Abstract
Background
Clinical trials in men with biochemically recurrent prostate cancer (BRPC) have been hampered by long survival times, making overall survival (OS) a difficult end point to reach. Intermediate end points are needed in order to conduct such trials within a more feasible time frame.
Patients and methods
This is a retrospective analysis of 450 men with BRPC following prostatectomy treated at a single institution between 1981 and 2010, of which 140 developed subsequent metastases. Androgen deprivation therapy (ADT) was deferred until after the development of metastases. Cox regression models were developed to investigate factors influencing OS.
Results
Median metastasis-free survival (MFS) was 10.2 years [95% confidence interval (CI) 7.6–14.0 years]; median OS after metastasis was 6.6 years (95%CI 5.8–8.4 years). Multivariable Cox regressions identified four independently prognostic variables for OS: MFS (HR 0.77; 95% CI 0.63–0.94), number of metastases (≤3 versus ≥4; HR 0.50; 95% CI 0.29–0.85), pain (absent versus present; HR 0.43; 95% CI 0.25–0.72), and bisphosphonate use (yes versus no; HR 0.60; 95% CI 0.37–0.98).
Conclusions
MFS emerged as an independent predictor of OS in men with BRPC treated with deferred ADT after the development of metastases. MFS may be a reasonable intermediate end point in future clinical trials. This observation requires prospective validation.
Keywords: clinical trial end points, metastasis-free survival, prostate cancer
introduction
An estimated 241 740 cases of prostate cancer were diagnosed in the United States in 2012, with 28 170 of those individuals dying as a result of their disease [1]. The clinical course of patients with prostate cancer is varied, and while the majority of patients will be cured after local therapy, about 20%–40% will develop a prostate-specific antigen (PSA) recurrence by 10 years postprostatectomy [2, 3]. A similar proportion of those patients will develop distant metastases, accounting for the majority of the morbidity and mortality from this disease [3, 4].
Defining the optimal treatment of men with PSA recurrence after local therapy has proven difficult. Management options for these men are numerous and include salvage pelvic irradiation (in select patients), continuous androgen deprivation therapy (ADT) initiated at the time of PSA recurrence, deferred ADT reserved until the onset of distant metastases, intermittent ADT, or clinical trial enrollment [5–14]. The lack of a standard treatment approach for these patients relates to the heterogeneity of this population, the existence of strong patient and physician preferences regarding the implementation of ADT, and the absence of well-conducted comparative clinical trials in these patients [3, 4, 9, 15]. Challenges in designing informative trials for men with PSA-recurrent prostate cancer abound, including the length of follow-up needed to assess clinically-relevant end points (e.g. metastasis and death), and the large sample sizes required to make statistically meaningful conclusions.
The need for surrogate end points is paramount for conducting trials in this patient population within a feasible time frame. We have previously described the natural history of patients with biochemically recurrent prostate cancer after radical prostatectomy in whom additional therapy was deferred until the development of radiographic metastases [4, 9, 16]. The objective of this analysis was to examine the association between metastasis-free survival (the time interval from PSA recurrence to first radiographic metastasis) and overall survival (the time interval from first metastasis to death). This study was not intended to develop a formal prediction model for overall survival (OS), but rather, serves as an important first step towards establishing MFS as a potential meaningful end point in future trials.
patients and methods
patients
Among patients undergoing radical prostatectomy at Johns Hopkins Hospital between July 1981 and July 2010, 1973 men subsequently developed a biochemical recurrence (defined as a postoperative PSA of ≥0.2 ng/mL). Our cohort of interest was the subset who did not receive any additional adjuvant/neoadjuvant or salvage therapies before the development of radiographic metastases (N = 642). Of these, only 450 patients had sufficient clinical information to be included in the present analysis. Further details on this patient population are presented as online supplements. Follow-up data was collected through July 2012.
This was a retrospective analysis of prospectively collected data. Data was derived from the Johns Hopkins Master Prostatectomy Database which stores clinical, pathological, and demographic information under a consent waiver allowing its use for future research without disclosing patient identifiers [16]. This database is approved by the Johns Hopkins institutional review board, and meets the requirements of the Health Insurance Portability and Accountability Act.
Patients were generally followed post-prostatectomy with PSA evaluation and digital rectal examination every 3 months for the first year, every 6 months for the second year, and every 12 months thereafter. Upon biochemical recurrence, PSA was measured approximately every 3–6 months. No patient received ADT until after the development of metastases. Per the standard practices of the treating physicians, computed tomography (CT) and technetium-99 bone scans were typically conducted at baseline and then yearly (or sooner if symptoms warranted). Precise data regarding the frequency of imaging were not captured in the database. Variations in postoperative follow-up practices were largely a result of patients opting to be followed outside of Johns Hopkins. Metastatic disease was defined on imaging modalities, most often using CT or bone scan.
PSA doubling time (PSADT) following biochemical recurrence was calculated as previously described [15]. A minimum of two PSA measurements collected ≥3 months apart were required.
statistical analysis
The primary end point of this study was OS, defined as the interval from the date of the first radiographic metastasis to the date of death from any cause. MFS was defined as the interval from biochemical recurrence (PSA ≥0.2 ng/ml) to first metastasis. The cause of death was determined from death certificates, and was coded as ‘prostate cancer related’ or ‘nonprostate cancer related’.
The association of risk of death with MFS and other potential prognostic clinical factors were assessed first using univariate Cox proportional hazards models and then multivariable models. Unadjusted hazard ratios were computed together with 95% confidence intervals (CIs). Clinical factors that were considered in the model are listed in Table 1. Log-transformation was applied to MFS to reduce skewness in its distribution. Time from prostatectomy to PSA relapse, PSADT, MFS, and number of metastases were considered both as continuous and binary variables. When transforming continuous variables to binary variables, optimized cut-off points were determined based on a modified log-rank test statistic [17, 18]. The cut-points used for Gleason score, time from prostatectomy to PSA relapse, and PSADT were chosen based on previously published work [4, 16].
Table 1.
Patient characteristics of our study cohort, comprising men who developed radiographic metastases after undergoing prior radical prostatectomy between July 1981 and July 2010
| Variables | Total sample size (N = 140) |
|---|---|
| Age in years, mean (SD) | 60 (5.2) |
| Race, N (%) | |
| Caucasian | 132 (94.3%) |
| Non-Caucasian | 8 (5.7%) |
| Clinical stage, N (%) | |
| cT1 | 32 (22.9%) |
| cT2 | 97 (69.3%) |
| cT3 | 9 (6.4%) |
| Preoperative PSA in ng/ml, mean (SD) | 13.6 (14.3) |
| Gleason score, N (%) | |
| ≤6 | 5 (3.6%) |
| 7 | 69 (49.3%) |
| 8–10 | 66 (47.1%) |
| Organ-confined disease, N (%) | |
| Yes | 13 (9.3%) |
| No | 127 (90.7%) |
| Extracapsular penetration, N (%) | |
| Yes | 106 (75.7%) |
| No | 34 (24.3%) |
| Seminal vesicle invasion, N (%) | |
| Yes | 64 (45.7%) |
| No | 76 (54.3%) |
| Lymph node involvement, N (%) | |
| Yes | 52 (37.1%) |
| No | 88 (62.9%) |
| Surgical margins, N (%) | |
| Positive | 46 (32.9%) |
| Negative | 94 (67.1%) |
| Time from prostatectomy to PSA recurrence, N (%) | |
| ≤3 years | 101 (72.1%) |
| >3 years | 39 (27.9%) |
| PSA doubling time (PSADT), N (%) | |
| <3 months | 30 (21.4%) |
| 3–9 months | 60 (42.9%) |
| 9–15 months | 25 (17.9%) |
| ≥15 months | 25 (17.9%) |
| Time from PSA recurrence to metastasis, N (%) | |
| ≤3 years | 81 (57.9%) |
| >3 years | 59 (42.1%) |
| Receipt of hormonal therapy (after metastasis), N (%) | |
| Yes | 129 (92.1%) |
| No | 11 (7.9%) |
| Numbera of metastases at first presentation, N (%) | |
| ≤3 | 97 (72.9%) |
| ≥4 | 36 (27.1%) |
| Site of first metastasis, N (%) | |
| Bone | 124 (88.6%) |
| Liver/lung/node | 16 (11.4%) |
| Painb with first metastasis, N (%) | |
| Yes | 38 (29.2%) |
| No | 92 (70.8%) |
| Receipt of bisphosphonate (after metastasis), N (%) | |
| Yes | 62 (53.0%) |
| No | 55 (47.0%) |
| ECOG status at first metastasis, N (%) | |
| ≥1 | 27 (20.8%) |
| 0 | 103 (79.2%) |
aThe number of metastases was taken to be the combined number of soft tissue and osseous metastases as determined by CT and bone scan, respectively.
bThe presence of cancer-related pain was based on a pain score of ≥1/10 on a standard 0–10 pain scale.
For the multivariable analysis, a backward elimination technique was applied to select variables from all potential covariates; those with P-values ≤0.10 were retained in the final model. Bootstrapping was used to validate the model [19]. The predictive discrimination of the model was assessed using c-index [19]. Median MFS was estimated for the whole cohort of 450 men using the Kaplan–Meier method. Likewise, OS was estimated for the 140 men with metastases using the Kaplan–Meier method. Differences in OS were evaluated using the log-rank test.
All tests were two sided and considered statistically significant at P ≤ 0.05. Statistical analyses were carried out using software R (version 2.15.1, Bethesda, MD) and SAS (version 9.2, Cary, NC).
results
Of the 450 men with PSA-recurrent prostate cancer included in this analysis, 140 developed distant metastases. Median MFS was 10.4 years (95% CI 7.6–14.0 years). The disease characteristics of these 140 men are summarized in Table 1. Metastases were most often to bone (124 patients; 88.6%). The remaining 16 patients (11.4%) developed metastases in extra-pelvic lymph nodes (5.7%), lungs (3.6%), or liver (2.1%). The median PSA at the time of initial metastasis was 24.8 ng/ml (range, 0.2–798.5 ng/ml).
As of July 2012, 78 patients (55.7%) have died after a median follow-up of 5.5 years from the date of first metastasis. Seventy-one deaths were due to metastatic prostate cancer; the other seven subjects died of other causes. Median OS was 6.6 years (95% CI 5.8–8.4 years) (Figure 1A). In univariate proportional hazards analysis, six variables were significantly associated with OS: MFS, PSADT after biochemical recurrence, number of metastases, site of metastases, pain with metastases, and bisphosphonate use after metastasis (Table 2).
Figure 1.
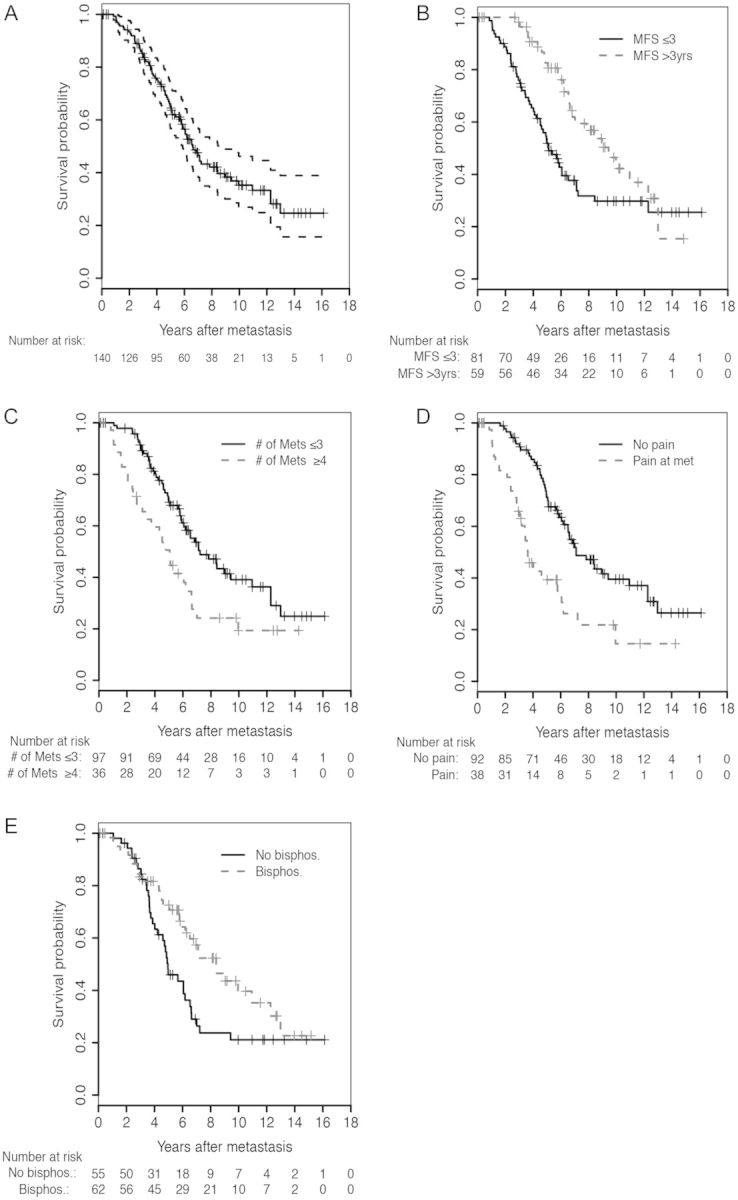
Kaplan-Meier survival curves for patients with (A) metastatic prostate cancer (N = 140) following radical prostatectomy and stratified by the following: (B) time from PSA recurrence to metastasis (i.e. MFS), (C) number of metastases at first presentation, (D) pain at the time of metastasis, and (E) bisphosphonate use after metastasis.
Table 2.
Univariate Cox proportional hazards model predicting OS from the time of first metastasis
| Variables | HR for death (95% CI) | P-value |
|---|---|---|
| Age | ||
| Continuous variable | 1.02 (0.97, 1.07) | 0.423 |
| Race | ||
| Caucasian | 1 [reference] | |
| Non-Caucasian | 1.07 (0.43, 2.65) | 0.888 |
| Clinical stage | ||
| cT1 | 1 | |
| cT2 | 1.05 (0.38, 2.86) | 0.919 |
| cT3 | 1.16 (0.67, 2.00) | 0.592 |
| Preoperative PSA | ||
| Continuous variable | 1.01 (0.99, 1.03) | 0.457 |
| Gleason score | ||
| ≤6 | 1 | |
| 7 | 1.01 (0.31, 3.28) | 0.994 |
| 8–10 | 1.29 (0.40, 4.16) | 0.675 |
| Organ-confined disease | ||
| Yes | 1 | |
| No | 1.75 (0.64, 4.76) | 0.271 |
| Extracapsular penetration | ||
| No | 1 | |
| Yes | 1.07 (0.63, 1.81) | 0.811 |
| Seminal vesicle invasion | ||
| No | 1 | |
| Yes | 1.05 (0.67, 1.65) | 0.821 |
| Lymph node involvement | ||
| No | 1 | |
| Yes | 1.04 (0.66, 1.64) | 0.851 |
| Surgical margins | ||
| Negative | 1 | |
| Positive | 1.33 (0.83, 2.11) | 0.233 |
| Time from surgery to PSA recurrence | ||
| Continuous variable | 0.96 (0.86, 1.08) | 0.525 |
| Categorical variable (years) | ||
| >3 | 1 | |
| ≤3 | 1.19 (0.71, 2.00) | 0.504 |
| PSA doubling time | ||
| Continuous variable | 0.99 (0.98, 1.00) | 0.146 |
| Categorical variable (months) | ||
| ≥15 | 1 | |
| 9–15 | 2.65 (1.17, 5.99) | 0.019 |
| 3–9 | 3.83 (1.57, 9.37) | 0.003 |
| <3 | 5.47 (2.31, 12.92) | <0.001 |
| Time from PSA recurrence to metastasis | ||
| Continuous variable (Log-transformed) | 0.79 (0.67, 0.93) | 0.005 |
| Categorical variable (years) | ||
| >3 | 1 | |
| ≤3 | 1.89 (1.18, 3.03) | 0.008 |
| Receipt of hormonal therapy? | ||
| Yes | 1 | |
| No | 1.69 (0.53, 5.38) | 0.378 |
| Number of metastases | ||
| Continuous variable | 1.10 (1.02, 1.18) | 0.013 |
| Categorical variable | ||
| ≤3 | 1 | |
| ≥4 | 1.85 (1.15, 2.98) | 0.011 |
| Site of first metastasis | ||
| Bone | 1 | |
| Liver/lung/node | 2.86 (1.09, 7.69) | 0.040 |
| Pain with metastasis | ||
| No | 1 | |
| Yes | 2.40 (1.49, 3.86) | <0.001 |
| Bisphosphonate use? | ||
| Yes | 1 | |
| No | 1.67 (1.03, 2.70) | 0.038 |
| ECOG status at metastasis | ||
| 0 | 1 | |
| ≥1 | 1.49 (0.87, 2.56) | 0.148 |
Data are from 140 men with PSA recurrence after having radical prostatectomy from July 1981 to July 2010.
Caution should be taken not to over-interpret these results, including P-values, given that the patient numbers in some categories may be small and no correction for multiple testing was carried out. Bold values denote statistically significant results.
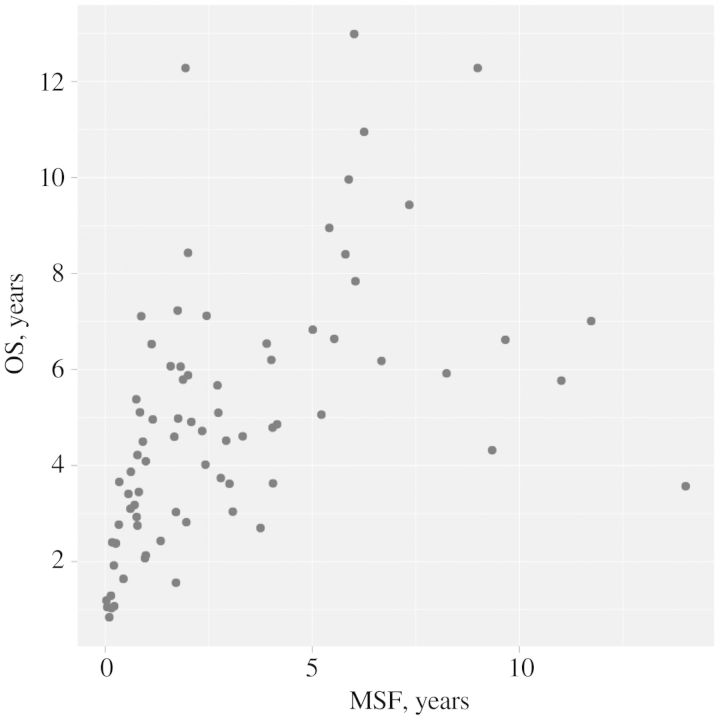
The multivariable model is presented in Table 3. MFS (Log-transformed), number of metastases, pain with metastasis. and bisphosphonate use after metastasis were all found to independently associate with OS. Figure 1B–E depicts OS Kaplan–Meier curves stratified by these significant prognostic variables. The c-index calculated from the model was 0.72 (95% CI 0.65–0.80). After penalizing for possible overfitting using 200 bootstrap samples, the estimate of c-index was 0.64. Figure 2 shows a scatter plot examining the relationship between MFS and OS in the 78 men who died with metastatic prostate cancer.
Table 3.
Multivariable Cox proportional hazards model for OS from the time of first metastasis
| Variables | HR for death (95% CI) | P–value |
|---|---|---|
| Log (MFS)a | 0.77 (0.63, 0.94) | 0.012 |
| Number of metastases | ||
| ≥4 | 1 [reference] | |
| ≤3 | 0.50 (0.29, 0.85) | 0.012 |
| Pain with metastases | ||
| Yes | 1 | |
| No | 0.43 (0.25, 0.72) | 0.002 |
| Bisphosphonate use | ||
| No | 1 | |
| Yes | 0.60 (0.37, 0.98) | 0.041 |
Data are from 140 men with PSA recurrence after undergoing radical prostatectomy from July 1981 to July 2010.
aMetastasis-free survival (MFS) is the time from PSA recurrence to first metastasis. MFS is considered as a continuous variable. Bold values denote statistically significant results.
Figure 2.
Scatter plot showing relationship between the time from PSA recurrence to metastasis (i.e. MFS) and the time from metastasis to death (i.e. OS) for patients who died with metastatic prostate cancer following PSA recurrence (N = 78).
discussion
While a number of predictive survival models exist for men with noncastrate biochemically recurrent prostate cancer, to our knowledge this is the first multivariable model that shows a significant association between MFS and OS. The conduct of informative clinical trials in this patient population has been challenged by the long natural history of this disease and the lack of validated surrogate end points. We and others have shown that OS in these patients may average 20 years or more from the time of PSA recurrence, while MFS may average 10 years [4, 15, 16]. Even if focusing on patients at highest risk of metastasis and death (e.g. those with a PSADT of ≤9 months), median OS and MFS are still estimated at 8 and 3 years, respectively [4, 16]. As a result, establishing that a given therapy leads to an OS benefit in this patient population has historically been difficult.
In order to advance the field, meaningful and realistic trial end points for men with biochemically recurrent prostate cancer are needed. Our group and others have shown that PSA kinetics are among the strongest predictors of metastasis and death in these patients [3, 4, 16]. However, while baseline PSA kinetics can serve as an invaluable tool in risk-stratifying patients before trial enrollment, post-treatment changes in PSA kinetics remain far from a validated intermediate end point [15]. MFS might be a more attractive proximal end point to use when designing trials in these patients. Compared with OS, MFS occurs earlier and is not influenced by therapies given after the development of metastases. Two prior studies suggested a possible association between MFS and OS among men with PSA-recurrent prostate cancer [3, 9]. However, this association did not remain significant in multivariable models. The present analysis has the advantage of longer follow-up and more patients with established metastatic disease. Here, we present data suggesting a significant association between MFS and OS in men with biochemically recurrent prostate cancer after controlling for known prognostic variables.
In addition, our multivariable model also revealed that the number of metastases at first presentation, the presence/absence of pain at metastasis, and the use of bisphosphonates after metastasis were all associated with OS. These parameters have previously been associated with OS, not only in prostate cancer but in a range of other solid tumors [20, 21]. Interestingly, our current analysis did not reveal a significant association between Gleason score and OS, despite such an association having been suggested in some, but not all, prior studies [2, 22, 23]. This suggests that in a group of patients who have already developed metastases, Gleason grade may no longer be as relevant. Finally, our analysis did not show an independent association between PSADT and OS after accounting for other covariates (including MFS), perhaps due to the close interconnection between PSADT and MFS [4].
It could be argued that the primary end point for trials targeting men with biochemically recurrent prostate cancer should have three characteristics, namely it should be: (i) clinically meaningful, (ii) occur in a reasonable time frame, and (iii) correlate with OS. It appears that MFS may fulfill all of these criteria. With the inherent pitfalls in designing studies in these patients, we need to be more thoughtful in choosing our end points. Investing in large phase III trials only to demonstrate that an intervention leads to favorable changes in PSA kinetics is not going to provide a great deal of clarity on how to manage these men, and it would therefore seem prudent to design future trials around more meaningful end point such as MFS. The current study is only the first step in potentially establishing MFS as a reasonable intermediate end point in such trials, and clearly falls short of satisfying surrogacy criteria.
This study has several shortcomings. Despite this being the largest study of its kind, the sample size remains relatively small, with only 140 men developing metastasis and only 78 deaths. These small patient numbers have led to a relatively low precision when estimating hazard ratios, as evidenced by wide CIs (see Table 3). Secondly, because of the retrospective nature of this analysis, patients had treatment deferred until the time of overt metastasis in a nonrandom fashion. This could potentially lead to exclusion of patients with more aggressive disease. Furthermore, given that the management of these patients was not prospectively defined, variability in follow-up and the utilization of diagnostics (i.e. CT and bone scans) was surely present. PSA changes most likely led to imaging in many patients, which in turn may have led to detection bias. In addition, the number of non-Caucasian men included in the analysis is much lower than the true racial mix of patients with prostate cancer and may not be reflective of the general US population [24]. Last, this study is based on a single-institution experience and needs validation across multiple centers, ideally in a prospective manner.
In conclusion, using a cohort of men with biochemically recurrent prostate cancer following prostatectomy treated with deferred ADT (initiated after metastasis), we have shown that MFS is significantly associated with OS after adjusting for other known prognostic variables. This suggests that MFS may be a reasonable proximal end point when evaluating novel agents in men with PSA recurrence after local therapy, and provides the impetus to validate this potential intermediate end point in prospective clinical trials.
funding
This work was partially funded by grants P30 CA006973 and P50 CA058236.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors acknowledge Bruce Trock for helpful discussions and revisions on the manuscript.
references
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J Urol. 2007;178:S20–S24. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 7.Tenenholz TC, Shields C, Ramesh VR, et al. Survival benefit for early hormone ablation in biochemically recurrent prostate cancer. Urol Oncol. 2007;25:101–109. doi: 10.1016/j.urolonc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarov DV, Humphreys EB, Mangold LA, et al. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161. doi: 10.1016/j.juro.2007.08.133. discussion 161. –152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2010;57:49–59. doi: 10.1016/j.eururo.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Yu EY, Gulati R, Telesca D, et al. Duration of first off-treatment interval is prognostic for time to castration resistance and death in men with biochemical relapse of prostate cancer treated on a prospective trial of intermittent androgen deprivation. J Clin Oncol. 2010;28:2668–2673. doi: 10.1200/JCO.2009.25.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Eisenberger M, D'Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 15.Antonarakis ES, Zahurak ML, Lin J, et al. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 2012;118:1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 17.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–270. [Google Scholar]
- 18.Williams BA, Mandrekar JN, Mandrekar SJ, et al. Technical Reports, edition. Rochester, MN: Department of Health Science Research, Mayo Clinic; 2006. Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-Event Outcomes; pp. 1–26. 79 (ed) [Google Scholar]
- 19.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Garrett-Mayer ES, Yang YC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 21.Gripp S, Moeller S, Bolke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–3320. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 22.Kim-Sing C, Pickles T. Intervention after PSA failure: examination of intervention time and subsequent outcomes from a prospective patient database. Int J Radiat Oncol Biol Phys. 2004;60:463–469. doi: 10.1016/j.ijrobp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Tollefson MK, Slezak JM, Leibovich BC, et al. Stratification of patient risk based on prostate-specific antigen doubling time after radical retropubic prostatectomy. Mayo Clin Proc. 2007;82:422–427. doi: 10.4065/82.4.422. [DOI] [PubMed] [Google Scholar]
- 24.Platz EA, Rimm EB, Willett WC, et al. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000;92:2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.