Abstract
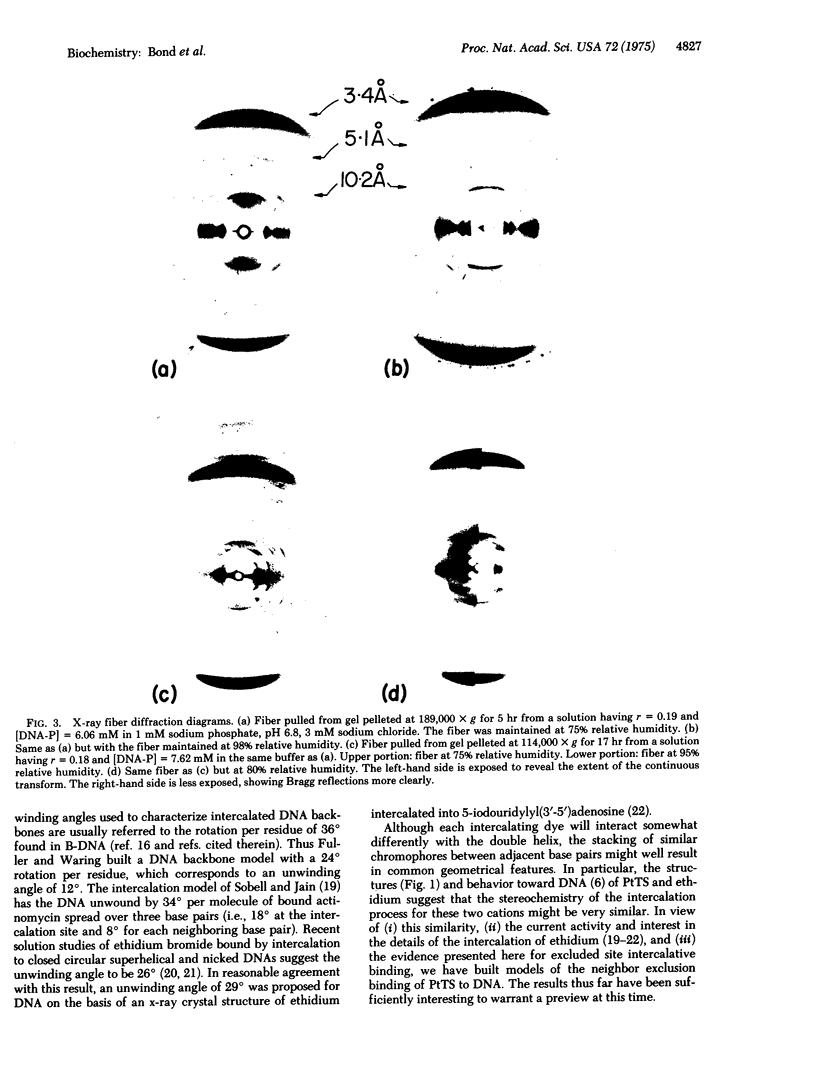
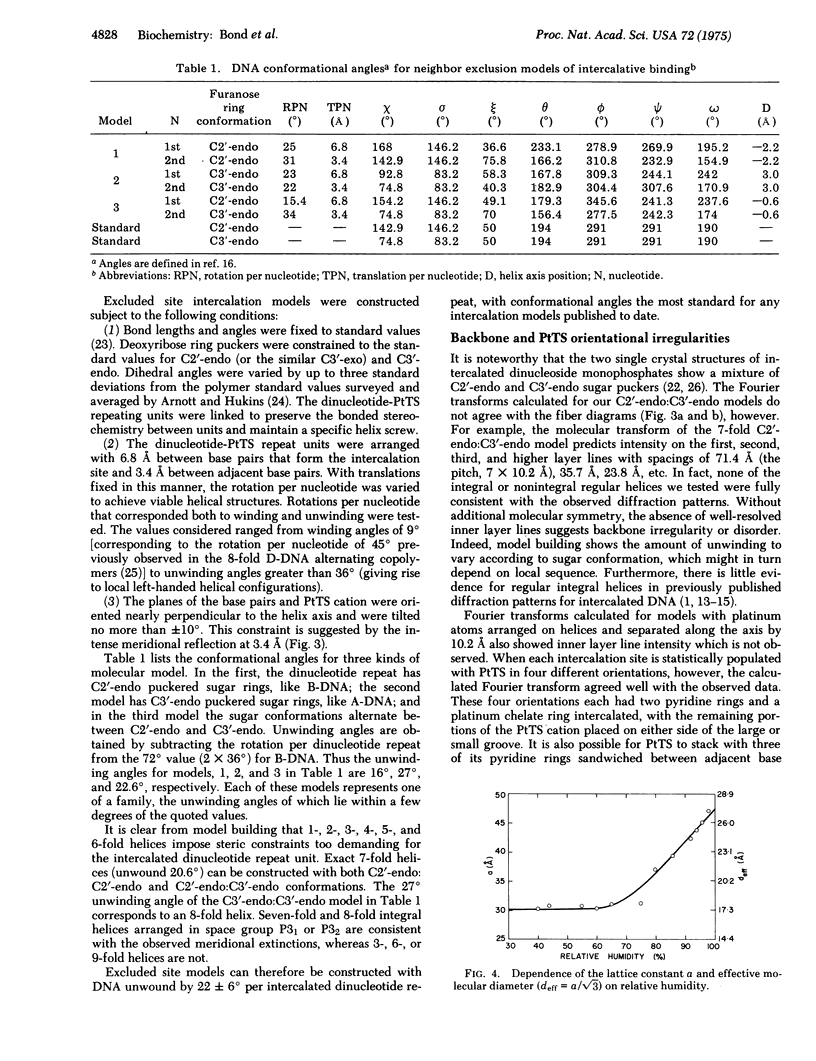
Good quality x-ray diffraction patterns have been obtained of polycrystalline fibers containing 2-hydroxyethanethiolato(2,2'2"-terpyridine)platinum(II) bound to calf thymus DNA by intercalation. The photographs strongly support the neighbor exclusion binding model in which electron-dense platinum atoms are regularly distributed at 10.2 A intervals, every other interbase pair site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hukins D. W., Smith P. J., Watts L. Structural details of double-helix observed for DNAs containing alternating purine and pyrimidine sequences. J Mol Biol. 1974 Sep 15;88(2):523–533. doi: 10.1016/0022-2836(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Conservation of conformation in mono and poly-nucleotides. Nature. 1969 Nov 29;224(5222):886–888. doi: 10.1038/224886a0. [DOI] [PubMed] [Google Scholar]
- Arnott S. The geometry of nucleic acids. Prog Biophys Mol Biol. 1970;21:265–319. doi: 10.1016/0079-6107(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The application of autoradiography to the study of DNA viruses. Cold Spring Harb Symp Quant Biol. 1962;27:311–318. doi: 10.1101/sqb.1962.027.001.029. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Jennette K. W., Lippard S. J., Vassiliades G. A., Bauer W. R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2',2'-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3839–3843. doi: 10.1073/pnas.71.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Davies D. R. The interaction of acridine dyes with DNA: an x-ray diffraction and optical investigation. J Mol Biol. 1966 May;17(1):57–74. doi: 10.1016/s0022-2836(66)80094-3. [DOI] [PubMed] [Google Scholar]
- Pigram W. J., Fuller W., Hamilton L. D. Stereochemistry of intercalation: interaction of daunomycin with DNA. Nat New Biol. 1972 Jan 5;235(53):17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Day R. O., Rich A. Nucleic acid-mutagen interactions: crystal structure of adenylyl-3',5'-uridine plus 9-aminoacridine. Nature. 1975 Jan 31;253(5490):324–327. doi: 10.1038/253324a0. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C. Stereochemistry of actinomycin binding to DNA. II. Detailed molecular model of actinomycin-DNA complex and its implications. J Mol Biol. 1972 Jul 14;68(1):21–34. doi: 10.1016/0022-2836(72)90259-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. X-ray crystallographic visualization of drug-nucleic acid intercalative binding: structure of an ethidium-dinucleoside monophosphate crystalline complex, Ethidium: 5-iodouridylyl (3'-5') adenosine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):628–632. doi: 10.1073/pnas.72.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARING M. J. COMPLEX FORMATION WITH DNA AND INHIBITION OF ESCHERICHIA COLI RNA POLYMERASE BY ETHIDIUM BROMIDE. Biochim Biophys Acta. 1964 Jun 22;87:358–361. doi: 10.1016/0926-6550(64)90238-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Zasedatelev A. S., Gurskii G. V., Vol'kenshtein M. V. Theory of one-dimensional adsorption. I. Adsorption of small molecules on a homopolymer. Mol Biol. 1971 Mar-Apr;5(2):194–198. [PubMed] [Google Scholar]