In a human challenge model with Campylobacter jejuni CG8421, primary infection caused campylobacteriosis and immune responsiveness measured by specific immunoglobuling (Ig) A and IgG, antibody-secreting cells, fecal IgA, and interferon γ production. Unexpectedly, after rechallenge, subjects were not protected from clinical illness.
Keywords: Campylobacterosis, human challenge model, Campylobacter jejuni, homologous protection
Abstract
Background. Campylobacter jejuni is a common cause of diarrhea and is associated with serious postinfectious sequelae. Although symptomatic and asymptomatic infections are recognized, protective immunity is not well understood. Previous data suggests that interferon γ (IFN-γ) may be associated with protection. To better define the clinical and immunologic development of protective immunity to C. jejuni, we assessed the ability of an initial infection to prevent clinical illness after a second experimental infection.
Methods. Subjects with no clinical or immunologic evidence of prior infection with C. jejuni received an initial challenge with C. jejuni CG8421 with rechallenge 3 months later. The primary endpoint was campylobacteriosis, as defined by diarrhea and/or systemic signs. Close inpatient monitoring was performed. Serum immunoglobulin A (IgA) and immunoglobulin G (IgG), fecal IgA, IgA antibody-secreting cells (ASCs), and IFN-γ production were evaluated. All subjects were treated with antibiotics and were clinically well at discharge.
Results. Fifteen subjects underwent a primary infection with C. jejuni CG8421; 14 (93.3%) experienced campylobacteriosis. Eight subjects received the second challenge, and all experienced campylobacteriosis with similar severity. Immune responses after primary infection included serum IgA, IgG, ASC, and IFN-γ production. Responses were less robust after secondary infection.
Conclusions. In naive healthy adults, a single infection with CG8421 did not protect against campylobacteriosis. Although protection has been demonstrated with other strains and after continuous environmental exposure, our work highlights the importance of prior immunity, repeated exposures, and strain differences in protective immunity to C. jejuni.
Clinical Trials Registration. NCT01048112
Campylobacter jejuni is among the most common causes of enteric infection worldwide, and its complex relationship with the human host is just starting to be understood. C. jejuni causes inflammatory enteritis manifested by diarrhea or dysentery, fever, and abdominal cramping. Asymptomatic infection/colonization is also common, as described in children after repeated exposure to C. jejuni in resource-poor countries [1, 2]. Recently, incidence estimates of 1 symptomatic or asymptomatic C. jejuni infection every 2 years have also been reported in adults in developed countries [2].
C. jejuni infections have strong associations with postinfectious sequelae, strain variability, and increasing resistance to antibiotics. These include the demyelinating neurologic syndrome Guillain-Barré, chronic gastrointestinal symptoms, and postinfectious arthritis [3–6]. The kinetics and composition of the human immune response to C. jejuni are poorly understood and difficult to evaluate in field settings because of the inability to know onset of infection, strain differences, and previous exposures. Human challenge models, in contrast, provide a controlled method to understand and define immunologic responses to infection and/or correlates of protection [7–9].
We and others have described the human challenge model development of C. jejuni, most recently using strain CG8421 [9]. This strain, which lacks ganglioside mimicry in its lipo-oligosaccaharide, replaced strain 81-176, which expresses ganglioside 2 and ganglioside 3 and was epidemiologically linked to Guillain-Barré [7, 10]. Previous human challenge studies with 81-176 and A3249 demonstrated that Campylobacter-specific immunoglobulin A (IgA) and interferon γ (IFN-γ) are associated with resistance to clinical disease, suggesting these components might be important markers of protective immunity [7, 8].
To better define protective immunity to C. jejuni and to further develop the model, we challenged healthy, immunologically naive adults with C. jejuni CG8421 and then rechallenged subjects 3 months later with the same strain. As with previous challenge trials, this study was performed with the expectation that a primary infection would afford significant, if not complete, clinical protection after rechallenge [7, 8].
METHODS
The study was an open-label, inpatient trial of oral inoculation of C. jejuni strain CG8421. After comprehensive screening, naive subjects received 5.5 × 105 colony-forming units (CFUs) of C. jejuni CG8421, based on previous experimental studies [9]. Three months later, the same veteran individuals were chosen to receive a second inoculation of CG8421, at the same dose, and with identical follow-up. Three additional naive subjects were challenged with the veteran group. The clinical protocol was approved by all institutional review boards (Clinical Trials.gov: NCT01048112). An independent data safety and monitoring board was convened.
Endpoints and Definitions
The primary study endpoint was campylobacteriosis, defined as a clinical illness with documented C. jejuni infection, occurring within 144 hours (6 days) of dosing. Clinical illness included either diarrhea or a febrile illness (≥38°C) without diarrhea but with at least 2 associated gastrointestinal symptoms (vomiting, abdominal cramping, tenesmus). Infection with C. jejuni was defined as a positive stool culture occurring >24 hours after dosing regardless of symptoms. All stools passed were documented for time, weight, and blood. Specimens were graded 1–5 as described, with grades 3–5 defined as diarrhea [9]. Diarrhea was defined as mild (one loose/ liquid stool ≥300 g, or ≥2 loose/liquid stools ≥200 g in any 48-hour period, or ≥3 loose/liquid stools in a 24-hour period), moderate (4–5 diarrheal stools in 24 hours or 401–800 g within 24 hours) or severe (≥6 loose/liquid stools in 24 hours or >800 g of loose/liquid stools in 24 hours). Dysentery was ≥2 episodes of gross blood in a loose stool. All symptoms were classified as mild (noticeable, short-lived, not requiring intervention or changing activities); moderate (interrupting some activities), or severe (interrupting all activities). An additional index, which uses both measures of systemic illness (eg, fever) and gastrointestinal symptoms (eg, diarrhea severity, cramping), was used to measure severity of the overall clinical illness [8].
Subject Recruitment/Eligibility
Subjects were healthy, aged 18–50 years, with no evidence of prior C. jejuni exposure. Extensive screening procedures have been described [9]. Exclusions included gastrointestinal, neurologic, or rheumatologic disease. Immunologic exclusions were a serologic response to C. jejuni CG8421 glycine extracted antigens (IgA >1:2000 by reciprocal endpoint titer) or IFN-γ >400 pg/mL after in vitro stimulation of peripheral blood mononuclear cells (PBMCs) with formalin-fixed whole-cells of C. jejuni CG8421 [8]. Volunteers meeting the endpoint of campylobacteriosis in the first inoculation were eligible to receive the second. This group was rescreened using parameters above (except C. jejuni immuno-assays) to confirm continued eligibility. Any volunteer experiencing a serious adverse event or recrudescence of infection was ineligible for the second challenge.
Challenge Strain and Dosing Procedures
C. jejuni strain CG8421 (serotype Penner heat-stable 23, 36) was isolated and characterized as previously described. The lipo-oligosaccaharide core of this strain lacks all ganglioside mimicry and the genes needed for synthesis of N-acetyl neuraminic acid, necessary for glycolipid mimicry associated with Guillain-Barré [11]. The master seed lot was grown under Good Manufacturing Practice, conditions for growth and preparation of the challenge inoculum, as described [9]. On the day of dosing (day 0), subjects fasted for 90 minutes, then drank 120 mL of sterile USP-grade bicarbonate solution, followed 1 minute later by the inoculum in 30 mL of bicarbonate solution. Subjects fasted for an additional 90 minutes after dosing. The dosing and follow-up procedures were the same for both inpatient periods [9].
Clinical Monitoring/Management
Subjects were continuously monitored as inpatients, as described, for either a single challenge episode or two identical challenge episodes [9]. All diarrheal losses were replaced with oral rehydration solution. Intravenous fluids were used if subjects met criteria for abrupt onset of voluminous diarrhea (>300 g single stool or >400 g over 2 hours) or hypovolemia or at physician discretion. Blood cultures were performed for fever >38°C. Electrolyte levels were monitored if intravenous fluids were used. Stool microbiology to detect C. jejuni shedding was performed on the first 2 stools of each day, as described [9].
Subjects were treated with ciprofloxacin (500 mg twice daily) and azithromycin (500 mg daily) for 5 days, starting no later than 144 hours (6 days) after challenge. Two antibiotics were given, as per US Food and Drug Administration guidance from past challenge models [9, 12]. Antibiotics were given earlier for moderate or severe diarrhea or for diarrhea of any severity with either ≥2 severe symptoms of abdominal pain/cramps, nausea, myalgias, arthralgias, or gross blood in stool or fever ≥38°C or any vomiting. Subjects were eligible for discharge after antibiotics had been started, 2 stool cultures (≥12 hours apart) were negative for C. jejuni, and symptoms were resolved. Stool cultures for C. jejuni were additionally performed on days 14, 21, 28, 35, 60, and 90 after dosing. Subjects were followed for safety for 6 months.
Any volunteer experiencing recrudescence of infection was confirmed to be shedding the challenge inoculum by polymerase chain reaction and had confirmation of antibiotic susceptibilities by minimal inhibitory concentration, as described [12, 13]. Unless antibiotic resistance had developed, oral ciprofloxacin and azithromycin were given for an additional 10 days. Subjects were monitored clinically for an additional 6 months and microbiologically for 5 weeks after shedding ended [12].
Immunological Studies
Sample Collection
For both dosing episodes, peripheral blood was collected in ethylenediaminetetraacetic acid tubes before and after dosing. Stool samples for fecal IgA were collected before and after infection (days 0, 4, 7, 9, 14, 28, 90) and frozen at −70°C within 2 hours of collection.
Assays methods have been described [9, 14]. Antigen-specific serum IgA and IgG were determined by enzyme-linked immunosorbent assay using homologous-strain glycine-extracted antigens. Total and antigen-specific fecal IgA levels to C. jejuni CG8421 glycine-extracted antigens) were determined by enzyme-linked immunosorbent assay. Antigen-specific fecal IgA is represented as a ratio of specific/total fecal IgA. Total IgA was determined using monoclonal IgA1/A2 antibodies. Antibody-secreting cell (ASC) responses were enumerated, as described [8]. Briefly, plates were coated with C. jejuni glycine-extracted antigens (3 µg/mL) and incubated with 3.3 × 105 PBMCs per well in complete media. Secreted antibodies were detected with goat antihuman antibodies (0.25 µg/mL) and visualized with nitroblue tetrazolium(10 µg/mL)-5-bromo-4-chloro-3-indoylphosphate (5 µg/mL). Plates with spot counts ≥5/106 PBMCs were considered positive [8]. For systemic cytokine responses, peripheral mononuclear cells (screening and 0, 28, 69, 90 days after infection) were incubated for 48 hours; supernatants were used to determine IFN-γ.
Data Analysis
All data were entered into a Microsoft Access database with 100% verification. Proportion of subjects meeting the primary endpoint, campylobacteriosis, was compared across baseline Campylobacter status, naive versus veteran. Analyses of secondary clinical outcomes included comparisons of the proportion with clinical outcomes as well as the distribution of diarrhea severity, stool frequency, stool volume, maximum temperature, time to illness, and time to infection across study groups.
Immunologic response definitions were consistent with prior studies of this strain [9]. Briefly, serologic and cell-mediated immunity responses were defined as a ≥4-fold and ≥2-fold increase over reciprocal baseline titers, respectively, whereas ASC response was defined as ≥5 ASCs per 106 PBMCs. Comparison between naive and veteran subjects were made using nonparametric tests (Kruskal–Wallis, continuous data) and Fisher exact test (categorical data) unless assumptions were fulfilled for Student t or Pearson χ2. Paired t tests were also used to compare postinoculation responses to baseline. All statistical analyses were performed using SAS version 8.2 and were interpreted in a two-tailed fashion using α= 0.05.
RESULTS
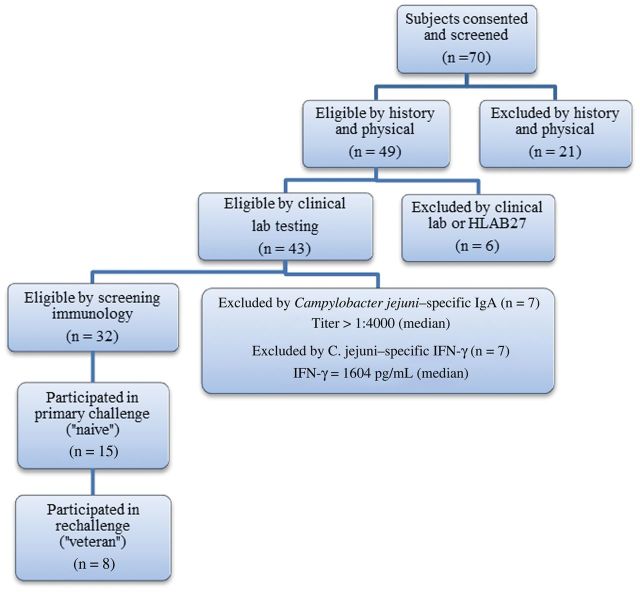
As shown in Figure 1, 70 healthy adult subjects were screened. Thirty-two subjects passed; 15 were enrolled and received a challenge inoculum of 5 × 105 CFUs C. jejuni CG8421. Twelve naive subjects were dosed during the first inpatient period. Eight veteran subjects and 3 naive subjects were dosed (or redosed) during the second inpatient period. The median age of the subjects was 23.2 years (interquartile range [IQR], 19.9–28.4). Twelve of 15 subjects were male. No subject experienced bacteremia, and all shed C. jejuni CG8421 after each dose.
Figure 1.
Screening eligibility and enrollment of volunteers participating in the Campylobacter jejuni challenge and rechallenge trial. Abbreviations: IFN-γ, interferon γ; IgA, immunoglobulin A.
Of the naive subjects, all met the campylobacteriosis endpoint except a single volunteer who shed C. jejuni but remained asymptomatic (92%). As shown in Table 1, clinical characteristics of naive volunteers, whether dosed in the first or second inpatient period, were similar. For this group, the median time to the campylobacteriosis endpoint was 52.3 (range, 32–62) hours. In addition to diarrhea (median volume, 1122 mL; range, 493–1621 mL), most volunteers experienced headache and abdominal cramping. Dysentery was seen in 2 (13.3%) subjects and fever in 8 (53%). Eight naive volunteers (53%) had severe diarrhea, and the median disease severity score was 9.5 (range, 0–14) [8]. All volunteers were asymptomatic at discharge and healthy upon readmission for the second dose.
Table 1.
Clinical Outcomes of Subjects After Challenge With Campylobacter jejuni CG8421
| Outcome | All Naive | Veteran |
|---|---|---|
| No. | 15 | 8 |
| Met primary endpoint of campylobacteriosis, No. (%) | 14 (93) | 8 (100) |
| Any diarrhea, No. (%) | 14 (93) | 8 (100) |
| Severe diarrhea, No. (%) | 8 (53) | 7 (88) |
| Time in hoursto campylobacteriosis, median (IQR) | 52.3 (46.9–56.1) | 53.7 (48.3–73.7) |
| Time in hours to first diarrheal stool, median (IQR) | 45.9 (43.7–51.2) | 51.1 (37.0–67.3) |
| Number of diarrhea stools, grade 3–5, median (IQR) | 14 (7–17) | 13 (11–16) |
| Highest 24-h stool output number, grade 3–5, median (IQR) | 7 (4–10) | 8 (6–10) |
| Highest 24-h stool output volume, mL, grade 3–5, median (IQR) | 661 (406–754) | 710 (574–859) |
| Total diarrhea volume, mL, median (IQR) | 1122 (493–1621) | 1122 (900–1669) |
| Time in hours to first antibiotic dose, median (IQR) | 57.0 (48.0–86.0) | 58.1 (46.7–76.4) |
| Gastrointestinal symptoms, No. (%) | ||
| Nausea | 11 (73)a | 2 (25)a |
| Vomiting | 0 (0.0) | 1 (13) |
| Abdominal pain/cramping, any | 14 (93) | 6 (75) |
| Abdominal pain/cramping, severe | 3 (20) | 0 (0) |
| Dysentery | 02 (13.3) | 1 (13) |
| Systemic symptoms, No. (%) | ||
| Fever | 8 (53) | 2 (25) |
| Headache | 12 (80) | 5 (63) |
| Diarrheal disease Severity index,b median (range) | 9.5 (01–14) | 8 (4–11) |
Abbreviation: IQR, interquartile range.
a No associations were statistically significant, except nausea. P = .04.
b See [8].
Unexpectedly, the attack rate of campylobacteriosis in veteran subjects was unchanged. Despite previous exposure to C. jejuni CG8421, volunteers had neither protection from reinfection nor an attenuated clinical illness (Table 1). The median time to first diarrhea stool was slightly longer in veteran subjects (51.1 hour; IQR, 37.0–67.3) than in naive subjects (45.9 hours; IQR, 43.7–51.2) as was the time to reach the endpoint of campylobacteriosis (naive subects, 52 hours; veteran subjects, 54 hours). Nevertheless, all (100%) veterans met the clinical endpoint of campylobacteriosis, and more veteran subjects experienced severe diarrhea (n = 7; 88%) than during primary infection. Total stool volume remained largely unchanged (median, 1122; range, 900–1669).
A few differences in clinical symptoms were noted; nausea, in particular, was more common in naive subjects (73% vs 25%; P = .039). Systemic complaints of fever and headache were also less frequent in veterans; however, numbers were small. As noted, more veteran subjects met the criteria for severe diarrhea (n = 7; 87.5%) than those receiving their first dose (n = 8; 53.3%), although this difference was not statistically different. When naive and veteran subjects were compared using a diarrheal disease severity index, no differences were evident [8].
One naive subject during the first inpatient period experienced an asymptomatic recrudescence on day 28; stool culture and polymerase chain reaction confirmed the CG8421 strain. Resolution was documented after additional antibiotics. No postinfectious complications were identified after 6 months of observation.
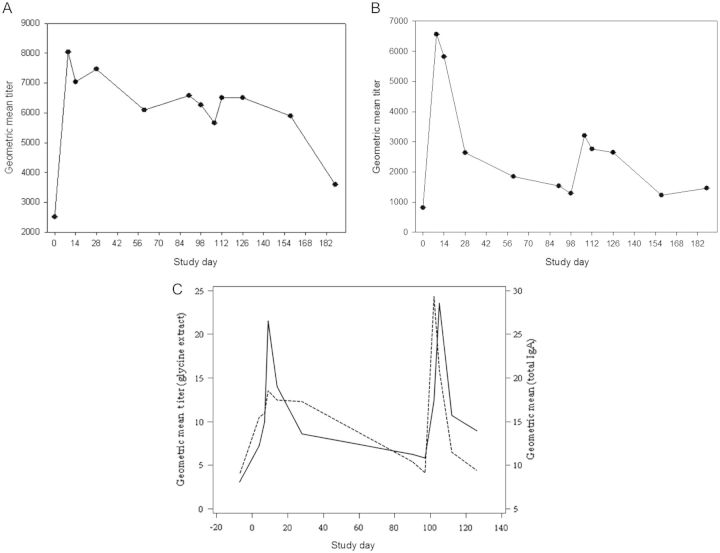
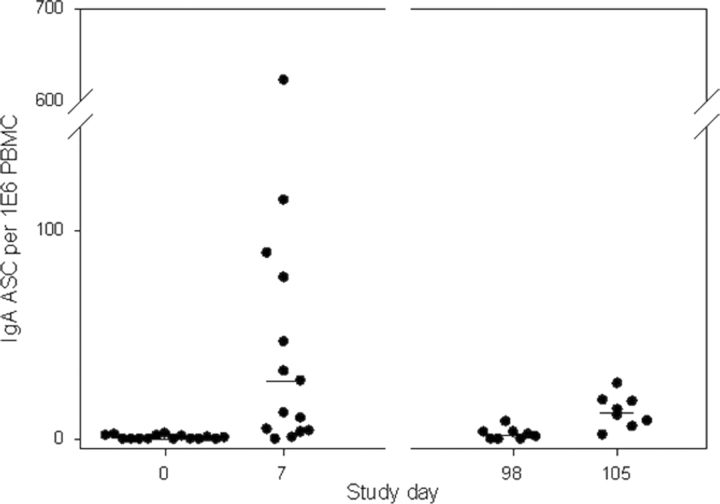
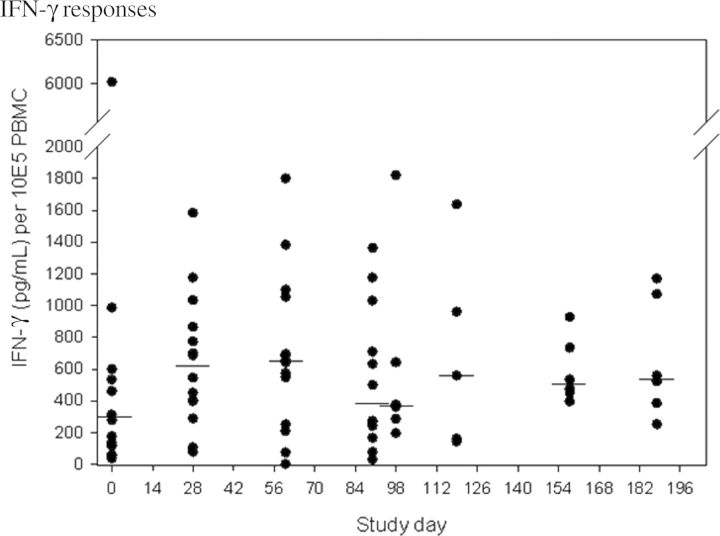
Immunologic responses were generally more robust after the first exposure to CG8421 (Table 2). Seroconversion (4-fold rise) was found in 67% (n = 10) and 53% (n = 7) of naive subjects after initial exposure to CG8421. After rechallenge, no veteran subjects had additional 4-fold rises. Of note, as seen in Figure 2A and 2B, most IgA responses had returned to baseline within 3 months, and responses to rechallenge were low (<4 fold-rises). In contrast, IgG levels did not fall to baseline after primary challenge but had minimal increases after the second dose and fell thereafter. For IgA ASCs, (Figure 3), 67% (n = 10) of naive subjects had a positive response, with a median spot number of 62 (Figure 3). Although most veterans had an ASC response, the maximal spot count was substantially lower (median, 14. 2). Similarly, as seen in Figure 4 and Table 2, approximately half of both naive and veteran subjects had a >2-fold rise in IFN-γ levels from baseline (P = .04), but no additional enhancement of IFN-γ response was seen after second intervention. IFN-γ levels fell close to baseline before the second dose in most subjects. Interestingly, despite a screening level of <400 pg/mL, one volunteer had an IFN-γ level at day 0 (before dosing) of 6000 pg/mL (suggesting exposure between screening and dosing), a level which would have excluded him from participation. This person (subject 011), was the only asymptomatic subject after dosing with C. jejuni CG8421.
Table 2.
Core Immunogenicity Assays After Challenge and Rechallenge With Campylobacter jejuni CG8421
| C. jejuni CG 8421 Antigen | Assay | Study Group |
|
|---|---|---|---|
| Initial Challenge, % (Peak Fold Rise) (n = 15) | Rechallenge, % (Peak Fold Rise) (n = 8) | ||
| Glycine extract | ASC (IgA)a | 66.7 (62.1) | 87.5 (14.2) |
| IgA (serum)b | 66.7 (10.3) | 0.0 | |
| IgG (serum)b | 53.3 (9.9) | 0.0 | |
| Glycine extract | IgA (Fecal)c | 66.7 (81.1) | 37.5 (16.4) |
| Whole cells | Interferon γd | 57.2 (4.2) | 50.0 (4.3) |
Response rates are denoted by the percentage of volunteers meeting the responder definition. The number in parentheses is the median peak fold rise from baseline titer among responders. Antibody-secreting cell (ASC) responses are summarized as percentage of responders and then median maximum number of ASC among responders in parentheses. Response rates for the rechallenge are based on study day 90 “baseline” values.
Abbreviations: ASC, antibody-secreting cell; IgA, immunoglobulin A; IgG, immunoglobulin G.
a Responder defined as ≥5 spots per 106 peripheral blood mononuclear cells.
b Responder defined as ≥4-fold rise in baseline titer and a postchallenge reciprocal titer >10.
c Responder defined as ≥4-fold rise total IgA adjusted baseline titer and a postchallenge total IgA adjusted reciprocal titer >10.
d Responder defined as ≥2-fold rise in interferon γ (pg/mL) per 106 peripheral blood mononuclear cells.
Figure 2.
Serum immunoglobulin G (IgG), immunoglobulin A (IgA), and fecal IgA responses after challenge (day 0) and secondary challenge (day 98) with Campylobacter jejuni CG8421. A, Serum IgG responses after primary (day 0) and secondary (day 98) challenge with C. jejuni CG8421. B, Serum IgA responses after primary (day 0) and secondary (day 98) challenge with C. jejuni CG8421. C, Fecal IgA responses after primary (day 0) and secondary (day 98) challenge with C. jejuni CG8421. Hatched lines represent total fecal IgA. Solid lines represent C. jejuni antigem-specific fecal IgA. Abbreviation: IgA, immunoglobulin A.
Figure 3.
Immunoglobulin A antibody-secreting cell responses after primary and secondary challenge with Campylobacter jejuni CG8421. Horizontal lines denote the median number of antibody-secreting cells for each day. Abbreviations: ASC, antibody-secreting cell; IgA, immunoglobulin A; PBMC, peripheral blood mononuclear cell.
Figure 4.
Interferon γ produced from peripheral blood mononuclear cells after primary and secondary challenge. Horizontal lines denote median interferon γ levels for each study day. Abbreviations: IFN-γ, interferon γ; PBMC, peripheral blood mononuclear cell.
DISCUSSION
We performed a rigorously monitored inpatient study in which primary infection with C. jejuni was followed 3 months later by an identical second challenge. Our data confirm the safety and reproducibility of the CG8421 challenge model; however, the most important finding was a complete absence of homologous protection after second challenge. Rechallenge was associated with diarrheal illnesses clinically indistinguishable from primary infection. Although the onset of illness was slightly delayed in the rechallenge subjects, the attack rate (percentage with campylobacteriosis), severity of infection, diarrhea volume, and duration of shedding were not different.
Immunologically, we demonstrated responsiveness to primary infection, with increases from baseline in serum IgG and IgA, fecal IgA, IgA ASCs, and C. jejuni–specific IFN-γ. With the exception of serum IgG, all immune parameters fell toward baseline in the 3 months between doses in most volunteers. After the second dose, “boosting” or enhancement of these immune responses was minimal; median IgG responses remained elevated but did not rise after second challenge, and serum IgA and fecal IgA measurements demonstrated only minimal increases. Although the ASC pattern was not surprising because these cells are home to the gut mucosa, the unresponsiveness of systemic and fecal antibody responses and the lack of clinical protection make it clear that there is much to be learned about the development of protective immunity, including the optimal timing and dose of subsequent infections, the role of antigenic tolerance, and which components of the innate and adaptive responses contribute to protection.
We expected that measures of C. jejuni–specific IFN-γ would yield informative data reiterating its role in protection from clinical disease, as suggested from a prior challenge model with 81-176 [8]. To explore this question, only subjects without evidence of exposure to C. jejuni were enrolled. IFN-γ levels increased after primary challenge, but most fell toward baseline before redosing and were not significantly changed after the rechallenge. Thus, although we cannot confirm whether high predose levels of IFN-γ are associated with protection, it is clear that a single primary infection with C. jejuni CG8421 at the 105 CFUs dose will not reliably prompt elevated and sustained IFN-γ levels. Evaluation of the contribution and kinetics of IFN-γ–producing T lymphocytes is ongoing (K. Fimlaid, unpublished data) and deserves further study in subjects with a range of IFN-γ levels at challenge and in subjects known to have multiple exposures.
Our data can be directly compared with 2 previous C. jejuni homologous strain re-challenge models using strains A3249 and 81-176: both demonstrated some protection from clinical disease [7, 8]. In the A3249 model, 2 volunteers were rechallenged with 106 CFUs 1 month after primary infection; neither developed illness. In a larger 81-176 trial, subjects were rechallenged with a higher dose of C. jejuni (109 CFUs) either 1–2 months (short-term veteran subjects) or 12 months (long-term veteran subject) after primary challenge. All short-term veteran subjects (n = 7) and 38% of long-term veteran subjects (n = 7) did not develop illness [9]. Notably, at similar doses (105–6 CFUs) the campylobacteriosis attack rates for primary infection for both A3249 and 81-176 are lower (19% and 60%, respectively) than at the 105 CFUs dose for CG8421 (93%). Taken together, although neither model excluded volunteers based on predosing IFN-γ (as in our work), these studies reiterate the significance of timing of subsequent infections in the development of protective immunity [15].
Our work contributes to recent contributions from large field trials, epidemiologic modeling studies, and experimental models [2, 16–18]. In addition to Campylobacter's known importance as a cause of symptomatic disease in children in resource-poor regions, the frequency of asymptomatic infection in all populations is becoming more recognized (Karen Kotloff [Global Enteric Multicenter Study] and William Petri Jr [Malnutrition and Enteric Diseases], personal communication) [1, 2, 19, 20]. Symptomatic disease wanes with age and repeated exposures, although protection is likely short-lived in the absence of continuous exposure [2]. In contrast, and as illustrated here, naive adults in developed countries without prior exposure in childhood may experience a severe clinical disease, akin to the first infection in childhood in resource-poor settings [1]. In this population, with less frequent exposures, it seems unlikely that long-lived protective immunity would be expected after a single exposure to C. jejuni.
Recent evaluations have described the innate and adaptive immune components of campylobacteriosis in a variety of models. Human ex vivo intestinal epithelial and dendritic cell coculture systems show expansion of T helper 1 and T helper 17T cells after C. jejuni infection at the mucosal surface and suggest the importance of IFN-γ, interleukin 22, and the interleukin 17 family in the acute and effector phases of C. jejuni infection [18]. C. jejuni also induces dendritic cell maturity and induces proinflammatory cytokines and STAT3 activation [16, 17]. Our observations from this human homologous rechallenge model caution that despite clinical and laboratory markers of inflammation, innate responses, and evidence of immunogenicity, clinical protection from disease (and corresponding adaptive immune responses) cannot be assumed to follow. Thus, it is increasingly important to identify which components of the innate immune response directly contribute to future protective responses.
Our data reiterate that protection from campylobacteriosis may require multiple (symptomatic or asymptomatic) infections; this was particularly evident when naive volunteers were chosen. Future investigations to better understand protective immunity, should include an evaluation (challenge model or birth cohort in endemic setting) of repeated exposures. Impact of strain differences on immune responses is also important; data show that capsule variations modulate intestinal colonization and cytokine expression [21]. Finally, detailed immunologic evaluations (systemically and mucosal) should confirm the significance of T-cell populations, dendritic cell maturation, and STAT activation. We were fortunate to work with a highly controlled human population and hope these observations provide avenues of inquiry toward a comprehensive understanding of protective immunity to C. jejuni in human populations.
Notes
Acknowledgments. We thank the study volunteers, the nurses and staff of the University of Vermont College of Medicine General Clinical Research Center and Vaccine Testing Center (C. Ventrone, J. Lindow) and Naval Medical Research Center research staff (C. L. Hill, L. Applebee, T. Vieten).
The study protocol was approved by the University of Vermont Committees on Human Research and the Naval Medical Research Center institutional review boards in compliance with all applicable federal regulations governing the protection of human subjects.
Authors are military service members (M. S. R. and D. H.) and employees of the US government (C. K. P., P. G., F. P., A. M., D. T.). This work was prepared as part of official duties. Title 17 USC. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC §101 defines a US government work as a work prepared by a military service member or employee of the US government as part of that person's official duties.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
Financial support. This work was supported by a grant from the National Institutes of Health National Center for Research Resources (5M01RR000109, support of General Clinical Research Center) and by the US Navy Medical Research Center work unit 6000.RAD1. DA3. A0308.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Glass RI, Stoll BJ, Huq MI, Struelens MJ, Blaser M, Kibriya AK. Epidemiologic and clinical features of endemic Campylobacter jejuni infection in Bangladesh. J Infect Dis. 1983;148:292–6. doi: 10.1093/infdis/148.2.292. [DOI] [PubMed] [Google Scholar]
- 2.Havelaar AH, van Pelt W, Ang CW, et al. Immunity to Campylobacter: its role in risk assessment and epidemiology. Critical Reviews in Microbiology. 2009;35:1–22. doi: 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- 3.Dorrell N, Wren BW. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr Opin Infect Dis. 2007;20:514–8. doi: 10.1097/QCO.0b013e3282a56b15. [DOI] [PubMed] [Google Scholar]
- 4.Zilbauer M, Dorrell N, Wren BW, Bajaj-Elliott M. Campylobacter jejuni–mediated disease pathogenesis: an update. Trans R Soc Trop Med Hyg. 2008;102:123–9. doi: 10.1016/j.trstmh.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick BD, Tribble DR. Update on human Campylobacter jejuni infections. Curr Opin Gastroent. 2011;27:1–7. doi: 10.1097/MOG.0b013e3283413763. [DOI] [PubMed] [Google Scholar]
- 6.Riddle MS, Gutierrez RL, Verdu EF, Porter CK. The chronic gastrointestinal consequences associated with campylobacter. Curr Gastroent Rep. 2012;14:395–405. doi: 10.1007/s11894-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–9. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 8.Tribble DR, Baqar S, Scott DA, et al. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78:1750–9. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tribble DR, Baqar S, Carmolli MP, et al. Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis. 2009;49:1512–9. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 10.Guerry P, Szymanski CM, Prendergast MM, et al. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun. 2002;70:787–93. doi: 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poly F, Read TD, Chen YH, et al. Characterization of two Campylobacter jejuni strains for use in volunteer experimental infection studies. Infect Immun. 2008;76:5655–67. doi: 10.1128/IAI.00780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baqar S, Tribble DR, Carmolli M, et al. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. Clin Vaccine Immunol. 2010;17:80–6. doi: 10.1128/CVI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindow JC, Poly F, Tribble DR, et al. Caught in the act: in vivo development of macrolide resistance to Campylobacter jejuni infection. J Clin Microbiol. 2010;48:3012–5. doi: 10.1128/JCM.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rautelin HI, Kosunen TU. Campylobacter etiology in human gastroenteritis demonstrated by antibodies to acid extract antigen. J Clin Microbiol. 1987;25:1944–51. doi: 10.1128/jcm.25.10.1944-1951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin A, Baraho-Hassan D, McSorley SJ. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol. 2009;183:1263–70. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu L, Bray MD, Geng Y, Kopecko DJ. Campylobacter jejuni–mediated induction of CC and CXC chemokines and chemokine receptors in human dendritic cells. Infect Immun. 2012;80:2929–39. doi: 10.1128/IAI.00129-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu L, Bray MD, Osorio M, Kopecko DJ. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun. 2006;74:2697–705. doi: 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards LA, Nistala K, Mills DC, et al. Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection. PloS One. 2010;5:e15398. doi: 10.1371/journal.pone.0015398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao MR, Naficy AB, Savarino SJ, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol. 2001;154:166–73. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 20.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case–control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 21.Maue AC, Mohawk KL, Giles DK, et al. The polysaccharide capsule of Campylobacter jejuni 81-176 modulates the host immune response. Infect Immun. 2013;81:665–72. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]