in inflammation and immune function. It promotes an inflammatory cascade, critical in the host response to infections and local injury. However, at high concentrations, TNF-α may lead to excess inflammation and organ damage as seen in JIA and pIBD. JIA patients can have increased TNF-α levels and other proinflammatory cytokines in the peripheral blood and synovial fluid, which may lead to tissue damage, cartilage loss, and bone destruction [5, 6]. In pIBD patients, unregulated TNF-α production can exacerbate disease through its proinflammatory effects and induction of apoptosis, compromising the gastrointestinal mucosa, specifically the barrier function of the endothelial and epithelial layers [7]. TNF-α inhibitors are thought to improve the patient's clinical course by impairing the ability of TNF-α to bind to its receptors, inactivating the sepsis cascade cytokines, inhibiting production of inflammatory cytokines, and preventing further inflammation and damage to tissue or joints. However, TNF-α inhibition can potentially make the patient more susceptible to infections due to this downregulation of the immune system [8].
INFLIXIMAB, ETANERCEPT, AND ADALIMUMAB
The 3 TNF-α inhibitors most commonly evaluated in pediatrics include 2 monoclonal antibodies, infliximab (Remicade) and adalimumab (Humira) and a soluble TNF receptor, etanercept (Enbrel). All 3 can bind to soluble and transmembrane TNF, thus attenuating TNF-α's biological effects. However, they differ in structure, half-life, dosage, and indications (Table 1). Infliximab is a chimeric mouse–human monoclonal immunoglobulin G1 (IgG1) antibody. It was first approved by the Food and Drug Administration (FDA) in 1998 for adults with Crohn's disease (CD). In 2006 infliximab was approved for pediatric CD, and by 2011 it was approved for pediatric ulcerative colitis (UC). It was approved for rheumatoid arthritis (RA) in 1999, but has no FDA-approved indications in JIA at this time, although often used off-label in this population.
Table 1.
TNF-α Inhibitors Used in Juvenile Idiopathic Arthritis and Pediatric Inflammatory Bowel Disease
| Biological Agent (Route Administered) | Composition | Half-life | Dosage and Timing | FDA-Approved JIA and pIBD Indications | EMEA-Approved JIA and pIBD Indications |
|---|---|---|---|---|---|
Infliximab (Remicade) (IV)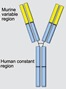
|
Chimeric IgG1 monoclonal antibody | Approximately 8–9.5 d | Initial 3 mg/kg, then 3 mg/kg at 2 and 6 wk Maintenance: 3–6 mg/kg every 8 wk |
Not approved in JIA | Not approved in JIA |
| CD: Initial 5 mg/kg, then 5 mg/kg at 2 and 6 wk Maintenance: 5 mg/kg every 8 wk. Can increase to 10 mg/kga |
Age ≥6 y with moderate to severe CD and UC | Age 6–17 y with CD and UCb | |||
Etanercept (Enbrel) (SQ)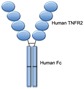
|
Human; fusion protein | Approximately 4.25 ± 1.25 d | 0.8 mg/kg weekly Or 0.4 mg/kg twice weekly |
Age ≥2 y with moderate to severe polyarticular JIA | Age ≥2 y with polyarticular JIAc |
| Age 12–17 y with psoriatic arthritisc | |||||
| Age 12–17 y with enthesitis-related arthritisc | |||||
| No role in IBD | No role in IBD | ||||
Adalimumab (Humira) (SQ)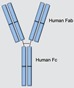
|
100% human | 10–20 d | JIA:15–29 kg: 20 mg every 2 wk ≥30 kg: 40 mg every 2 wk |
Age ≥4 y with moderate to severe polyarticular JIA | Age ≥4 y with polyarticular JIA |
| CD: Induction: <40 kg: 80 mg day 1, 40 mg day 14 >40 kg: 160 mg day 1, 80 mg day 14 Maintenance: <40 kg: 20 mg every 2 wk >40 kg: 40 mg every 2 wk |
Not approved in pIBD yet | Age 6–17 y with severe active CDb |
Abbreviations: CD, Crohn's disease; EMEA, European Medicines Agency; FDA, Food and Drug Administration; IBD, inflammatory bowel disease; IgG1, immunoglobulin G1; IV, intravenous; JIA, juvenile idiopathic arthritis; pIBD, pediatric inflammatory bowel disease; SQ, subcutaneous; UC, ulcerative colitis.
a If response is incomplete.
b In patients who have not responded to other therapy.
c In patients who have not responded to or cannot tolerate methotrexate.
Adalimumab, a fully humanized IgG1 monoclonal antibody, was initially approved for RA in 1998, with indications for the adult CD population by 2007. In 2008 it was approved for JIA patients age 4 and older. Recent studies have shown that adalimumab is also effective in inducing and maintaining remission in pediatric CD. In 2012 adalimumab was approved for pediatric CD in Europe, but it is not FDA approved for pIBD at this time.
Etanercept is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the TNF receptor linked to the Fc portion of the human IgG1. It was first approved for the treatment of RA in 1998 and for treatment of JIA 1 year later. Although etanercept has several approved indications in adults with inflammatory arthritis, it is ineffective for the treatment of IBD.
It is unknown how many unique pediatric patients have thus far been exposed to these 3 biologics. According to the FDA, approximately 9200 children and adolescents aged 0–17 years received etanercept through 2007 (source: Amgen), and 2636 patients aged 0–16 years received adalimumab for the 2-year period of 2006–2007 [9]. Approximately 19 000 patients aged 0–18 have been exposed to infliximab between 1998 and 2010 worldwide (source: Centocor). Despite the use of biologics by thousands of children and its important role in the management of patients with JIA and pIBD, the concerns of infectious risk with TNF-α inhibitor therapy continue to be largely extrapolated from adult studies.
METHODS
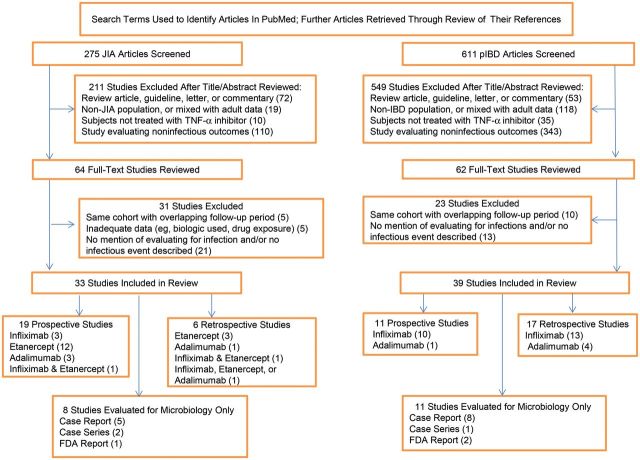
We performed a systematic literature review to find original studies that evaluated TNF-α inhibitor therapy (infliximab, etanercept, or adalimumab) in pediatric JIA or pIBD patients (Figure 1). We reviewed all references found through PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using a combination of the search terms JIA, CD, UC, IBD, infliximab, etanercept, and adalimumab. The search was limited to studies including human subjects, subjects aged 0–18 years, all clinical trials, case reports, and the English language that were published between 2000 and 2012. We then checked the references of each study to find any missed studies. Studies that did not mention collecting data on the occurrence of infection or that did not describe any infection in the manuscript were excluded. Case reports, case series, and FDA reports were included in the microbiology tables only in order to understand the breadth of serious infections as they are rare events that may be missed in the small pediatric studies available. We defined serious infections as life threatening, requiring hospitalization, requiring intravenous antimicrobial therapy, and/or characterized in individual studies as a serious infection or serious adverse event. Mild infections were defined as infections that did not require hospitalization and/or intravenous antimicrobial therapy. If infection data were unclear, an email was sent to the corresponding author.
Figure 1.
Flow diagram of literature search and selection process. Abbreviations: FDA, Food and Drug Administration; JIA, juvenile idiopathic arthritis; pIBD, pediatric inflammatory bowel disease; TNF-α, tumor necrosis factor alpha.
JUVENILE IDIOPATHIC ARTHRITIS
JIA is the most common chronic childhood rheumatic disease, affecting nearly 300 000 children in the United States. It is a heterogeneous autoimmune disease with 7 categories, which are based on the number of joints affected during the first 6 months of disease and extra-articular involvement. JIA is a diagnosis of exclusion, characterized by arthritis that persists for at least 6 weeks and occurs before age 16. The categories of oligoarticular, polyarticular rheumatoid factor negative, and polyarticular rheumatoid factor positive JIA comprise 65%–80% of all JIA patients [10]. The remaining 4 categories include systemic-onset JIA, enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis.
JIA is a multifactorial disease with many patients likely having a genetic predisposition, but with disease often developing after various environmental triggers that are not yet fully understood [11]. Although still labeled as a JIA subcategory at this time, systemic-onset JIA likely results from a distinct pathogenesis in comparison to all other subtypes [12]. Not surprisingly, treatment is also different for systemic-onset JIA, and TNF-α inhibitors are less efficacious in this cohort [13]. JIA patients of the remaining subtypes are thought to have an autoreactive immune response triggered by the adaptive immune system toward a self-antigen. This includes an uncontrolled response by Th1 and Th17 cells, leading to proliferation of T cells and proinflammatory cytokines with subsequent joint destruction. Recent studies have shown that biologics may lead to clinical response in rheumatologic patients by restoring the balance between specific T-cell populations [14–16]. However, these immune effects may in turn lead to an increased risk of infections from mycobacteria, intracellular bacteria, and fungi [17, 18].
EPIDEMIOLOGY OF INFECTIONS IN JIA PATIENTS TREATED WITH TNF-α INHIBITORS
Frequency and Sites of Mild and Severe Infections
Five case reports, 2 case series, 1 FDA report, 19 prospective studies, and 6 retrospective studies were reviewed (Table 2) [18–50]. Mild infections occurred more frequently and were observed in 8% (2/25) to 97% (31/32) of JIA patients treated with TNF-α inhibitors [31, 36]. Upper respiratory tract infections were most often reported (Table 3). Severe infections occurred in 0% (0/25) to 9% (3/32) of pediatric patients [31, 41]. The most commonly reported sites of severe infections were the respiratory tract and musculoskeletal system (Table 4). The incidence of severe infections in adult RA patients treated with biologics is similar at 3.8%–6.2%, with the organ systems most commonly affected being the respiratory tract and skin [2, 51–54].
Table 2.
Studies That Reported Infections in Juvenile Idiopathic Arthritis Patients Treated With Tumor Necrosis Factor–α Inhibitors
| Study, First Author | TNF-α Inhibitor | No. of Subjects | Age, y, Mean | Disease Duration, y, Mean | DMARDa, CS, or Both | Patient-years of Drug Exposure | Total No. of Infections (per Patient-year) | Total No. of Serious Infections (per Patient-year) |
|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||
| Lovell [34] | ET | 69 | 10.5 | 5.9 | CS | 0.54 | NS | 1 (1.85) |
| Lovell [32] | ET | 58 | 10.5 | 5.9 | Both | 53 | 78 (1.5) | 8 (0.15) |
| Lovell [45] | ET | 26 | 10.5 | 5.9 | Both | 126 | NS | 10 (0.03) |
| Rupertob [23] | INX | 117 | 11 | 4.2 | DMARD | 99 | 78 (0.78) | 7 (0.07) |
| Tzaribachev [41] | ET | 25 | 12 | 5 | Both | 39.6 | 2 (0.05) | 0 |
| Gerloni [25] | INX | 68 | 21.7 | 13.7 | Both | 126 | 7 (0.06) | 1 (0.008) |
| ET | 95 | 13.7 | 8.4 | 190 | 34 (0.18) | 2 (0.011) | ||
| Lovell [33] | AD | 86 | 11.1 | 3.6 | CS | 93 | 97 (0.66) | 4 (0.027) |
| AD + MTX | 85 | 11.4 | 4 | CS | 109 | 112 (0.65) | 3 (0.017) | |
| Giannini [26] | ET | 103 | 10.8 | 4.8 | Both | 224 | NS | 4 (0.018) |
| MTX | 197 | 9.0 | 1.7 | 388 | NS | 5 (0.013) | ||
| ET + MTX | 294 | 10.1 | 3.3 | 635 | NS | 13 (0.02) | ||
| Horneff [28] | ET | 100 | 13.8 | 5.5 | Both | NS | 34 | 25 |
| ET + MTX | 504 | 12.5 | 4.9 | NS | 50 | 1 | ||
| Horneff [29] | ET | 20 | 12.9 | 4.1 | Both | 4.6 | 13 (2.82) | 0 |
| Prince [30] | ET | 146 | 11c | 4.1c | Both | 312 | 21 (0.07) | 4 (0.013) |
| Ruperto [46] | INX | 78 | NS | NS | DMARD | 171 | 57 (0.33) | 2 (0.012) |
| Zuber [47] | ET | 188 | 19 | 4.3 | Both | 393 | 959 (2.44) | 16 (0.04) |
| Trachana [35] | AD | 26 | 12.6 | 7.1 | Both | 72 | 10 (0.14) | 3 (0.04) |
| Otten [27] | ET | 262 | 12.4c | 3.0 | Both | 323 | 99 (0.31) | 7 (0.03) |
| Tynjalab [24] | INX | 19 | 10.5 | 0.13 | DMARD | 19.7 | 36 (1.82) | 0 |
| COMBOd | 20 | 8.3 | 0.19 | 20 | 35 (1.75) | 0 | ||
| MTX | 20 | 10.1 | 0.15 | 20 | 48 (2.4) | 3 (0.15) | ||
| Wallaceb,e [48] | ET + MTX | 42 | 9.9 | 0.41 | Both | 24.8 | 16 (0.65) | NS |
| MTX | 43 | 11.1 | 0.43 | 20.6 | 18 (0.87) | NS | ||
| Mori [31] | ET | 19 | 13.7 | 6.1 | Both | 118 | 99 (0.84) | 3 (0.03) |
| Imagawab [49] | AD | 25 | 13 | 4.7 | Both | 29 | 21 (0.72) | 3 (0.1) |
| Retrospective studies | ||||||||
| Takei [38] | ET | 8 | 8.4 | 5.3 | DMARD | 10.3 | 3 (0.29) | 0 |
| Tynjala [39] | AD | 20 | 13.4 | 10 | DMARD | 31 | 30 (0.97) | 0 |
| Southwoodb [40] | ET | 483 | 12 | 5 | DMARD | 941 | 5 (0.005) | 5 (0.005) |
| Lamot [37] | INX, ET | 41 | 6.9 | 4.1 | DMARD | 106 | NS | 2 (0.02) |
| Beukelman [50] | ET, INX, or AD | 1315 | NS | 9.7 | NS | 1580 | NS | 55 (0.04) |
| Bracaglia [36] | ET | 25 | 3.3c | 1.2c | Both | NS | NS | 2 |
Abbreviations: AD, adalimumab; CS, corticosteroid; DMARD, disease-modifying antirheumatic drug; ET, etanercept; INX, infliximab; MTX, methotrexate; NS, not specified; TNF-α, tumor necrosis factor alpha.
a DMARD may include 5-aminosalicylic acid, 6-mercaptopurine, cyclosporine, azathioprine, or methotrexate.
b Excluded subjects previously exposed to any TNF-α inhibitor.
c Median.
d Patients on combination of DMARDs.
e Only study to include 2 or fewer JIA categories. All other studies included 3 or more JIA categories.
Table 3.
Mild Infections in Juvenile Idiopathic Arthritis Patients Treated With Tumor Necrosis Factor–α Inhibitors
| Infection Type (No. of Infections) | Infliximab (n = 296)a | Etanercept (n = 2465) | Adalimumab (n = 242) |
|---|---|---|---|
| Respiratory tract infections (1255) | |||
| URTIb (1251) | 73 | 1016c | 162 |
| LRTI (4) | 0 | 4 | 0 |
| Skin and soft tissue infections (43) | 0 | 39 | 4 |
| Gastrointestinal infections (59) | 3 | 56d | 0 |
| Genitourinary infections (28) | 0 | 28 | 0 |
| Viral infections (197) | |||
| Primary varicella (13) | 0 | 12 | 1 |
| Zoster (15) | 1 | 12 | 2 |
| HSV (49) | 1 | 47 | 1 |
| EBV (4) | 0 | 4 | 0 |
| Nonspecific (116) | 0 | 0 | 116 |
| Fungal infectionse (8) | 2 | 5 | 1 |
| Mycobacterial infections (1) | |||
| Pulmonary tuberculosis | 1 | 0 | 0 |
| Fever of unknown source (28) | 1 | 27 | 0 |
Abbreviations: EBV, Epstein-Barr virus; HSV, herpes simplex virus; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
a Total number of subjects included in the studies where microbiology and/or infection type was available.
b Includes pharyngitis, otitis, and sinusitis.
c Seven cases of influenza.
d One case of hepatitis A.
e Includes oropharyngeal and vulvovaginal candidiasis.
Table 4.
Severe Infections in Juvenile Idiopathic Arthritis Patients Treated With Tumor Necrosis Factor–α Inhibitors
| Infection Type (No. of Infections) | Infliximab (n = 296) | Etanercept (n = 2465) | Adalimumab (n = 242) |
|---|---|---|---|
| Respiratory tract infections (37) | |||
| URTI (14) | 0 | 12 | 2 |
| LRTI (23) | 5a | 15 | 3 |
| Sepsis/bacteremia (8) | 0 | 7b | 1c |
| CNS infections (4) | |||
| Meningitis (4) | 0 | 4d | 0 |
| Gastrointestinal infections (11) | |||
| Gastroenteritis/colitis (11) | 0 | 11e | 0 |
| Musculoskeletal infections (25) | |||
| Necrotizing fasciitis (2) | 0 | 2f | 0 |
| Abscess/cellulitis (15) | 1 | 13 | 2h |
| Pyomyositis (2) | 1 | 1 | 0 |
| Septic arthritis (3) | 0 | 3i | 0 |
| Osteomyelitis (2) | 1i | 1 | 0 |
| Osteomyelitis and septic arthritis (1) | 0 | 1i | 0 |
| Genitourinary infections (11) | 1 | 10 | 0 |
| Viral infections (17) | |||
| Primary varicella (2) | 0 | 2 | 0 |
| Zoster (9) | 2 | 5 | 2 |
| HSV (2) | 1 | 0 | 1 |
| CMV (2) | 1 | 1 | 0 |
| EBV (1) | 0 | 1 | 0 |
| HBV (1) | 0 | 0 | 1j |
| Fungal infections (4) | |||
| Histoplasmosis (4) | 2 | 2 | 0 |
| Mycobacterial infections (4) | |||
| Pulmonary tuberculosis (1) | 1 | 0 | 0 |
| Extrapulmonary tuberculosis (3) | 1 | 2 | 0 |
| Infectious fatalities (4) | 1 | 2 | 1 |
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; EBV, Epstein-Barr virus; HBV, hepatitis B virus; HSV, herpes simplex virus; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
a One case of Streptococcus pneumoniae.
b Two cases of group A Streptococcus (GAS) purpura fulminans, both fatal.
c Fatal infection.
d One case each of varicella-zoster virus (VZV) and EBV meningoencephalitis.
e One case each of Escherichia coli and Clostridium difficile colitis.
f One case of Staphylococcus aureus infection; 1 case secondary to VZV infection.
g One case of Enterococcus faecalis urachal cyst infection.
h Both cases due to S. aureus infection in same patient.
i One case of GAS infection.
j Primary HBV infection. Patient was negative for HBV infection prior to starting adalimumab.
Overall, the rates of mild and severe infections observed in JIA patients treated with biologics appear significant, but wide-ranging, especially for mild infections. The incidence of infections likely varied in the studies reviewed due to the inclusion of a heterogeneous JIA population with varying subtypes of JIA, and disease duration, small numbers of patients enrolled, use of concurrent DMARDs and/or corticosteroids, and limitations of study design that focused more on efficacy (Table 2). Importantly, it is unknown whether JIA itself further contributes to an increased risk of infection. A recent study found that there may be an increased rate of hospitalization with bacterial infections in JIA patients compared to healthy children [50]. These authors also found no increased rate of hospitalized bacterial infections among JIA patients treated with TNF-α inhibitors.
Microbiology
The microbiology of infections was unavailable in most of the pediatric studies reviewed. Bacterial pathogens most often identified were Streptococcus pyogenes and Staphylococcus aureus. Herpes simplex virus and varicella zoster virus (VZV) were the most frequently identified viral infections in both mild and severe illnesses. Three children who developed severe VZV disease (aseptic meningitis, disseminated varicella, and necrotizing fasciitis), were nonimmune prior to starting anti-TNF therapy [32, 36].
Fungal and mycobacterial infections were also reported. Progressive disseminated histoplasmosis was described in 4 patients and all lived in an endemic region [42]. Only 1 patient was on concurrent corticosteroids. Five cases of M. tuberculosis were reported [18, 23, 43]. These opportunistic infections have similarly been reported in adults [3, 42, 55]. Other opportunistic infections described in RA patients treated with TNF-α inhibitors include listeriosis, aspergillosis, and Pneumocystis jirovecii pneumonia, which have not yet been described in JIA patients [55, 56]. Differences in microbiology observed in RA compared to JIA patients is likely multifactorial, including longer disease duration, older age, higher cumulative exposure to immunosuppressive therapy, and the underlying disease [57].
Pediatric IBD
There are approximately 150 000 pIBD patients aged 0–17 years in the United States [58]. UC and CD are inflammatory bowel diseases characterized by inflammation of the gastrointestinal tract. UC involves recurring inflammation of the mucosal layer of the colon, almost invariably involving the rectum, but may affect any portion of the colon in a continuous fashion. CD is characterized by transmural inflammation of any component of the gastrointestinal tract from the oral cavity to the anus. Although UC and CD have distinct pathologic and clinical characteristics, both emerge from genetic and environmental influences that likely stem from an abnormality in mucosal immune function [59].
In comparison with adult-onset disease, pediatric UC patients tend to have more extensive intestinal involvement and a more severe disease course, and are more likely to be corticosteroid dependent [60]. This may also be true of pediatric CD, but has not been consistently shown [60, 61]. In pIBD, both the disease and corticosteroid therapy may result in significant long-term adverse effects such as poor weight gain and linear growth. Thus, treatment with TNF-α inhibitors has significantly altered the outcomes for pIBD patients by allowing for steroid-sparing regimens and improved linear growth [62].
Infliximab, adalimumab, and certolizumab have been shown to be efficacious in treating IBD. Because no studies evaluating certolizumab therapy in pIBD could be found at the time of this review, it is not included. Although recent studies have shown infliximab and adalimumab to be effective in inducing and maintaining remission in pIBD, only infliximab is currently FDA approved for pIBD (CD and UC) [63]. Nevertheless, adalimumab and certolizumab have been used off-label to treat pIBD.
EPIDEMIOLOGY OF INFECTIONS IN pIBD PATIENTS TREATED WITH TNF-α INHIBITORS
Frequency and Sites of Mild and Severe Infections
Eight case reports, 1 case series, 2 FDA reports, 11 prospective studies, and 17 retrospective studies describing the incidence of infections in pIBD patients treated with infliximab and/or adalimumab were reviewed (Table 5) [63–101]. Most infectious adverse events reported in pIBD patients were mild infections, specifically upper respiratory tract infections (Table 6). Sepsis, gastrointestinal, and soft tissue infections were the most common types of severe infections (Table 7).
Table 5.
Studies That Reported Infections in Pediatric Inflammatory Bowel Disease Patients Treated With TNF-α Inhibitors
| Study, First Author | IBD Type | TNF-α Inhibitor | No. of Subjects | Age, y, Mean | Disease Duration, y, Mean | DMARDa, CS, or Both | Patient-years of Drug Exposureb | Total No. of Infections (per Patient-year) | Total No. of Serious Infections (per Patient-year) |
|---|---|---|---|---|---|---|---|---|---|
| Prospective | |||||||||
| Kugathasan [77] | CD | INX | 15 | 12.8 | 10.8 | Both | NS | 0 | 0 |
| Baldassano [78] | CD | INX | 21 | 15.0c | 3.8c | Both | NS | 3 | 0 |
| Cezard [79] | CD | INX | 21 | 15.0c | 4.0 | Both | NS | 1 | 1 |
| Hyams [81] | CD | INX | 112 | 13.3 | 2.0 | Both | 85 | 70 (0.824) | 9 (0.106) |
| Hyams [72] | CD | INX | 202 | 12.7 | NS | Both | NS | 1 | 1 |
| Ruemmele [83] | CD | INX | 40 | 13.9 | 3.0 | Both | NS | 15 | 0 |
| Viola [89] | CD | ADA | 23 | 16.1c | 4.4 | Both | NS | 8 | 1 |
| Hyams [66] | UC | INX | 52 | 13.3 | NS | Both | NS | 0 | 0 |
| Crombe [71] | CD | INX | 120 | 14.5c | 9.3c | NS | 320d | 2 (0.006d) | 0 |
| Hyams [63] | CD | INX | 60 | 13.2 | 1.8 | Both | 98 | 54 (0.551) | 8 (0.082) |
| Hyams [85] | UC | INX | 60 | 14.5c | 1.4c | Both | 34 | 33 (0.971) | 6 (0.176) |
| Retrospective | |||||||||
| Serrano [100] | Both | INX | 18 | 15.5 | 4.9 | Both | 12d | 2 (0.168d) | 2 (0.168d) |
| Stephens [64] | CD | INX | 82 | 15.2 | NS | Both | 73d | 3 (0.041d) | 0 |
| De Ridder [76] | CD | INX | 30 | 14.1 | NS | Both | 63d | 1 (0.016d) | 1 (0.016d) |
| Friesen [65] | Both | INX | 111 | 13.4 | NS | Both | 176d | 4 (0.023d) | 0 |
| Lamireau [80] | CD | INX | 88 | 11.0 | 1.2 | NS | 29 | 2 (0.069) | 2 (0.069) |
| Mamula [67] | UC | INX | 8 | 15.3 | 1.5c | Both | 6 | 1 (0.159) | 0 |
| Eidelwein [68] | UC | INX | 12 | 12.5c | 1.6c | Both | NS | 1 | 1 |
| Wewer [75] | CD | INX | 24 | 15.4c | 2.2c | Both | NS | 1 | 0 |
| Cucchiara [69] | UC | INX | 22 | 12c (M); 14c (F) | NS | Both | NS | 0 | 0 |
| De Ridder [74] | CD | INX | 66 | 14.5 | 2.4 | Both | 159 | 10 (0.063) | 0 |
| McGinnis and Murray [70] | UC | INX | 40 | 12.9 | 0.54c | Both | 62d | 3 (0.049d) | 1 (0.016d) |
| Wynands [82] | CD | INX | 38 | 10.7 | 3.1 | NS | 22 | 1 (0.045) | 0 |
| Wyneski [99] | CD | ADA | 15 | 16.6 | 5.7 | Both | 12 | 4 (0.333) | 0 |
| Rosh [88] | CD | ADA | 115 | 11.1 | NS | Both | 127d | 2 (0.016d) | 1 (0.008d) |
| Rosenbach [87] | CD | ADA | 14 | 13.9c | 3.9c | NS | 17 | 1 (0.059) | 1 (0.059) |
| De Bie [73] | CD | INX | 152 | 15.0c | 1.8c | Both | 203 | 27 (0.133) | 2 (0.010) |
| Russell [86] | Both | ADA | 72 | 14.8c | 4.3c | NS | NS | 5 | 4 |
Abbreviations: ADA, adalimumab; CD, Crohn's disease; CS, corticosteroids; DMARD, disease-modifying antirheumatic drug; F, female; IBD, inflammatory bowel disease; INX, infliximab; M, male; NS, not specified; TNF-α; tumor necrosis factor alpha; UC, ulcerative colitis.
a DMARD may include 5-aminosalicylic acid, 6-mercaptopurine, cyclosporine, azathioprine, or methotrexate.
b Calculated based on patient-years of exposure to TNF-α inhibitor.
c Median years.
d Calculated based on patient-years of follow-up in study. Unable to determine patient-years of exposure to TNF-α inhibitor from study.
Table 6.
Mild Infections in Pediatric Inflammatory Bowel Disease Patients Treated With Tumor Necrosis Factor–α Inhibitors
| Infection Type (No. of Infections) | Infliximab (n = 1407) | Adalimumab (n = 241) |
|---|---|---|
| Respiratory tract infections (114) | ||
| URTI (105) | 94 | 11 |
| LRTI (9) | 9 | 0 |
| Skin and soft tissue infections (14) | 13a | 1b |
| Gastrointestinal infections (4) | 4 | 0 |
| Other viral infections (15) | ||
| Primary varicella (1) | 1 | 0 |
| Zoster (12) | 12 | 0 |
| HSV (1) | 1 | 0 |
| EBV (1) | 1 | 0 |
| Mycobacterial infections (1) | 0 | 1c |
| Mild infections not specified (71) | 71 | NR |
Abbreviations: EBV, Epstein-Barr virus; HSV, herpes simplex virus; LRTI, lower respiratory tract infection; NR, not reported; URTI, upper respiratory tract infection.
a Three cases of dermatophytoses.
b One case of Staphylococcus aureus.
c One case of Mycobacterium avium complex.
Table 7.
Severe Infections in Pediatric Inflammatory Bowel Disease Patients Treated With Tumor Necrosis Factor–α Inhibitors
| Infection Type (No. of Infections) | Infliximab (n = 1407) | Adalimumab (n = 241) |
|---|---|---|
| Respiratory tract infections (4) | ||
| URTI (1) | 1 | 0 |
| LRTI (3) | 3 | 0 |
| Sepsis/bacteremia (10) | ||
| Sepsis (9) | 7a | 2b |
| Bacteremia (1) | 1c | |
| CNS infections (2) | ||
| Meningitis (2) | 1d | 1d |
| Gastrointestinal infections (9) | ||
| Abdominal abscess (6) | 3 | 3 |
| Colitis (3) | 2 | 1e |
| Musculoskeletal infections (9) | ||
| Abscess/cellulitis (8) | 8c,f | 0 |
| Septic arthritis and osteomyelitis (1) | 1c | 0 |
| Genitourinary infections (1) | 1 | 0 |
| Primary varicella (3) | 3g | 0 |
| CMV (1) | 1h | 0 |
| EBV (1) | 1 | 0 |
| Fungal infections (9) | ||
| Histoplasmosis (6) | 4 | 2 |
| Aspergillosis (1) | 0 | 1i |
| PCP (1) | 1j | 0 |
| Candidemia (1) | 0 | 1k |
| Infectious fatalities (4) | 2h | 2b |
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; EBV, Epstein-Barr virus; LRTI, lower respiratory tract infection; PCP, Pneumocystis jirovecii pneumonia; URTI, upper respiratory tract infection.
a One case each of Escherichia coli and Staphylococcus aureus sepsis.
b Bacteremia associated with central lines; 1 case due to E. coli and 1 case due to coagulase-negative Staphylococcus infection. Both were fatal infections.
c One case each of S. aureus infection.
d One case each of Listeria monocytogenes.
e One case of Clostridium difficile colitis.
f One case of Enterococcus faecalis infection.
g Disseminated varicella-zoster virus infection in 2 patients.
h Disseminated CMV infection that was fatal.
I Had concurrent coagulase-negative Staphylococcus line sepsis.
j Concurrent PCP and histoplasmosis in same patient.
k Associated with central line infection.
In pIBD patients treated with either adalimumab or infliximab, the incidence of mild infections ranged from 3% (1/38) to 77% (46/60), and from 0% (0/66) to 10% (6/60) for serious infections [63, 65, 74]. In adult IBD patients, the risk of serious infections with TNF-α inhibitor therapy is between 2.2% to 5% [102–104]. However, the adult IBD studies have had conflicting results because these patients may also be at increased risk of serious infections due to the underlying disease and advanced age [105]. It is unknown if pIBD patients have a similar increased risk of infections because of their underlying illness.
Microbiology
The microbiologic results of mild and severe infections in pIBD patients treated with TNF-α inhibitors were extremely limited; most studies had no information on mild infections or pathogens involved. For both mild and severe infections, VZV was the most commonly identified viral pathogen. There were 2 reports of disseminated varicella and both patients either had evidence of immunity or prior exposure [84].
Bacterial and fungal infections also contributed to severe infections in pIBD patients. There were 2 cases of Listeria monocytogenes meningitis in 1 CD patient and 1 UC patient treated with infliximab and concurrent DMARDs [92, 101]. Histoplasmosis was described in 6 patients, all of whom lived in an endemic region and 5 of whom were on concurrent DMARDs [94–96]. One patient was on infliximab only and presented concurrently with P. jirovecii pneumonia [95]. A recent study in the murine model found that TNF-α inhibitors may alter protective immunity and dampen the response to Histoplasma capsulatum infection [106]. There was 1 report of systemic Mycobacterium avium complex infection [93].
Two infectious fatalities from infliximab were reported in CD patients: 1 due to disseminated cytomegalovirus and 1 due to bacterial sepsis [73, 97]. There were 2 infectious fatalities with adalimumab [86]. Both patients required central venous catheters for parenteral nutrition and were on other DMARDs. One patient had coagulase-negative staphylococcal bacteremia followed by pulmonary invasive aspergillosis.
Approaches to Screening and Prevention of Infections in Children With JIA or pIBD Treated With TNF-α Inhibitors
Recommendations for screening strategies, vaccinations, and safety monitoring of pediatric patients on TNF-α antagonists are scarce and largely extrapolated from adult studies. The American College of Rheumatology recommends that JIA patients be screened for latent tuberculosis, and if high risk, chronic hepatitis B (HBV) and hepatitis C (HCV) infection, before starting biologics [13]. Similarly, pIBD patients should be screened for latent tuberculosis prior to initiating TNF-α inhibitor therapy, and then annually. This has likely helped minimize the incidence of these opportunistic infections in pediatric patients, with only 5 cases of M. tuberculosis and no reports of HBV or HCV reactivation found in this cohort.
Vaccine recommendations are inadequately detailed for pIBD and JIA patients who are starting TNF-α inhibitors. For adults with RA or IBD, there are more specific preventive measures, including the recommendation to provide influenza vaccine annually, as well as pneumococcal, HBV, and human papillomavirus (HPV) vaccines [107, 108]. In pIBD patients, the European Crohn's and Colitis Organization recommends administering routine inactivated vaccines, including HBV and HPV [109]. In pediatrics, only 1 of 3 JIA guidelines found mentioned the importance of updating the patient on vaccines prior to initiation of TNF-α inhibitors [13, 110, 111]. Although there may be a concern for flares in JIA patients with vaccine administration, studies have shown that JIA patients who received hepatitis A, HBV, meningococcal C, or measles-mumps-rubella (MMR) vaccines were not at increased risk of reactivation of their underlying disease [112–115]. Ideally, children with pIBD and JIA should adhere to the immunization schedule recommended by the American Academy of Pediatrics, except for the administration of live vaccines, which are currently contraindicated while on biologics. JIA and pIBD patients should be caught up with vaccines and complete the regimen of live attenuated vaccines prior to immunosuppressive therapy whenever possible.
In our practice, we evaluate the patient's varicella and measles immune status prior to initiation of TNF-α inhibitor therapy. We encourage VZV vaccination if a patient is nonimmune prior to TNF-α inhibitor therapy, given the risk of severe varicella disease and zoster as described above. If a patient is MMR nonimmune and he/she has only received 1 dose, we recommend administering the second MMR dose at least 4 weeks prior to initiating TNF-α inhibitor therapy. This is especially important given recent measles outbreaks in the United States and possible diminished immunogenicity to vaccines while on biologics [116].
Because of the incidence of histoplasmosis observed with TNF-α inhibitor therapy, all patients should be screened for risk factors prior to starting therapy, and then annually. This includes questioning patients about travel to endemic regions and participation in activities that may increase risk of exposure. Although there are no formal recommendations on screening strategies in high-risk patients, these patients may warrant closer monitoring.
CONCLUSIONS
Pediatric patients with JIA and IBD can frequently develop mild infections and, less commonly, severe infections when treated with infliximab, etanercept, or adalimumab. Bacterial, viral, and fungal infections were all important etiologies of serious infections. Importantly, few pediatric patients developed M. tuberculosis, likely owing to effective screening for latent tuberculosis. Unfortunately, the majority of the studies reviewed had significant limitations, making it difficult to adequately assess the extent of infections that occurred in this cohort. This includes limited information about the frequency, sites of infection, and microbiology. As mentioned, studies included heterogeneous populations on concomitant immunosuppressive therapy and were not designed to effectively evaluate for mild and/or severe infections. Future prospective studies with larger patient populations, more frequent follow-up, and a more thorough assessment of mild and serious infections would significantly help clinicians better understand the implications of starting a patient with JIA or pIBD on TNF-α inhibitors, and ultimately help to improve prevention strategies and management.
Notes
Financial support. T. J. W. is a Scholar of the Henry Schueler Foundation and a Scholar of Pediatric Infectious Diseases of the Sharpe Family Foundation, and receives support from the SOS Kids Foundation (grant numbers: R34HL117352, 1R01AI103315-01A1).
Potential conflicts of interest. T. J. W. is a board member of iCo; has served as a consultant for Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius; has received research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Martin-Mola E, Balsa A. Infectious complications of biologic agents. Rheum Dis Clin North Am. 2009;35:183–99. doi: 10.1016/j.rdc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Favalli EG, Desiati F, Atzeni F, et al. Serious infections during anti-TNFalpha treatment in rheumatoid arthritis patients. Autoimmun Rev. 2009;8:266–73. doi: 10.1016/j.autrev.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–44. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 4.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 5.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–98. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Ham HJ, de Jager W, Bijlsma JW, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology. 2009;48:899–905. doi: 10.1093/rheumatology/kep125. [DOI] [PubMed] [Google Scholar]
- 7.Ngo B, Farrell CP, Barr M, et al. Tumor necrosis factor blockade for treatment of inflammatory bowel disease: efficacy and safety. Curr Mol Pharmacol. 2010;3:145–52. doi: 10.2174/1874467211003030145. [DOI] [PubMed] [Google Scholar]
- 8.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Questions and answers—TNF blockers (25 August 2009) Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm180694.htm?utm_camcampaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=etanercept pediatric juvenile arthritis &utm_content=10 . Accessed 30 April 2012.
- 10.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 11.Prakken BJ, Albani S. Using biology of disease to understand and guide therapy of JIA. Best Pract Res Clin Rheumatol. 2009;23:599–608. doi: 10.1016/j.berh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Lin YT, Wang CT, Gershwin ME, Chiang BL. The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun Rev. 2011;10:482–9. doi: 10.1016/j.autrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Beukelman T, Patkar NM, Saag KG, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022–10. doi: 10.1016/j.jaci.2009.08.046. e1–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calleja S, Cordero-Coma M, Rodriguez E, Llorente M, Franco M, Ruiz de Morales JG. Adalimumab specifically induces CD3(+) CD4(+) CD25(high) Foxp3(+) CD127(-) T-regulatory cells and decreases vascular endothelial growth factor plasma levels in refractory immuno-mediated uveitis: a non-randomized pilot intervention study. Eye (Lond) 2012;26:468–77. doi: 10.1038/eye.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vigna-Perez M, Abud-Mendoza C, Portillo-Salazar H, et al. Immune effects of therapy with adalimumab in patients with rheumatoid arthritis. Clin Exp Immunol. 2005;141:372–80. doi: 10.1111/j.1365-2249.2005.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filler SG, Yeaman MR, Sheppard DC. Tumor necrosis factor inhibition and invasive fungal infections. Clin Infect Dis. 2005;41(suppl 3):S208–12. doi: 10.1086/430000. [DOI] [PubMed] [Google Scholar]
- 18.Armbrust W, Kamphuis SS, Wolfs TW, et al. Tuberculosis in a nine-year-old girl treated with infliximab for systemic juvenile idiopathic arthritis. Rheumatology. 2004;43:527–9. doi: 10.1093/rheumatology/keh074. [DOI] [PubMed] [Google Scholar]
- 19.Morishita K, Petty R, Cairns R, Bolaria R, Cabral D, Turvey S. Serious musculoskeletal infections in children receiving anti-tumor necrosis factor-alpha therapy: a case series. Clin Rheumatol. 2010;29:677–81. doi: 10.1007/s10067-010-1410-x. [DOI] [PubMed] [Google Scholar]
- 20.Elwood RL, Pelszynski MM, Corman LI. Multifocal septic arthritis and osteomyelitis caused by group A Streptococcus in a patient receiving immunomodulating therapy with etanercept. Pediatr Infect Dis J. 2003;22:286–8. [PubMed] [Google Scholar]
- 21.Fitch PG, Cron RQ. Septic abscess in a child with juvenile idiopathic arthritis receiving anti-tumor necrosis factor-alpha. J Rheumatol. 2006;33:825. author reply 826–7. [PubMed] [Google Scholar]
- 22.Renaud C, Ovetchkine P, Bortolozzi P, Saint-Cyr C, Tapiero B. Fatal group A Streptococcus purpura fulminans in a child receiving TNF-alpha blocker. Eur J Pediatr. 2011;170:657–60. doi: 10.1007/s00431-010-1341-1. [DOI] [PubMed] [Google Scholar]
- 23.Ruperto N, Lovell DJ, Cuttica R, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 24.Tynjala P, Vahasalo P, Tarkiainen M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70:1605–12. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 25.Gerloni V, Pontikaki I, Gattinara M, Fantini F. Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis. 2008;67:1145–52. doi: 10.1136/ard.2007.069484. [DOI] [PubMed] [Google Scholar]
- 26.Giannini EH, Ilowite NT, Lovell DJ, et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2794–804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- 27.Otten MH, Prince FH, Armbrust W, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306:2340–7. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- 28.Horneff G, De Bock F, Foeldvari I, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA registry. Ann Rheum Dis. 2009;68:519–25. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
- 29.Horneff G, Ebert A, Fitter S, et al. Safety and efficacy of once weekly etanercept 0.8 mg/kg in a multicentre 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatology (Oxford) 2009;48:916–9. doi: 10.1093/rheumatology/kep122. [DOI] [PubMed] [Google Scholar]
- 30.Prince FH, Twilt M, ten Cate R, et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009;68:635–41. doi: 10.1136/ard.2007.087411. [DOI] [PubMed] [Google Scholar]
- 31.Mori M, Takei S, Imagawa T, et al. Safety and efficacy of long-term etanercept in the treatment of methotrexate-refractory polyarticular-course juvenile idiopathic arthritis in Japan. Mod Rheumatol. 2012;22:720–6. doi: 10.1007/s10165-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 32.Lovell DJ, Giannini EH, Reiff A, et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48:218–26. doi: 10.1002/art.10710. [DOI] [PubMed] [Google Scholar]
- 33.Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–20. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 34.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 35.Trachana M, Pratsidou-Gertsi P, Pardalos G, Kozeis N, Badouraki M, Kanakoudi-Tsakalidou F. Safety and efficacy of adalimumab treatment in Greek children with juvenile idiopathic arthritis. Scand J Rheumatol. 2011;40:101–7. doi: 10.3109/03009742.2010.517546. [DOI] [PubMed] [Google Scholar]
- 36.Bracaglia C, Buonuomo PS, Tozzi AE, et al. Safety and efficacy of etanercept in a cohort of patients with juvenile idiopathic arthritis under 4 years of age. J Rheumatol. 2012;39:1287–90. doi: 10.3899/jrheum.111555. [DOI] [PubMed] [Google Scholar]
- 37.Lamot L, Bukovac LT, Vidovic M, Frleta M, Harjacek M. The “head-to-head” comparison of etanercept and infliximab in treating children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2011;29:131–9. [PubMed] [Google Scholar]
- 38.Takei S, Groh D, Bernstein B, Shaham B, Gallagher K, Reiff A. Safety and efficacy of high dose etanercept in treatment of juvenile rheumatoid arthritis. J Rheumatol. 2001;28:1677–80. [PubMed] [Google Scholar]
- 39.Tynjala P, Kotaniemi K, Lindahl P, et al. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford) 2008;47:339–44. doi: 10.1093/rheumatology/kem356. [DOI] [PubMed] [Google Scholar]
- 40.Southwood TR, Foster HE, Davidson JE, et al. Duration of etanercept treatment and reasons for discontinuation in a cohort of juvenile idiopathic arthritis patients. Rheumatology. 2011;50:189–95. doi: 10.1093/rheumatology/keq308. [DOI] [PubMed] [Google Scholar]
- 41.Tzaribachev N, Kuemmerle-Deschner J, Eichner M, Horneff G. Safety and efficacy of etanercept in children with juvenile idiopathic arthritis below the age of 4 years. Rheumatol Int. 2008;28:1031–4. doi: 10.1007/s00296-008-0563-2. [DOI] [PubMed] [Google Scholar]
- 42.Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Wheat LJ. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85–92. doi: 10.1086/648724. [DOI] [PubMed] [Google Scholar]
- 43.Mohan AK, Cote TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295–9. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- 44.Holl-Wieden A, Beer M, Marx A, Bonfig R, Tappe D, Girschick HJ. Infection of an urachal cyst during etanercept therapy in juvenile idiopathic arthritis. Rheumatology Int. 2008;28:819–22. doi: 10.1007/s00296-008-0521-z. [DOI] [PubMed] [Google Scholar]
- 45.Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 46.Ruperto N, Lovell DJ, Cuttica R, et al. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis. 2010;69:718–22. doi: 10.1136/ard.2009.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuber Z, Rutkowska-Sak L, Postepski J, et al. Etanercept treatment in juvenile idiopathic arthritis: the Polish registry. Med Sci Monit. 2011;17:SR35–42. doi: 10.12659/MSM.882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64:2012–21. doi: 10.1002/art.34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imagawa T, Takei S, Umebayashi H, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumat. 2012;31:1713–21. doi: 10.1007/s10067-012-2082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beukelman T, Xie F, Chen L, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64:2773–80. doi: 10.1002/art.34458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane MA, McDonald JR, Zeringue AL, et al. TNF-alpha antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore) 2011;90:139–45. doi: 10.1097/MD.0b013e318211106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 53.Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 54.Komano Y, Tanaka M, Nanki T, et al. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety. J Rheumat. 2011;38:1258–64. doi: 10.3899/jrheum.101009. [DOI] [PubMed] [Google Scholar]
- 55.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 56.Salmon-Ceron D, Tubach F, Lortholary O, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–23. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- 57.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 58.Crohn's and Colitis Foundation. Available at http://www.ccfa.org. Accessed 25 April 2012.
- 59.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 60.Jakobsen C, Bartek J, Jr, Wewer V, et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease—a population-based study. Aliment Pharmacol Ther. 2011;34:1217–24. doi: 10.1111/j.1365-2036.2011.04857.x. [DOI] [PubMed] [Google Scholar]
- 61.Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16:953–61. doi: 10.1002/ibd.21152. [DOI] [PubMed] [Google Scholar]
- 62.Malik S, Ahmed SF, Wilson ML, et al. The effects of anti-TNF-alpha treatment with adalimumab on growth in children with Crohn's disease (CD) J Crohns Colitis. 2012;6:337–44. doi: 10.1016/j.crohns.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Hyams J, Walters TD, Crandall W, et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn's disease in children: REACH open-label extension. Curr Med Res Opin. 2011;27:651–62. doi: 10.1185/03007995.2010.547575. [DOI] [PubMed] [Google Scholar]
- 64.Stephens MC, Shepanski MA, Mamula P, Markowitz JE, Brown KA, Baldassano RN. Safety and steroid-sparing experience using infliximab for Crohn's disease at a pediatric inflammatory bowel disease center. Am J Gastroenterol. 2003;98:104–11. doi: 10.1111/j.1572-0241.2003.07161.x. [DOI] [PubMed] [Google Scholar]
- 65.Friesen CA, Calabro C, Christenson K, et al. Safety of infliximab treatment in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:265–9. doi: 10.1097/00005176-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Hyams JS, Lerer T, Griffiths A, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430–6. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 67.Mamula P, Markowitz JE, Cohen LJ, von Allmen D, Baldassano RN. Infliximab in pediatric ulcerative colitis: two-year follow-up. J Pediatr Gastroenterol Nutr. 2004;38:298–301. doi: 10.1097/00005176-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Eidelwein AP, Cuffari C, Abadom V, Oliva-Hemker M. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis. 2005;11:213–8. doi: 10.1097/01.mib.0000160803.44449.a5. [DOI] [PubMed] [Google Scholar]
- 69.Cucchiara S, Romeo E, Viola F, et al. Infliximab for pediatric ulcerative colitis: a retrospective Italian multicenter study. Dig Liver Dis. 2008;40(suppl 2)):S260–4. doi: 10.1016/S1590-8658(08)60535-6. [DOI] [PubMed] [Google Scholar]
- 70.McGinnis JK, Murray KF. Infliximab for ulcerative colitis in children and adolescents. J Clin Gastroenterol. 2008;42:875–9. doi: 10.1097/MCG.0b013e3181354417. [DOI] [PubMed] [Google Scholar]
- 71.Crombe V, Salleron J, Savoye G, et al. Long-term outcome of treatment with infliximab in pediatric-onset Crohn's disease: a population-based study. Inflamm Bowel Dis. 2011;17:2144–52. doi: 10.1002/ibd.21615. [DOI] [PubMed] [Google Scholar]
- 72.Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009;15:816–22. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 73.De Bie CI, Hummel TZ, Kindermann A, et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn's disease is limited. Aliment Pharmacol Ther. 2011;33:243–50. doi: 10.1111/j.1365-2036.2010.04507.x. [DOI] [PubMed] [Google Scholar]
- 74.de Ridder L, Rings EH, Damen GM, et al. Infliximab dependency in pediatric Crohn's disease: long-term follow-up of an unselected cohort. Inflamm Bowel Dis. 2008;14:353–8. doi: 10.1002/ibd.20329. [DOI] [PubMed] [Google Scholar]
- 75.Wewer V, Riis L, Vind I, Husby S, Munkholm P, Paerregaard A. Infliximab dependency in a national cohort of children with Crohn's disease. J Pediatr Gastroenterol Nutr. 2006;42:40–5. doi: 10.1097/01.mpg.0000189137.06151.33. [DOI] [PubMed] [Google Scholar]
- 76.de Ridder L, Escher JC, Bouquet J, et al. Infliximab therapy in 30 patients with refractory pediatric Crohn disease with and without fistulas in the Netherlands. J Pediatr Gastroenterol Nutr. 2004;39:46–52. doi: 10.1097/00005176-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response to infliximab in early but not late pediatric Crohn's disease. Am J Gastroenterol. 2000;95:3189–94. doi: 10.1111/j.1572-0241.2000.03263.x. [DOI] [PubMed] [Google Scholar]
- 78.Baldassano R, Braegger CP, Escher JC, et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98:833–8. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 79.Cezard JP, Nouaili N, Talbotec C, et al. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors (Remicade) in severe pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2003;36:632–6. doi: 10.1097/00005176-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Lamireau T, Cezard JP, Dabadie A, et al. Efficacy and tolerance of infliximab in children and adolescents with Crohn's disease. Inflamm Bowel Dis. 2004;10:745–50. doi: 10.1097/00054725-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Wynands J, Belbouab R, Candon S, et al. 12-month follow-up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:293–8. doi: 10.1097/MPG.0b013e31815604cd. [DOI] [PubMed] [Google Scholar]
- 83.Ruemmele FM, Lachaux A, Cezard JP, et al. Efficacy of infliximab in pediatric Crohn's disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis. 2009;15:388–94. doi: 10.1002/ibd.20788. [DOI] [PubMed] [Google Scholar]
- 84.Kunz AN, Rajnik M. Disseminated cutaneous varicella zoster virus infections during infliximab therapy for Crohn's disease: case report of two pediatric patients at one institution. Clin Pediatr (Phila) 2011;50:559–61. doi: 10.1177/0009922810380452. [DOI] [PubMed] [Google Scholar]
- 85.Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391–9. doi: 10.1016/j.cgh.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 86.Russell RK, Wilson ML, Loganathan S, et al. A British Society of Paediatric Gastroenterology, Hepatology and Nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:946–53. doi: 10.1111/j.1365-2036.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 87.Rosenbach Y, Hartman C, Shapiro R, Hirsch A, Avitzur Y, Shamir R. Adalimumab treatment in children with refractory Crohn's disease. Dig Dis Sci. 2010;55:747–53. doi: 10.1007/s10620-009-0791-7. [DOI] [PubMed] [Google Scholar]
- 88.Rosh JR, Lerer T, Markowitz J, et al. Retrospective Evaluation of the Safety and Effect of Adalimumab Therapy (RESEAT) in pediatric Crohn's disease. Am J Gastroenterol. 2009;104:3042–9. doi: 10.1038/ajg.2009.493. [DOI] [PubMed] [Google Scholar]
- 89.Viola F, Civitelli F, Di Nardo G, et al. Efficacy of adalimumab in moderate-to-severe pediatric Crohn's disease. Am J Gastroenterol. 2009;104:2566–71. doi: 10.1038/ajg.2009.372. [DOI] [PubMed] [Google Scholar]
- 90.Nasir A, El Bahesh E, Whitten C, Lawson A, Udall JN., Jr Pityrosporum folliculitis in a Crohn's disease patient receiving infliximab. Inflamm Bowel Dis. 2010;16:7–8. doi: 10.1002/ibd.20928. [DOI] [PubMed] [Google Scholar]
- 91.Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schonlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101. doi: 10.1097/RHU.0b013e31819bca9e. [DOI] [PubMed] [Google Scholar]
- 92.Chuang MH, Singh J, Ashouri N, Katz MH, Arrieta AC. Listeria meningitis after infliximab treatment of ulcerative colitis. J Pediatr Gastroenterol Nutr. 2010;50:337–9. doi: 10.1097/MPG.0b013e3181a70f3a. [DOI] [PubMed] [Google Scholar]
- 93.Jordan N, Waghmare A, Abi-Ghanem AS, Moon A, Salvatore CM. Systemic Mycobacterium avium complex infection during antitumor necrosis factor-alpha therapy in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;54:294–6. doi: 10.1097/MPG.0b013e31822938c3. [DOI] [PubMed] [Google Scholar]
- 94.Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–70. doi: 10.1002/art.10583. [DOI] [PubMed] [Google Scholar]
- 95.Tschudy J, Michail S. Disseminated histoplasmosis and pneumocystis pneumonia in a child with Crohn disease receiving infliximab. J Pediatr Gastroenterol Nutr. 2010;51:221–2. doi: 10.1097/MPG.0b013e3181c2c10d. [DOI] [PubMed] [Google Scholar]
- 96.Dotson JL, Crandall W, Mousa H, et al. Presentation and outcome of histoplasmosis in pediatric inflammatory bowel disease patients treated with antitumor necrosis factor alpha therapy: a case series. Inflamm Bowel Dis. 2011;17:56–61. doi: 10.1002/ibd.21378. [DOI] [PubMed] [Google Scholar]
- 97.Pickering O, Weinstein T, Rubin LG. Fatal disseminated cytomegalovirus infection associated with infliximab and 6-mercaptopurine therapy in a child with Crohn disease. Pediatr Infect Dis J. 2009;28:556. doi: 10.1097/INF.0b013e3181a39571. [DOI] [PubMed] [Google Scholar]
- 98.Reichardt P, Dahnert I, Tiller G, Hausler HJ. Possible activation of an intramyocardial inflammatory process (Staphylococcus aureus) after treatment with infliximab in a boy with Crohn disease. Eur J Pediatr. 2002;161:281–3. doi: 10.1007/s00431-002-0925-9. [DOI] [PubMed] [Google Scholar]
- 99.Wyneski MJ, Green A, Kay M, Wyllie R, Mahajan L. Safety and efficacy of adalimumab in pediatric patients with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;47:19–25. doi: 10.1097/MPG.0b013e318174e886. [DOI] [PubMed] [Google Scholar]
- 100.Serrano MS, Schmidt-Sommerfeld E, Kilbaugh TJ, Brown RF, Udall JN, Jr., Mannick EE. Use of infliximab in pediatric patients with inflammatory bowel disease. Ann Pharmacother. 2001;35:823–8. doi: 10.1345/aph.10395. [DOI] [PubMed] [Google Scholar]
- 101.Slifman NR, Gershon SK, Lee JH, Edwards ET, Braun MM. Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor alpha-neutralizing agents. Arthritis Rheum. 2003;48:319–24. doi: 10.1002/art.10758. [DOI] [PubMed] [Google Scholar]
- 102.Colombel JF, Loftus EV, Jr., Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 103.Lawrance IC, Radford-Smith GL, Bampton PA, et al. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J Gastroenterol Hepatol. 2010;25:1732–8. doi: 10.1111/j.1440-1746.2010.06407.x. [DOI] [PubMed] [Google Scholar]
- 104.Lofberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn's disease: results from CARE. Inflamm Bowel Dis. 2012;18:1–9. doi: 10.1002/ibd.21663. [DOI] [PubMed] [Google Scholar]
- 105.Epple HJ. Therapy- and non-therapy-dependent infectious complications in inflammatory bowel disease. Dig Dis. 2009;27:555–9. doi: 10.1159/000233297. [DOI] [PubMed] [Google Scholar]
- 106.Deepe GS, Jr, Gibbons RS. TNF-alpha antagonism generates a population of antigen-specific CD4+CD25+ T cells that inhibit protective immunity in murine histoplasmosis. J Immunol. 2008;180:1088–97. doi: 10.4049/jimmunol.180.2.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D'Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2010;106:199–212. doi: 10.1038/ajg.2010.392. quiz 3. [DOI] [PubMed] [Google Scholar]
- 109.Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214–23. doi: 10.1038/ajg.2010.464. quiz 24. [DOI] [PubMed] [Google Scholar]
- 110.Yokota S, Mori M, Imagawa T, et al. Guidelines on the use of etanercept for juvenile idiopathic arthritis in Japan. Mod Rheumatol. 2010;20:107–13. doi: 10.1007/s10165-009-0259-9. [DOI] [PubMed] [Google Scholar]
- 111.Dueckers G, Guellac N, Arbogast M, et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin Immunol. 2012;142:176–93. doi: 10.1016/j.clim.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Erguven M, Kaya B, Hamzah OY, Tufan F. Evaluation of immune response to hepatitis A vaccination and vaccine safety in juvenile idiopathic arthritis. J Chin Med Assoc. 2011;74:205–8. doi: 10.1016/j.jcma.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Kasapcopur O, Cullu F, Kamburoglu-Goksel A, et al. Hepatitis B vaccination in children with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1128–30. doi: 10.1136/ard.2003.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zonneveld-Huijssoon E, Ronaghy A, Van Rossum MA, et al. Safety and efficacy of meningococcal c vaccination in juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:639–46. doi: 10.1002/art.22399. [DOI] [PubMed] [Google Scholar]
- 115.Borte S, Liebert UG, Borte M, Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology. 2009;48:144–8. doi: 10.1093/rheumatology/ken436. [DOI] [PubMed] [Google Scholar]
- 116.Dell'Era L, Corona F, Daleno C, Scala A, Principi N, Esposito S. Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. Vaccine. 2012;30:936–40. doi: 10.1016/j.vaccine.2011.11.083. [DOI] [PubMed] [Google Scholar]