Abstract
Purpose
This study aimed at examining the relationship between quality of life (QOL) in prostate cancer (PCa) patients and partners and how baseline demographics, cancer-related factors, and time-varying psychosocial and symptom covariates affect their QOL over time.
Methods
Guided by a modified Stress-Coping Model, this study used multilevel modeling to analyze longitudinal data from a randomized clinical trial that tested a family-based intervention to improve QOL in couples managing PCa. Patients and partners from the usual-care control group (N = 134 dyads) independently completed the measurements at baseline, and at 4-, 8-, and 12-month follow-ups.
Results
Correlations of QOL between patients and partners over time were small to moderate. Patients’ lower education level, partners’ older age, higher family income, and localized cancer at baseline were associated with better QOL in couples. Over time, couples’ QOL improved as their social support and cancer-related dyadic communication increased and as couples’ uncertainty, general symptoms, and patients’ prostate cancer-related sexual and hormonal symptoms decreased.
Conclusions
Evidence indicates that couples’ QOL during cancer survivorship is affected by multiple contextual factors (e.g., baseline demographics and time-varying psychosocial factors and symptoms). Intervention research is needed to explore comprehensive strategies to improve couples’ QOL during the continuum of PCa survivorship.
Keywords: Prostate cancer, Symptom, Family, Quality of life, Multilevel model, Communication, Uncertainty
Introduction
The diagnosis of prostate cancer (PCa) represents a major threat to the patient and his family members [1, 2]. For patients who are married or in an intimate relationship, PCa and treatment-related side effects (e.g., impotence and incontinence) affect that intimate relationship [3] and cause declines in both partners’ quality of life (QOL) [4–7]. Yet, limited research has examined the interdependence of partners’ experiences as they manage the demands of PCa [8–10]. Separate investigation into isolated perceptions of individuals (often the patient) fails to provide reference to the interaction in the family system [11].
Few studies have examined couples’ QOL trajectory while considering their responses to the impact of PCa over time. According to Lewis, cancer “is not a single stressful event, [but] rather is characterized as a series of multiple, interwoven, and layered psychosocial transitions” [12] during which a family (e.g., a couple) constantly modifies its adaptation efforts [13]. Examination of couples’ cancer experiences and QOL in a longitudinal context, i.e., during the continual psychosocial adaptation process, is essential to identification of strategies to maximize their well-being. Kershaw et al. [8] examined the longitudinal effects of psychosocial variables on couples’ QOL (all variables were measured at one point in time). Building on their findings, we examined how selected variables measured at four data points affected the patterns of change in couples’ QOL over time.
This study aimed at examining (1) whether the QOL of PCa patients and partners was related over time and (2) whether selected baseline and time-varying demographics, psychosocial, and cancer-related factors affected couple’s QOL over time. The selection of factors was based on a theoretical framework adapted from the modified Stress-Coping Model [14]. Integrated with family systems theory [13, 15], this model conceptualizes the couple as a unit and contends that the stress and demands of cancer affects both patients and partners. It also suggests that personal, family/social, and cancer-related factors affect couples’ QOL.
Literature review
QOL in PCa patients and their partners
QOL is a multidimensional construct that includes physical, emotional, functional, and social well-being. Prostate cancer and its treatment often introduce symptoms and difficulties that affect different aspects of QOL in men and their spouses [4, 9, 10, 16]. The QOL of patient and partner may be related, with partners sometimes reporting lower QOL or more psychological distress than patients, especially partners of men with localized [17] and advanced PCa [10, 18].
Most research examining QOL of patients with PCa and partners is retrospective and/or cross-sectional. Such designs overlook the fact that cancer involves a continual process of mapping the illness and its changing contingencies onto a family’s operations [13]. Family members become involved and come to personal terms with the meaning of cancer during constant cognitive-emotional transitions [12]. Research with a longitudinal perspective is needed to concurrently examine partners’ QOL while accounting for the effects of contextual factors (e.g., time-varying physical–psychosocial factors) during the continuum of cancer survivorship.
Factors affecting QOL in PCa patients and partners
Among demographic factors, younger age in patients was associated with better physical QOL [19], but with worsening mental well-being over time [16, 19]. Younger partners also reported better physical QOL [8]. Middle-aged couples experienced more PCa-related distress than older couples [20]. Higher household income and education were related to higher QOL in patients [19].
Some psychosocial factors have been related to QOL in PCa patients and partners. Social support positively affected patients’ QOL directly and indirectly in cross-sectional and longitudinal studies [8, 21–23]. Among spouses, less social support was associated with more distress [23, 24] but baseline social support did not predict their QOL 8 months later [8], suggesting that couples may have different support needs at different phases of survivorship.
Open communication, verbal exchange of cancer-related information and personal experiences between patients and partners, was related to QOL. Couples facing heterogeneous cancers (e.g., prostate and breast) who openly communicated about the illness reported better adjustment [25, 26]. Inadequate communication (e.g., hiding feelings), conversely, was associated with lower QOL and more distress in both partners [24, 27, 28].
Uncertainty about the illness was associated with lower QOL [29] and poorer psychological adjustment in patients and spouses in cross-sectional studies [29, 30]. In a recent study, however, baseline uncertainty reported by PCa patients and spouses did not predict their QOL 8 months later [8]. Such findings may suggest that the relationship between uncertainty and QOL is concurrent rather than long-lasting as couples experience different uncertainty at different phases of survivorship.
The relationships between QOL and cancer-related factors are often examined without concurrently accounting for the effects of psychosocial factors. The QOL of patients with localized PCa usually declines initially but improves over time [31–33], whereas QOL of patients with advanced cancer continues to decline, especially in the emotional and social domains [16, 34–36].
The effect of time since diagnosis on QOL has been mixed. Earlier research showed QOL of PCa survivors was more likely to decline with time when compared to patients with colorectal and lung cancers [37]. These effects were independent of the cancer stage [35] and/or the type of treatment patients received [31, 32]. Research on QOL of healthy spouses is limited and mostly cross-sectional [38, 39]. Their psychological well-being typically improves over time [40].
Finally, PCa-related symptoms and general symptoms were associated with couples’ QOL [8]. Patients with more symptoms and deteriorating physical function experienced more problems in adaptation [9]. Different types of comorbidities (e.g., physical vs psychological) had different effects on men’s QOL [19, 37, 41, 42]. Spouses’ distress was highly predicted by patient’s urinary and bowel dysfunction and diminished mental health [9, 24].
Methods
This research is a secondary analysis of longitudinal data from a randomized clinical trial (RCT) testing a family-based, nursing intervention to improve QOL for couples managing PCa. To eliminate the intervention effects on study variables, only dyads of patients and partners from the usual-care control group were included. The sampling and data collection procedures [10, 43, 44] and the subject-tracking consort table [44] were published previously. Regarding the three phases of illness, patients were eligible only if they were 2–4 months after: completion of primary treatment (newly diagnosed localized), or two consecutive rises in their PSA post-primary treatment (biochemical recurrence), or diagnosis of metastatic disease or progression of advanced disease (advanced). This limited window of eligibility was used to reduce heterogeneity by identifying couples within each phase who were dealing with similar phase-related issues [10]. Patients and partners independently completed all measurements.
Measures
Two types of factors were included: (1) time-invariant factors obtained at baseline: role (patient or partner), age, education, family income, time since diagnosis, and phase of cancer (localized, biochemical recurrence, or advanced); and (2) time-varying factors that were measured at baseline and at 4-, 8-, and 12-month follow-ups (QOL, social support, open dyadic communication about cancer, uncertainty about the illness, and symptom distress).
Quality of Life, the outcome variable, was measured using the 27-item Functional Assessment of Chronic Illness Therapy general scale (FACT-G) [45]. It measures general QOL in physical, social/family, emotional, and functional domains [46]. The total score was used. Spouses reported their own QOL using the spouse’s version of FACT-G with slightly modified wording [47]. Cronbach’s alpha was .90 for both patients and partners at baseline in this study.
Demographics, including age, education, and family income, were obtained using the demographic section of the Risk for Distress scale (RFD, previously known as Omega Screening Questionnaire [OSQ]) [14, 48]. Among psychosocial factors, social support was assessed using the Personal Resource Questionnaire (PRQ) [49], a 15-item measure using Likert response scales across items. The PRQ measures the amount of general social support from others (e.g., friends and relatives). The baseline Cronbach’s alpha was .89 and .90 for patients and partners, respectively. Open dyadic cancer-related communication was measured using the Lewis Mutuality and Interpersonal Sensitivity Scale (MIS) [50]. This 23-item scale assesses each partner’s perception of extent to which they share cancer-related issues with one another. The baseline Cronbach’s alpha was .90 and .91 for patients and partners, respectively. Uncertainty about the illness was measured by the 28-item Mishel Uncertainty in Illness Scale (MUIS) [51]. Its baseline Cronbach’s alpha was .91 for both partners.
Among cancer-related factors, time since diagnosis and phase of illness were obtained from patients’ medical records. Symptom distress included PCa-specific symptoms and general symptoms. PCa-specific symptoms in patients (i.e., bowel, hormonal, sexual, or urinary symptoms) were measured using the 50-item Expanded PCa Index Composite (EPIC) that assesses four symptom subscales [52]. The baseline Cronbach’s alpha for these subscales ranged from .74 to .90 for patients. Partners completed a four-item EPIC spousal version, which assessed how much of a problem their husbands’ symptoms were for them. General symptoms (e.g., fatigue, pain, and insomnia) were measured with the 16-item Symptom Scale, a subscale of the RFD [48]. Patients and partners separately rated their own symptoms; the baseline Cronbach’s alpha was .80 and .76 for patients and partners, respectively.
Data analysis
Within-couple differences of demographic characteristics were assessed with paired t tests for continuous variables and McNemar’s test for categorical variables. Multilevel modeling (MLM) (i.e., SAS 9.2 PROC MIXED) [53] for longitudinal data was used to achieve the research aims. Compared to conventional multivariate methods, the MLM method uses variables measured at multiple points in time, which allows the examination of patterns of change in couples’ QOL while controlling for time-varying and time-invariant covariates. It also has the advantage of handling missing data: partial data are not discarded, nor is it necessary to impute values for missing outcomes [54].
In each model, the fixed effects were estimated for all predictors to describe marginal relationships between QOL and each predictor; the random effects described random deviations of a given subject or cluster from the overall fixed effects at three levels: intra-personal, intra-couple, and inter-couple [54]. Time was calculated as the months since diagnosis at baseline plus the months of the follow-up interviews, i.e., 0, 4, 8, and 12, respectively. Both the linear and quadratic effects of time were included in model fitting to capture possible curvilinear patterns of QOL over time.
To improve interpretation and reduce potential multicollinearity [55], continuous predictors (e.g., month since diagnosis, age, education, uncertainty, social support, communication, and general symptoms) were group-centered by subtracting the group mean of patients (or partners) across time from each observed score. EPIC measures for patients and partners were standardized within individuals across time to control for their sizeable discrepancy. The categorical variables were coded with the value of reference group as 0.
A series of models were fitted. First, in order to guide the specification of the combined model for couples, separate QOL models for patients and partners were fitted as a function of measurements of their own predictors. These models examined how their QOL was affected as individuals over time. Second, a combined full model of couples’ QOL was fitted to examine how QOL of patients and partners was related while accounting for contextual factors of survivorship. To ensure inclusiveness, variables with P values ≤ .10 in the separate models were selected as covariates for the full model. The interactions between role, phase of illness, and time were retained, whenever statistically significant, to examine whether couples’ QOL varied by role and phase of illness over time. Due to consideration of power and the available sample size, interactions between role and other predictors were excluded. Finally, the parsimonious combined model was fitted by eliminating non-significant interactions and predictors in the full model. Competing models were compared using the Akaike information criterion (AIC), Bayesian information criterion (BIC), and the likelihood ratio test (LRT) [54].
Results
Demographics and clinical characteristics
In the control group, of the 134 patient–partner dyads that completed the baseline assessment, 124, 123, and 114 completed the assessments at 4-, 8-, and 12-month follow-ups, respectively. Over 83% of the patients and partners were white, 13% were African Americans, and 4% from other racial groups. Patients were diagnosed with localized (65%), biochemical recurrent (12%), or advanced (23%) cancer. Patients’ mean months since diagnosis at baseline was 28.96 (SD = 39.66) months. Table 1 lists other characteristics of participants, and Table 2 displays descriptive analysis results of QOL and predictors by time.
Table 1.
Descriptive statistics for research participants at baseline
| Characteristics | Patient (N = 134) | Spouse (N = 134) | P |
|---|---|---|---|
| Mean age (year) (SD, range) | 62.57 (9.22, 42–90) | 58.92 (9.65, 34–84) | <.001* |
| Mean education (year) (SD, range) | 16.13 (3.63, 8–29) | 14.68 (2.68, 8–22) | <.001* |
| Mean years of marriage (SD, range) | 31.75 (14.26, .33–65) | – | |
| Family income (%) | |||
| <$30,000 | 6.5 | – | |
| $30,001–$50,000 | 22.0 | ||
| $50,001–$75,000 | 18.7 | ||
| >$75, 001 | 52.8 | ||
| % Presently working | .01** | ||
| No | 54.5 | 42.5 | |
| Yes | 45.5 | 57.5 | |
| % Having other health problems | .17** | ||
| No | 41.8 | 50.7 | |
| Yes | 58.2 | 49.3 |
by paired t test
by McNemar’s test
Table 2.
Descriptive analysis results
| Variables | Baseline | 4-month follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Partner | Patient | Partner | |||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Quality of life1 | 134 | 85.20 | 13.09 | 134 | 85.63 | 11.87 | 124 | 84.95 | 12.39 | 124 | 83.82 | 13.07 |
| Social support1 | 134 | 88.18 | 12.12 | 134 | 85.24 | 14.25 | 124 | 86.69 | 13.13 | 124 | 84.52 | 15.12 |
| Uncertainty2 | 134 | 60.54 | 15.53 | 134 | 61.84 | 16.05 | 124 | 60.31 | 17.16 | 124 | 62.90 | 16.91 |
| Levels of dyadic communication1 | 130 | 3.72 | .66 | 132 | 3.70 | .63 | 120 | 3.63 | .73 | 122 | 3.51 | .78 |
| Pca-specific symptoms_urine1 | 133 | 77.29 | 16.01 | 134 | 4.08 | 1.18 | 124 | 81.19 | 15.85 | 124 | 4.18 | 1.13 |
| Pca-specific symptoms_bowel1 | 131 | 88.71 | 12.01 | 134 | 4.58 | .87 | 122 | 90.05 | 12.73 | 124 | 4.65 | .82 |
| Pca-specific symptoms_sexual1 | 130 | 26.76 | 21.92 | 132 | 3.30 | 1.49 | 123 | 29.13 | 23.04 | 123 | 3.16 | 1.60 |
| PCa-specific symptoms_hormonal1 | 134 | 82.16 | 15.01 | 134 | 4.08 | 1.19 | 124 | 83.09 | 15.13 | 124 | 4.07 | 1.27 |
| General symptom score2 | 134 | 7.07 | 4.35 | 134 | 5.50 | 3.82 | 124 | 6.43 | 4.24 | 124 | 6.23 | 4.48 |
| Variables | 8-month follow-up | 12-month follow-up | F ratio (df=3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Partner | Patient | Partner | Patient | Partner | |||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | Time Effect | Time Effect | |
| Quality of life1 | 122 | 86.54 | 12.83 | 122 | 83.85 | 14.27 | 114 | 86.52 | 12.60 | 119 | 84.01 | 14.93 | .54 | .54 |
| Social support1 | 122 | 86.99 | 12.02 | 122 | 83.98 | 14.41 | 114 | 87.34 | 13.50 | 119 | 84.10 | 16.05 | .34 | .19 |
| Uncertainty2 | 122 | 58.78 | 14.91 | 121 | 61.66 | 16.98 | 114 | 57.08 | 15.62 | 114 | 59.95 | 16.69 | 1.23 | .63 |
| Levels of dyadic communication1 | 119 | 3.73 | .64 | 120 | 3.50 | .73 | 111 | 3.64 | .68 | 116 | 3.45 | .76 | .77 | 2.93* |
| Pca-specific symptoms_urine1 | 120 | 82.79 | 14.15 | 121 | 4.22 | 1.10 | 113 | 83.57 | 13.93 | 114 | 4.30 | 1.05 | 4.34** | .81 |
| Pca-specific symptoms_bowel1 | 120 | 89.96 | 12.39 | 121 | 4.62 | .82 | 112 | 90.63 | 11.13 | 114 | 4.61 | .82 | .55 | .16 |
| Pca-specific symptoms_sexual1 | 119 | 29.53 | 23.65 | 120 | 3.23 | 1.57 | 112 | 32.43 | 24.49 | 112 | 3.21 | 1.58 | 1.20 | .18 |
| PCa-specific symptoms_hormo1 | 122 | 85.02 | 14.53 | 120 | 4.12 | 1.20 | 111 | 85.71 | 13.75 | 114 | 4.07 | 1.17 | 1.55 | .04 |
| General symptom score2 | 122 | 6.30 | 4.55 | 122 | 6.19 | 4.31 | 114 | 5.82 | 4.08 | 119 | 6.04 | 4.55 | 1.76 | .81 |
Higher scores indicate more positive results: better QOL, more communication, more support from others, and less Pca-specific symptoms
Higher scores indicate more negative results: more uncertainty about the illness and more symptom distress such as fatigue, nausea, and difficulty sleeping
P < .05;
P < .001
The main reasons for attrition were patients’ death, lost contacts, or couples’ lack of time. There were no significant differences in the demographics and months since diagnosis between patients who withdrew and those who completed the study. However, more patients with advanced cancer were lost from the study than patients with other phases of illness (χ2 = 18.002, df = 2, P <.001). For partners, there were no significant differences between respondents who were retained and those who withdrew from the study.
Results of separate models (Table 3)
Table 3.
Separate models of QOL for patients and partners
| Effect | Patients (df = 186)* | Partners (df = 195)* | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | Pr > |t| | Estimate | SE | Pr > |t| | |
| Intercept | 79.89 | 2.55 | <.0001 | 72.71 | 3.49 | <.0001 |
| Age | 0.18 | 0.05 | 0.0008 | 0.21 | 0.07 | 0.0019 |
| Education | −0.31 | 0.12 | 0.0119 | −0.47 | 0.25 | 0.0627 |
| Family income | 0.80 | 0.48 | 0.0971 | 1.68 | 0.65 | 0.0108 |
| Uncertainty2 | −0.21 | 0.03 | <.0001 | −0.15 | 0.03 | <.0001 |
| Social support1 | 0.21 | 0.03 | <.0001 | 0.15 | 0.03 | <.0001 |
| Communication1 | 3.65 | 0.57 | <.0001 | 4.64 | 0.68 | <.0001 |
| Time since diagnosis | −0.006 | 0.04 | 0.9064 | 0.002 | 0.04 | 0.9607 |
| Time since diagnosis_sq | −0.0004 | 0.0004 | 0.3951 | 0.00005 | 0.0004 | 0.9020 |
| Phase of illness (referent: advanced) | ||||||
| Localized | 1.81 | 1.43 | 0.2083 | 3.42 | 1.87 | 0.0683 |
| Recurrent | 5.59 | 2.22 | 0.0128 | 4.80 | 2.62 | 0.0684 |
| General symptoms2 | −1.02 | 0.12 | <.0001 | −1.33 | 0.11 | <.0001 |
| Pca3 symptoms_urine1 | 0.11 | 0.40 | 0.7775 | 0.44 | 0.40 | 0.2742 |
| Pca3 symptoms_bowel1 | −0.05 | 0.35 | 0.8836 | −0.61 | 0.41 | 0.1416 |
| Pca3 symptoms_sexual1 | 0.52 | 0.19 | 0.0067 | −0.31 | 0.34 | 0.3677 |
| Pca3 symptoms_hormonal1 | 1.98 | 0.44 | <.0001 | 0.48 | 0.41 | 0.2457 |
Bold numbers are significant findings
Separate models of QOL for patients and partners were fitted as a function of measurements of their own predictors
Sample sizes of patients and partners differ due to different attrition rates, 9 partners of patients who dropped out of the study remained in the study
Higher scores indicate more positive results: more support from others, more communication, and less prostate cancer-specific symptoms
Higher scores indicate more negative results: more uncertainty about the illness and more symptom distress such as pain, fatigue, and insomnia
Prostate cancer-specific
The results of the separate model for patients indicated that better patients’ QOL was associated with their older age (P < .001), less education (P < .05), and having biochemical recurrent cancer (P < .05) (rather than advanced cancer). Patients’ QOL improved with an increase in their social support (P < .001) and their open communication with partners (P < .001), and with a decrease in their uncertainty (P < .001), general symptoms (P < .001), and PCa-specific sexual (P < .01) and hormonal symptoms (P < .001). Time effects (linear or quadratic) were non-significant, suggesting that patient’s QOL was relatively stable over time, when controlling for all covariates.
The results of the model for partners indicated that better QOL in partners was associated with older age (P < .001) and higher family income (P < .05). QOL of partners improved as their social support (P < .001) and open communication (P < .001) increased and as their own uncertainty (P < .001) and general symptoms (P < .001) decreased. Partner’s QOL, like patient’s, did not change over time, when holding other covariates constant.
Results of combined models (Table 4)
Table 4.
Combined models of QOL for couples
| Effect | Full model | Final model* | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | Pr > |t| | Estimate | SE | Pr > |t| | |
| Intercept | 76.78 | 2.70 | <.0001 | 76.90 | 2.37 | <.0001 |
| Role (referent: partner): patient | 1.63 | 1.38 | 0.2395 | 1.40 | 0.66 | 0.0345 |
| Partner age | 0.21 | 0.08 | 0.0095 | 0.17 | 0.04 | <.0001 |
| Patient education | −0.33 | 0.13 | 0.0113 | −0.36 | 0.12 | 0.0031 |
| Patient age | −0.05 | 0.09 | 0.5776 | |||
| Partner education | −0.09 | 0.19 | 0.6389 | |||
| Family income | 1.00 | 0.49 | 0.0422 | 1.00 | 0.45 | 0.0281 |
| Uncertainty | −0.19 | 0.02 | <.0001 | −0.19 | 0.02 | <.0001 |
| Social support | 0.19 | 0.02 | <.0001 | 0.19 | 0.02 | <.0001 |
| Open dyadic communication | 4.13 | 0.46 | <.0001 | 4.15 | 0.46 | <.0001 |
| Phase of illness (referent: advanced) | ||||||
| Localized cancer | 2.49 | 1.32 | 0.0587 | 2.37 | 1.03 | 0.0223 |
| Recurrent cancer | 4.50 | 1.88 | 0.0168 | 4.27 | 1.46 | 0.0035 |
| General symptoms | −1.26 | 0.07 | <.0001 | −1.26 | 0.07 | <.0001 |
| Pca symptoms_sexual | 0.31 | 0.16 | 0.0461 | 0.32 | 0.16 | 0.0441 |
| Pca symptoms_hormonal | 1.05 | 0.30 | 0.0004 | 1.05 | 0.29 | 0.0004 |
| Role* phase of illness (referent: patient* advanced & partner* all phases | ||||||
| Patient* localized | −0.27 | 1.61 | 0.8679 | |||
| Patient* recurrent | 0.47 | 2.40 | 0.8448 | |||
| −2 log likelihood | 5666.2 | 5664.8 | ||||
| AIC** | 5672.2 | 5670.2 | ||||
| BIC** | 5682.7 | 5681.3 | ||||
Bold numbers are significant findings
df = 601
Although couples with missing data tended to have advanced cancer, fixed effects of the variables of interest did not change after controlling for missing data
Smaller is better when comparing two models
In the combined full model, the majority of above-mentioned predictors, except patient’s age and partner’s education level, remained statistically significant. The interactions between role and phase were non-significant, suggesting that the effects of phase of illness did not vary by role. The role effect became significant (P < .05) after eliminating the role–phase interactions and, thus, was retained to compare the differences in QOL between patients and partners.
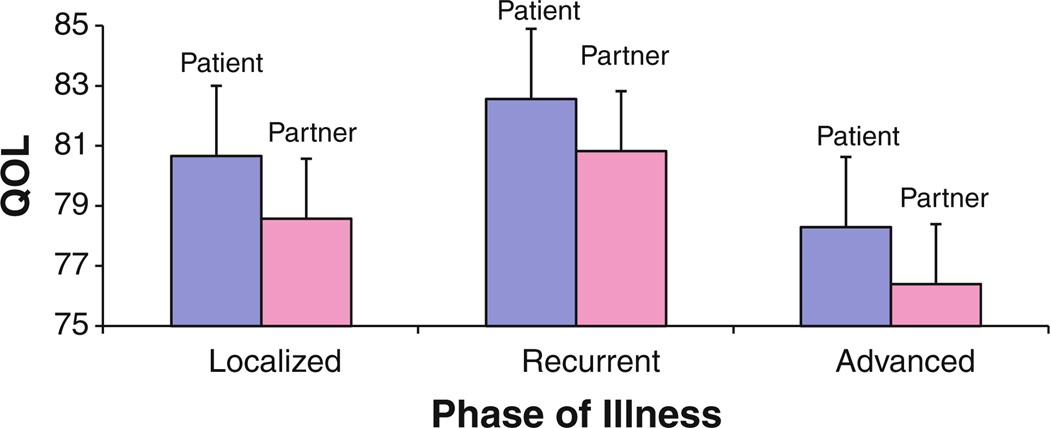
The combined final model, containing all the statistically significant variables in the combined full model, indicated that couples’ QOL was associated with baseline variables such as role (patient vs partner) (P < .05), localized (P < .01) or recurrent cancer (P < .05), partner’s age (P < .001), patient’s education (P < .01), and family income (P < .05). As shown in Fig. 1, when holding other covariates constant, QOL of patients is about 1.4 points higher than that of their partners. This relationship holds for couples facing different phases of illness. Higher family incomes and lower education in patients also predicted better QOL in couples.
Fig. 1.
Estimated differences in QOL scores between patients and partners facing different phases of cancer. The results were obtained by holding all other covariates constant. (Note: Higher scores indicate better QOL)
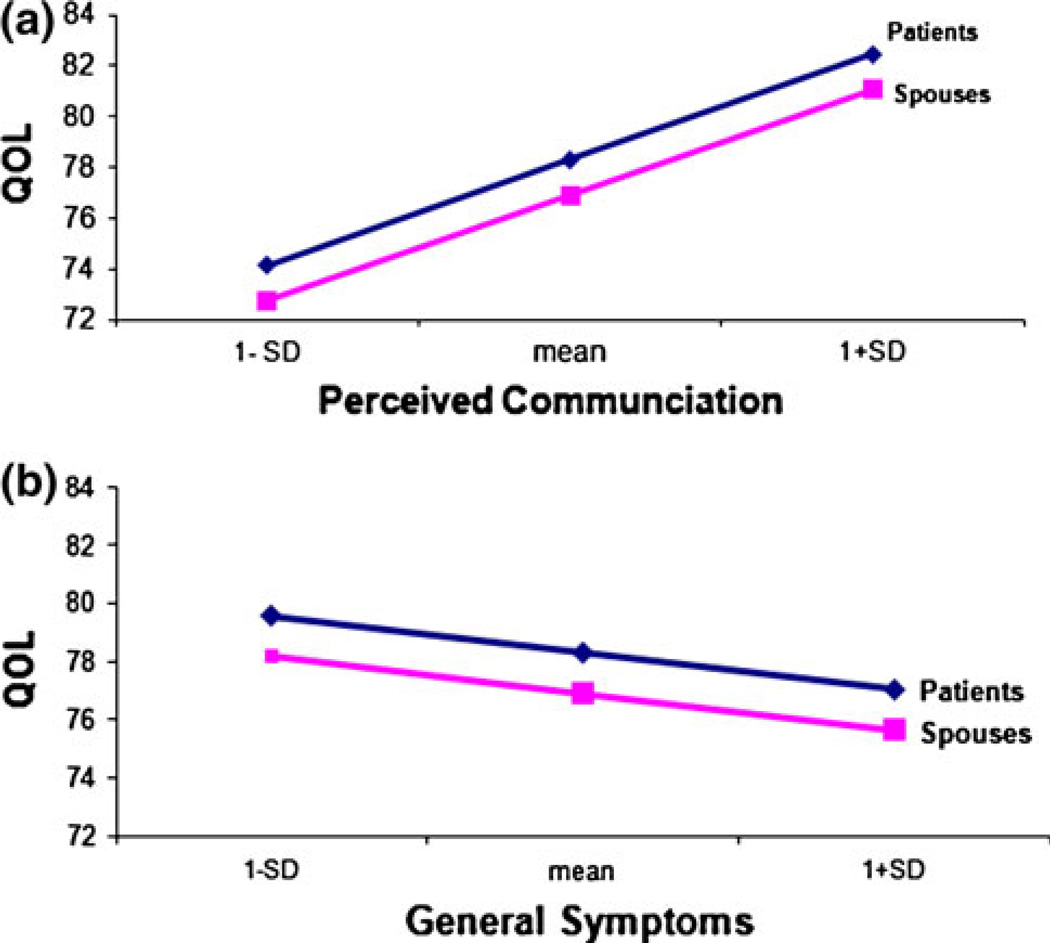
Couples’ QOL was also associated with time-varying factors. Specifically, couples’ QOL improved with an increase in their social support (P < .001) and open communication (P < .001), and with a decrease in their uncertainty (P < .001), PCa-specific hormonal (P < .001) and sexual symptoms (P < .05) in the patient, and general symptoms (P < .001) in both partners. Figure 2 demonstrates some of these results, e.g., when holding other covariates constant, QOL scores for couples increased by about 4.2 points when their open communication scores increased by one standard deviation.
Fig. 2.
Estimated trajectories of changes in patients’ and partners’ QOL. a As dyadic communication about cancer-related issues between patients and partners increased, their QOL improved. b As the general symptoms in patients and partners increased, their QOL decreased. (Note: Higher score of the general symptom scale suggested more symptoms such as pain and insomnia)
Regarding model integrity evaluation, the AIC and BIC statistics (Table 4) supported the final model. The likelihood ratio test (χ2 = 1.4, df = 4, P = .84, Nobservation = 871) further indicated the fit of the final model was as good as that of the full model. Yet, it is more parsimonious, i.e., with fewer predictors, than the full model.
The correlation coefficients between QOL in patients and partners, after controlling for the covariates, were .25, .24, .23, and .23 at baseline, 4-, 8-, and 12-month follow-ups, respectively. These results indicate small to moderate correlations of QOL between patients and partners that remained consistent during PCa survivorship.
Discussion
One of the important contributions of this study is that it examined how the QOL in PCa patients correlated with the QOL of their partners while taking into consideration both partners’ baseline and time-varying demographic, cancer-related, and psychosocial variables. Different from previous studies using measures at one time point to model QOL of patients and partners separately (e.g., Kershaw et al. [8]), we used a more comprehensive analytical method, i.e., MLM, to examine how QOL of patients and partners measured at four data points correlated. We evaluated both the lag effects of baseline demographic and cancer-related factors, and the concurrent effects of time-varying psychosocial factors on QOL. The effects of time were non-significant after controlling for time-varying antecedents, suggesting that the patterns of change in couple’s QOL over time are a function of survivorship context (e.g., the challenges and resources couples have at different phases of survivorship), rather than the time variable itself. These results provide evidence that otherwise might have gone undetected or misrepresented by traditional analytical methods, e.g., repeated-measure ANOVA [54, 55].
This study expanded our knowledge about the complexity of cancer experience in the context of family. Our findings of small to moderate correlations of QOL between PCa patients and partners, after accounting for the effects of covariates, are consistent with the results of a recent meta-analysis of couples’ distress during cancer survivorship [56]. In our study, the QOL scores of patients, similar to those reported in earlier research using FACT-G [36], were better than those of their partners. These results suggest that partners are “team members” in managing PCa, underscoring the interdependence within couples as they cope with the demands of cancer [57]. Such evidence alerts clinicians to the need for caring for the health of the spouse, who often acts as the primary caregiver for patients by providing major emotional and tangible support on a daily basis [58]. However, the possible influence of gender on participants’ responses to the QOL measure cannot be ruled out. Significant gender differences in reporting distress were found in couples adjusting to colon cancer, a non-gender-specific illness [59, 60].
Certain baseline demographics and time-varying physical and psychosocial variables affected couples’ QOL. Consistent with the conclusions of a prior meta-analysis based on 33 studies [61], the association between couples’ perceived open communication and their QOL in this study confirms that open communication about cancer is beneficial to couples managing PCa: couples may maintain or improve QOL through open communication about cancer. Aging persons more frequently interact with their families, especially their spouses [62]; their QOL may be closely related to how well they maintain those close relationships. Demands of cancer, treatment-related side effects, and age-related chronic health problems [63] limit couples’ physical interactions and leisure activities. Open communication becomes even more important for couples to obtain emotional and tangible support from each other.
In this study, couples’ QOL was positively affected by social support from other people. This result expanded previous research findings of direct and/or indirect effects of social support on mental well-being (e.g., less depression and anxiety) in patients and partners [8, 22, 64] to general QOL domains. We also confirmed that uncertainty about the illness was another factor affecting couple’s QOL; more uncertainty was associated with lower QOL. This finding was consistent with Mishel’s uncertainty theory [65] and extended findings of previous cross-sectional studies [29]. It also provided empirical support for interventions that aim at improving QOL through uncertainty management for both partners.
Another clinically relevant finding was that, among all PCa-specific symptoms, only patients’ sexual and hormonal symptoms (e.g., impotence and hot flashes) significantly affected couples’ QOL. Previous qualitative research reported that PCa patients considered their current QOL had little to do with their cancer or its treatment [66]. Yet, the debilitating symptoms of PCa and treatment side effects pose major threats to patients’ masculine image and sense of control [67]. Fewer problems in sexual and hormonal functions in patients, thus, provide reassurance to couples, especially men, and preserve their feelings of normalcy. These findings extended prior work in assessing the effects of hormonal symptoms on couples’ QOL, a rarely reported area [52].
We validated the results of previous investigations that cancer patients, especially the elderly with various symptoms, had poorer physical, mental, and social QOL [8, 68]. It also explored the negative effects of partners’ symptoms on their QOL, which was often ignored in the past because QOL and survival of cancer patients have been the foci of clinical practice and research. Family caregivers of cancer patients have significantly poorer global QOL than caregivers of non-cancer patients. Spouse caregivers, in particular, have significantly poorer QOL than non-spousal caregivers (e.g., adult children, relatives or friends) [69]. This research reminded us that partners provide care and support to cancer patients while managing the demands of their own health and age-related problems. Health professionals, thus, need to attend to spousal caregivers’ health needs while caring for cancer patients.
Regarding demographics, we found that couples’ QOL was related to partner’s age, patient’s education level, and family income. The association between partners’ older age and better QOL in couples may be related to the fact that, among our research participants, most (84.8%) of the younger partners (<60 years of age) worked outside the home while less than 20% of partners 60 years and older worked. Younger partners may have to care for the patient while dealing with competing demands of employment and child care. Consistent with Kim et al.’s findings [70], this result may suggest that the more social roles and responsibilities a caregiver carries, the more likely the caregiver experiences stress and negative adjustment.
The positive association between partners’ older age and couples’ better QOL may be related to the fact that the majority of couples were married, with an average of 32 years of relationship. In this relatively elderly population with long-standing relationships, partners have developed a deeper understanding of each other, enabling their better adaptation to cancer. It is noteworthy that sexuality may no longer be considered of primary importance among some elderly, particularly in aged women [71]. The pressure that older PCa patients put upon themselves to perform sexually may not be as great as that of younger patients who are more sexually active.
Several studies reported associations between baseline income and patients’ QOL at baseline and over time [72, 73]. Our results indicate higher family income predicted better QOL in both patients and partners. Financial concerns are prevalent among cancer patients and family members [74]. More income allows couples to afford materials and human resources to better manage cancer and caregiving and thus gives couples a sense of security that reduces stress and improves QOL. The negative effects of patients’ education level on couples’ QOL are inconsistent with the results of previous studies, especially those including participants with diverse education backgrounds [75–78]. However, participants of this study have relatively high education levels (mean = 16 years); our finding may not be generalized to diverse populations.
Finally, this study has limitations that warrant discussion. First, the treatment information was not included. Many individuals underwent more than one treatment. Classification would have resulted in too many treatment combinations to be analyzed with the existing sample size, i.e., too few subjects to utilize multivariate analyses to separate treatment effects statistically. Second, more patients with advanced cancer dropped from the study due to patients’ deaths. Third, participants were primarily well-educated, middle-class Caucasians. The limited number of participants from different ethnic groups or those with low socioeconomic status lessens the generalizability of our findings. Next, we could not differentiate the influences of gender from that of role (patient or partner) on couples’ QOL as PCa is a gender-specific illness. Finally, this study did not address the change of the outcome variable (i.e., QOL) as a function of the changes in predictors.
In conclusion, this study, using MLM, provided integral evidence on QOL in PCa patients and partners while controlling for baseline and time-varying contextual factors of both partners. Capturing couples’ complicated survivorship experiences, both as individuals and as a pair, this research provides information on modifiable factors that can be targeted for intervention. Research is needed to examine the effects of family-focused comprehensive interventions, e.g., uncertainty reduction, support mobilization from internal (e.g., open communication between partners) and external sources (e.g., assistance from other relatives, friends, and health providers), and symptom management. These efforts will strengthen couples’ adaptive abilities and ultimately improve their QOL.
Acknowledgments
The authors gratefully acknowledge the expert guidance and contributions of Mr. Brady West at the University of Michigan Center for Statistical Consultation and Research, Drs. George Knafl and Merle Mishel at the University of North Carolina-Chapel Hill School of Nursing. The study in this report was funded in part by grant F31NR010990 from the National Institute of Nursing Research (L. Song, Prinicipal Investigator) and R01CA10738 from National Cancer Institute (L. Northouse, Principal Investigator). L. Song is currently supported by a postdoctoral training grant at the University of North Carolina School of Nursing (5T32NR007091, M. Mishel, Principal Investigator).
Abbreviations
- PCa
Prostate cancer
- QOL
Quality of life
- MLM
Multilevel modeling
Contributor Information
Lixin Song, Email: lsong@unc.edu, University of North Carolina-Chapel Hill School of Nursing, 101 Carrington Hall, Chapel Hill, NC 27599, USA.
Laurel L. Northouse, University of Michigan School of Nursing, Ann Arbor, MI, USA
Thomas M. Braun, University of Michigan School of Public Health, Ann Arbor, MI, USA
Lingling Zhang, University of Michigan, Center for Statistical Consultation and Research, Ann Arbor, MI, USA.
Bernadine Cimprich, University of Michigan School of Nursing, Ann Arbor, MI, USA.
David L. Ronis, University of Michigan School of Nursing, Ann Arbor, MI, USA VA Ann Arbor Healthcare System, Health Services Research & Development, Ann Arbor, MI, USA.
Darlene W. Mood, College of Nursing, Wayne State University, Detroit, MI, USA
References
- 1.Litwin MS, Pasta DJ, Yu J, et al. Urinary function and bother after radical prostatectomy or radiation for prostate cancer: A longitudinal, multivariate quality of life analysis from the cancer of the prostate strategic urologic research endeavor. Journal of Urology. 2000;164:1973–1977. doi: 10.1016/s0022-5347(05)66931-5. [DOI] [PubMed] [Google Scholar]
- 2.Gray RE, Fitch M, Phillips C, et al. Managing the impact of illness: The experiences of men with prostate cancer, their spouses. Journal of Health Psychology. 2000;5:531. doi: 10.1177/135910530000500410. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. What you need to know about prostate cancer? 2005 [Google Scholar]
- 4.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: A review of the literature. Psycho-Oncology. 2002;11:307–326. doi: 10.1002/pon.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White WM, Sadetsky N, Waters WB, et al. Quality of life in men with locally advanced adenocarcinoma of the prostate: An exploratory analysis using data from the CaPSURE database. Journal of Urology. 2008;180:2409–2413. doi: 10.1016/j.juro.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 6.Sonn G, Sadetsky N, Presti J, et al. Differing perceptions of quality of life in patients with prostate cancer and their doctors. Journal of Urology. 2009;182:2296–2302. doi: 10.1016/j.juro.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: Population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw T, Mood D, Newth G, et al. Longitudinal analysis of a model to predict quality of life in prostate cancer patients and their spouses. Annals of Behavioral Medicine. 2008;36:117–128. doi: 10.1007/s12160-008-9058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornblith AB, Herr HW, Ofman US, et al. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73:2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Northouse LL, Mood D, Montie JE, et al. Living with prostate cancer: Patients’ and spouses’ psychosocial status and quality of life. Journal of Clinical Oncology. 2007;25:4171–4177. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 11.Fisher L. Alternative strategies for creating ‘relational’ family data. Family Process. 1985;24:213–224. doi: 10.1111/j.1545-5300.1985.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis FM. Psychosocial transitions, the family’s work in adjusting to cancer. Seminars in Oncology Nursing. 1993;9:127. doi: 10.1016/s0749-2081(05)80109-3. [DOI] [PubMed] [Google Scholar]
- 13.Lewis FM. Advancing family focused oncology nursing research. In: Phillips JM, King CR, editors. Advancing oncology nursing science. Pittsburgh, Pennsylvania: Oncology Nursing Society Publishing Division; 2009. pp. 409–434. [Google Scholar]
- 14.Northouse L, Kershaw T, Mood D, et al. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psycho-Oncology. 2005;14:478–491. doi: 10.1002/pon.871. [DOI] [PubMed] [Google Scholar]
- 15.Galvin KM, Dickson FC, Marrow SR. Systems theory: Patterns and (W)holes in family communication. In engaging theories in family communication—Multiple perspectives. Thousand Oaks, California: Sage Publications, Inc.; 2006. p. 364. [Google Scholar]
- 16.Lintz K, Moynihan C, Steginga S, et al. Prostate cancer patients’ support and psychological care needs: Survey from a non-surgical oncology clinic. Psycho-Oncology. 2003;12:769–783. doi: 10.1002/pon.702. [DOI] [PubMed] [Google Scholar]
- 17.Soloway CT, Soloway MS, Kim SS, et al. Sexual, psychological and dyadic qualities of the prostate cancer ‘couple’. BJU International. 2005;95:780–785. doi: 10.1111/j.1464-410X.2005.05400.x. [DOI] [PubMed] [Google Scholar]
- 18.Rees J, Clarke MG, Waldron D, et al. The measurement of response shift in patients with advanced prostate cancer, their partners. Health & Quality of Life Outcomes. 2005;3:21. doi: 10.1186/1477-7525-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: Results from CaPSURE. Journal of Urology. 2004;171:703–707. doi: 10.1097/01.ju.0000107964.61300.f6. [DOI] [PubMed] [Google Scholar]
- 20.Harden JK, Northouse LL, Mood DW. Qualitative analysis of couples’ experience with prostate cancer by age cohort. Cancer Nursing. 2006;29:367–377. doi: 10.1097/00002820-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Rondorf-Klym LM, Colling J. Quality of life after radical prostatectomy. Oncology Nursing Forum Online. 2003;30:E24–E32. doi: 10.1188/03.ONF.E24-E32. [DOI] [PubMed] [Google Scholar]
- 22.Scholz U, Knoll N, Roigas J, et al. Effects of provision and receipt of social support on adjustment to laparoscopic radical prostatectomy. Anxiety, Stress, & Coping. 2008;21:227–241. doi: 10.1080/10615800801983759. [DOI] [PubMed] [Google Scholar]
- 23.Mellon S, Northouse LL, Weiss LK. A population-based study of the quality of life of cancer survivors and their family caregivers. Cancer Nursing. 2006;29:120–131. doi: 10.1097/00002820-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Eton DT, Lepore SJ, Helgeson VS. Psychological distress in spouses of men treated for early-stage prostate carcinoma. Cancer. 2005;103:2412–2418. doi: 10.1002/cncr.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotcher JM. Well-adjusted and maladjusted cancer patients: An examination of communication variables. Health Communication. 1995;7:21–33. [Google Scholar]
- 26.Gotcher JM. Interpersonal communication and psychosocial adjustment. Journal of Psychosocial Oncology. 1992;10:21–39. [Google Scholar]
- 27.Ko CM, Malcarne VL, Varni JW, et al. Problem-solving and distress in prostate cancer patients and their spousal caregivers. Supportive Care in Cancer. 2005;13:367–374. doi: 10.1007/s00520-004-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepore SJ, Silver RC, Wortman CB, et al. Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. Journal of Personality and Social Psychology. 1996;70:271–282. doi: 10.1037//0022-3514.70.2.271. [DOI] [PubMed] [Google Scholar]
- 29.Wallace M. Uncertainty and quality of life of older men who undergo watchful waiting for prostate cancer. Oncology Nursing Forum Online. 2003;30:303–309. doi: 10.1188/03.onf.303-309. [DOI] [PubMed] [Google Scholar]
- 30.Germino BB, Mishel MH, Belyea M, et al. Uncertainty in prostate cancer. Ethnic and family patterns. Cancer Practice. 1998;6:107–113. doi: 10.1046/j.1523-5394.1998.1998006107.x. [DOI] [PubMed] [Google Scholar]
- 31.Litwin MS, McGuigan KA, Shpall AI, et al. Recovery of health related quality of life in the year after radical prostatectomy: Early experience. Journal of Urology. 1999;161:515–519. [PubMed] [Google Scholar]
- 32.Lubeck DP, Litwin MS, Henning JM, et al. Changes in health-related quality of life in the first year after treatment for prostate cancer: Results from CaPSURE. Urology. 1999;53:180–186. doi: 10.1016/s0090-4295(98)00408-7. [DOI] [PubMed] [Google Scholar]
- 33.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the prostate cancer outcomes study. Journal of the National Cancer Institute. 2000;92:1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 34.Albertsen PC, Aaronson NK, Muller MJ, et al. Health-related quality of life among patients with metastatic prostate cancer. Urology. 1997;49:207–216. doi: 10.1016/S0090-4295(96)00485-2. [DOI] [PubMed] [Google Scholar]
- 35.Cassileth BR, Soloway MS, Vogelzang NJ, et al. Quality of life and psychosocial status in stage D prostate cancer. Zoladex prostate cancer study group. Quality of Life Research. 1992;1:323–329. doi: 10.1007/BF00434946. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld B, Roth AJ, Gandhi S, et al. Differences in health-related quality of life of prostate cancer patients based on stage of cancer. Psycho-Oncology. 2004;13:800–807. doi: 10.1002/pon.797. [DOI] [PubMed] [Google Scholar]
- 37.Schag CA, Ganz PA, Wing DS, et al. Quality of life in adult survivors of lung, colon and prostate cancer. Quality of Life Research. 1994;3:127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 38.Baider L, Ever-Hadani P, Goldzweig G, et al. Is perceived family support a relevant variable in psychological distress? A sample of prostate and breast cancer couples. Journal of Psychosomatic Research. 2003;55:453–460. doi: 10.1016/s0022-3999(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 39.Banthia R, Malcarne VL, Varni JW, et al. The effects of dyadic strength, coping styles on psychological distress in couples faced with prostate cancer. Journal of Behavioral Medicine. 2003;26:31. doi: 10.1023/a:1021743005541. [DOI] [PubMed] [Google Scholar]
- 40.Kornblith AB, Herndon JE, Zuckerman E, et al. The impact of docetaxel, estramustine, and low dose hydrocortisone on the quality of life of men with hormone refractory prostate cancer and their partners: A feasibility study. Annals of Oncology. 2001;12:633–641. doi: 10.1023/a:1011102619058. [DOI] [PubMed] [Google Scholar]
- 41.Green HJ, Pakenham KI, Headley BC, et al. Coping and health-related quality of life in men with prostate cancer randomly assigned to hormonal medication or close monitoring. Psycho-Oncology. 2002;11:401–414. doi: 10.1002/pon.599. [DOI] [PubMed] [Google Scholar]
- 42.Borghede G, Karlsson J, Sullivan M. Quality of life in patients with prostatic cancer: Results from a Swedish population study. Journal of Urology. 1997;158:1477–1485. doi: 10.1016/s0022-5347(01)64247-2. [DOI] [PubMed] [Google Scholar]
- 43.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 44.Northouse LL, Rosset T, Phillips L, et al. Research with families facing cancer: The challenges of accrual and retention. Research in Nursing and Health. 2006;29:199–211. doi: 10.1002/nur.20128. [DOI] [PubMed] [Google Scholar]
- 45.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 46.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 47.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology. 2002;20:4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 48.Mood D, Song L, Kershaw T, et al. Assessing risk for distress in cancer patients and family caregivers. Oncology Nursing Forum. 2007;34(1):233. [Google Scholar]
- 49.Brandt PA, Weinert C. The PRQ—a social support measure. Nursing Research. 1981;30:277–280. [PubMed] [Google Scholar]
- 50.Lewis FM. Family home visitation study final report. Bethesda, MD: National Cancer Institute, National Institutes of Health; 1996. [Google Scholar]
- 51.Mishel M, Epstein D. Uncertainty in illness scales: Manual. Tucson, AZ: University of Arizona; 1990. [Google Scholar]
- 52.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 53.SAS Institute Inc. SAS 9. 2. Cary, NC, USA: SAS Institute Inc.; 2008. [Google Scholar]
- 54.West BT, Welch KB, Galecki A. Linear mixed models: A practical guide using statistical software. Boca Raton, Florida: Chapman & Hall/CRC: Taylor & Francis Group; 2007. [Google Scholar]
- 55.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. In advanced quantitative techniques in the social sciences series. Thousand Oaks, California: Sage Publications, Inc.; 2002. [Google Scholar]
- 56.Hagedoorn M, Sanderman R, Bolks HN, et al. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychological Bulletin. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Northouse LL, McCorkle R. Spouse caregivers of cancer patient. In: Holland JC, Breitbart WS, Jacobsen PB, et al., editors. Psycho-oncology. New York: Oxford University Press, Inc.; 2010. pp. 516–521. [Google Scholar]
- 58.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–4372. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 59.Goldzweig G, Hubert A, Walach N, et al. Gender and psychological distress among middle- and older-aged colorectal cancer patients and their spouses: An unexpected outcome. Critical Reviews in Oncology/hematology. 2009;70:71–82. doi: 10.1016/j.critrevonc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 60.Northouse LL, Mood D, Templin T, et al. Couples’ patterns of adjustment to colon cancer. Social Science and Medicine. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 61.Roesch SC, Adams L, Hines A, et al. Coping with prostate cancer: A meta-analytic review. Journal of Behavioral Medicine. 2005;28:281–293. doi: 10.1007/s10865-005-4664-z. [DOI] [PubMed] [Google Scholar]
- 62.Steverink N, Westerhof GJ, Bode C, et al. The personal experience of aging, individual resources, and subjective well-being. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2001;56:P364–P373. doi: 10.1093/geronb/56.6.p364. [DOI] [PubMed] [Google Scholar]
- 63.Harden J. Developmental life stage and couples’ experiences with prostate cancer: A review of the literature. Cancer Nursing. 2005;28:85–98. doi: 10.1097/00002820-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Jones RA, Taylor AG, Bourguignon C, et al. Family interactions among African American prostate cancer survivors. Family & Community Health. 2008;31:213. doi: 10.1097/01.FCH.0000324478.55706.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishel MH. Uncertainty in illness. Image—The Journal of Nursing Scholarship. 1988;20:225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 66.Foley KL, Farmer DF, Petronis VM, et al. A qualitative exploration of the cancer experience among long-term survivors: Comparisons by cancer type, ethnicity, gender, age. Psycho-Oncology. 2006;15:248. doi: 10.1002/pon.942. [DOI] [PubMed] [Google Scholar]
- 67.Clark JA, Wray N, Brody B, et al. Dimensions of quality of life expressed by men treated for metastatic prostate cancer. Social Science and Medicine. 1997;45:1299–1309. doi: 10.1016/s0277-9536(97)00058-0. [DOI] [PubMed] [Google Scholar]
- 68.Thom ÃB, Dykes A-K, Hallberg IR. Quality of life in old people with and without cancer. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation. 2004;13:1067. doi: 10.1023/B:QURE.0000031342.11869.2f. [DOI] [PubMed] [Google Scholar]
- 69.Luria DLL. An investigation of caregiver burden in primary caregivers of home hospice patients with cancer versus non-cancer, non-AIDS diagnoses. US: ProQuest Information & Learning; 2001. [Google Scholar]
- 70.Kim Y, Baker F, Spillers RL, et al. Psychological adjustment of cancer caregivers with multiple roles. Psycho-Oncology. 2006;15:795–804. doi: 10.1002/pon.1013. [DOI] [PubMed] [Google Scholar]
- 71.Dello Buono M, Zaghi PC, Padoani W, et al. Sexual feelings and sexual life in an Italian sample of 335 elderly 65 to 106-year-olds. Archives of Gerontology and Geriatrics. 1998;26(Suppl 1):155–162. [Google Scholar]
- 72.Penson DF, Stoddard ML, Pasta DJ, et al. The association between socioeconomic status, health insurance coverage, and quality of life in men with prostate cancer. Journal of Clinical Epidemiology. 2001;54:350–358. doi: 10.1016/s0895-4356(00)00312-7. [DOI] [PubMed] [Google Scholar]
- 73.Zavala MW, Maliski SL, Kwan L, et al. Longitudinal quality of life in low-income men in a state-funded prostate cancer treatment program. Journal of Health Care for the Poor and Underserved. 2008;19:200. doi: 10.1353/hpu.2008.0026. [DOI] [PubMed] [Google Scholar]
- 74.Devins GM, Bezjak A, Mah K, et al. Context moderates illness-induced lifestyle disruptions across life domains: A test of the illness intrusiveness theoretical framework in six common cancers. Psycho-Oncology. 2006;15:221–233. doi: 10.1002/pon.940. [DOI] [PubMed] [Google Scholar]
- 75.Knight SJ, Latini DM, Hart SL, et al. Education predicts quality of life among men with prostate cancer cared for in the Department of Veterans Affairs: A longitudinal quality of life analysis from CaPSURE. Cancer. 2007;109:1769–1776. doi: 10.1002/cncr.22597. [DOI] [PubMed] [Google Scholar]
- 76.Brar R, Maliski SL, Kwan L, et al. Changes in quality of life among low-income men treated for prostate cancer. Urology. 2005;66:344–349. doi: 10.1016/j.urology.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 77.Lepore SJ, Helgeson VS, Eton DT, et al. Improving quality of life in men with prostate cancer: A randomized controlled trial of group education interventions. Health Psychology. 2003;22:443–452. doi: 10.1037/0278-6133.22.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: An examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92:1451–1459. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]