Abstract
Increasing evidence demonstrates that commensal microorganisms in the human skin microbiome help fight pathogens and maintain homeostasis of the microbiome. However, it is unclear how these microorganisms maintain biological balance when one of them overgrows. The overgrowth of Propionibacterium acnes (P. acnes), a commensal skin bacterium, has been associated with the progression of acne vulgaris. Our results demonstrate that skin microorganisms can mediate fermentation of glycerol, which is naturally produced in skin, to enhance their inhibitory effects on P. acnes growth. The skin microorganisms, most of which have been identified as Staphylococcus epidermidis (S. epidermidis), in the microbiome of human fingerprints can ferment glycerol and create inhibition zones to repel a colony of overgrown P. acnes. Succinic acid, one of four short-chain fatty acids (SCFAs) detected in fermented media by nuclear magnetic resonance (NMR) analysis, effectively inhibits the growth of P. acnes in vitro and in vivo. Both intralesional injection and topical application of succinic acid to P. acnes-induced lesions markedly suppress the P. acnes-induced inflammation in mice. We demonstrate for the first time that bacterial members in the skin microbiome can undergo fermentation to rein in the overgrowth of P. acnes. The concept of bacterial interference between P. acnes and S. epidermidis via fermentation can be applied to develop probiotics against acne vulgaris and other skin diseases. In addition, it will open up an entirely new area of study for the biological function of the skin microbiome in promoting human health.
Keywords: Acne, Fermentation, P. acnes, Probiotic, S. epidermidis, Skin Microbiome
INTRODUCTION
Fermentation of milk with gut-friendly bacteria, i.e. yogurt, which is the best example of bacterial interference through fermentation, is an excellent aid to balance the bacteriological ecosystem in the human intestine. Bacterial interference via fermentation occurs in natural ecosystems as well. For example, microorganisms both on and inside fruits consume sugars that were converted from starch during ripening to produce fermentation products including ethanol and short-chain fatty acids (SCFAs). Production of SCFAs and ethanol by fermentative yeasts is, in fact, part of an evolved strategy to compete with other microbes for access to sugars (Dudley 2004). It is not fully clear whether friendly microbes in human skin possess the fermentation activity and whether ferments including SCFAs of these microbes have probiotic activities to maintain the homeostasis of the skin microbiome. Reports show that SCFAs produced by fermentation of microorganisms have been detected in pus from a deep-seated abscess, an anaerobic microenvironment in the context of human bacterial infection (Demaerel et al. 1994; Gorbach et al. 1976; Menon et al. 2007). Like a ripening fruit, an acne lesion, particularly a closed comedone or deep-seated abscess in an open comedone, creates an anaerobic microenvironment which facilitates overgrowth of Propionibacterium acnes (P. acnes). It has been reported that P. acnes, Staphylococcus epidermidis (S. epidermidis), and other skin microflora co-exist in acne lesions (Nishijima et al. 2000). We thus envision that the anaerobic acne microenvironment triggers human skin microflora to undergo fermentation, and that these skin microflora utilize fermentation to rein in the overgrowth of P. acnes within acne lesions.
A number of SCFAs are naturally produced by skin cells and commensal bacteria in relatively low concentrations (Burtenshaw 1942). It has been reported that SCFAs exert antimicrobial activities (Ryssel et al. 2009; Ushijima et al. 1984). Several SCFAs have been approved by the United States Environmental Protection Agency (EPA) as active ingredients for use as fungicides and bactericides on stored grains, poultry litter, and drinking water for poultry and livestock (Sebastian et al. 1996). The Food and Drug Administration (FDA) has approved succinic acid (C4H6O4), one of the SCFAs, as a flavor enhancer, miscellaneous and general purpose food chemical, neutralizing agent, and pH control agent (2011-10-27). SCFAs (e.g. lactic acid) and glycerol are ingredients in many skin care products, where they serve as moisturizers or anti-inflammatory agents.
Acne vulgaris is an inflammatory skin disease associated with the overgrowth of P. acnes. Around 40 to 50 million Americans suffer from acne vulgaris each year (Imahiyerobo-Ip and Dinulos 2011). Many treatment options are available for acne, however none of them completely cure the disease in all patients, and many of them also have significant side effects. Antibiotics, for example, have been used for treating acne vulgaris, but these are non-specific and have a risk of creating antibiotic-resistant bacteria (Haider and Shaw 2004). The oxidizing agent benzoyl peroxide (BPO) is one of the most frequently used topical medications for acne treatment. It is available over-the-counter and is generally well-tolerated by patients. Isotretinoin, another acne treatment option, is a powerful and effective medication derived from vitamin A (Layton et al. 2006). However, it is strictly regulated due to the induction of serious side effects including congenital anomalies. Finally, intralesional corticosteroid injections are an important adjunct in the treatment of painful nodulocystic acne lesions. However, the injection can cause local side effects including linear hypopigmentation and atrophy (Levine and Rasmussen 1983). None of these treatments use a person’s endogenous molecules to treat acne despite the fact that these molecules may have fewer side-effects and a less of a chance of developing antibiotic-resistant microbes. Acne vaccines selectively targeting P. acnes-induced inflammation, not P. acnes bacterial particles, are actively being developed in our laboratory (Huang et al. 2008; Liu et al. 2011; Lo et al. 2011; Nakatsuji et al. 2008a; Nakatsuji et al. 2008b; Nakatsuji et al. 2008c; Nakatsuji et al. 2011). However, the vaccine may be mainly for preventive treatments. Here we introduce the concept of treating acne with probiotics or prebiotics, which include three main products: 1) anti-P. acnes SCFAs, 2) glycerol, which is known as a fermentation inducer and a healing enhancer (Fluhr et al. 2008), and 3) live fermenting microorganisms with the ability to inhibit the growth of P. acnes. The use of probiotic skin microorganisms or their fermentation products as innate anti-P. acnes therapeutics is in compliance with evolutionary medicine. It may also have a lower risk of inducing resistant strains of P. acnes and causing side-effects since P. acnes/skin commensal interference may occur naturally within lesions of acne vulgaris.
MATERIALS AND METHODS
Ethics statement
Experiments involving mice were performed at the University of California, San Diego (UCSD). The UCSD ethics committee specifically approved this study under an approved Institutional Animal Care and Use Committee (IACUC) protocol (no. S10058). The Institutional Review Board (IRB) at UCSD approved the consent procedure and bacterial sampling under approved protocols (no. 100473 and 121230). The written consents from all participants were obtained before conducting bacterial sampling.
Culture of microorganisms
P. acnes (ATCC6919) was cultured in Reinforced Clostridium Medium (RCM, Oxford, Hampshire, England) under anaerobic conditions using Gas-Pak (BD, Sparks, MD, USA) at 37°C as previously described (N akatsuji et al. 2008a). Human skin microorganisms were isolated by moving a sterile inoculating loop (Fisher Scientific, San Diego, CA, USA) along the surface of the nose of a male subject without acne vulgaris. The isolated skin microorganisms containing a mixture of various microbes were cultured in tryptic soy broth (TSB) (Sigma, St. Louis, MO, USA). Overnight cultures were diluted 1:100 and cultured to an absorbance at 600 nm [optical density (OD)600]=1.0. Microorganisms were harvested by centrifugation at 5,000 g for 10 min, washed with phosphate buffered saline (PBS), and suspended in PBS.
P. acnes growth in a homogeneous microbial lawn
The skin microorganisms or P. acnes [105 colony forming unit (CFU] were mixed with 1% molten (w/v) agar (Oxoid. Ltd., London, UK) with/without glycerol (20 g/l) in TSB. The microbial suspension/agar was poured into plates to produce a homogeneous lawn of microbes. P. acnes or skin microorganisms with a serial dilution (5 × 106– 5 × 101 CFU in 5 μl in PBS) were spotted on top of the microbial lawn under anaerobic conditions at 30°C. CFUs were counted on day 6 after spotting.
Bacterial interference in the fermented skin fingerprints
Fingerprints of index, middle, and ring fingers were pressed onto the surfaces of agar plates composed of rich medium (10 ml) [10 g/l yeast extract (Biokar Diagnostics, Beauvais, France), 5 g/l TSB, 2.5 g/l K2HPO4 and 1.5 g/l KH2PO4] supplemented with/without glycerol (20 g/l). To mimic the overgrowth of P. acnes in lesions of acne vulgaris, a high dose of P. acnes (107 CFU in 5 μl PBS) was spotted on the central portion of fingerprints and grown for six days at 30°C under anaerobic conditions using Gas-Paks. Three subjects (2 males and 1 female) participated in fingerprinting on agar plates. All subjects were asked not to wash their hands before pressing their fingerprints. Fingers in the right hand were pressed on the glycerol-containing plates and fingers in the left hand were pressed on glycerol-free plates. The sequence analysis of 16S rRNA genes (Lindh et al. 2005) was performed to identify the microorganisms in fingerprints.
Nine single colonies of microorganisms, which created inhibition zones in three glycerol-containing plates derived from three subjects, were picked up by sterile toothpicks and boiled at 100°C for DNA extraction. The polymerase chain reaction (PCR) with 16S rRNA 27F and 534R primers in addition to sequencing of PCR products were conducted as previously described (Lindh et al. 2005). The 16S rRNA gene sequences were analyzed using the basic local alignment search tool (BLASTn).
Fermentation of microorganisms
The skin microorganisms (105 CFU/ml) isolated from the surface of the human nose by tape stripping using a D-Squame Standard Sampling Discs adhesive tape strip (CuDerm Corporation, Dallas, TX, USA) with a diameter of 2.0 cm were incubated in rich medium in the absence and presence of 20 g/l glycerol under anaerobic conditions at 30°C. Rich medium plus 20 g /l glycerol without microorganisms was included as a control. The 0.001% (w/v) phenol red (Sigma, St. Louis, MO, USA) in rich medium with 20 g/l glycerol served as an indicator, converting from red-orange to yellow when fermentation occurs.
Identification of SCFAs in the fermented media of microorganisms by nuclear magnetic resonance (NMR) analysis
The skin microorganisms isolated from the surface of the human nose were incubated in phenol red-free rich medium with 13C3-glycerol (20 g/l) (Cambridge Isotope Laboratories, Andover, MA, USA) for six days. After that, microorganisms were discarded by centrifugation at 5,000 g for 30 min. Fermented media were then passed through 0.2-μm-pore-size filters. SCFAs and other metabolites in the microorganism-free media were identified by NMR analysis. The one-dimensional (1-D) NMR spectra were measured on a JEOL-ECS NMR spectrometer operating at a resonance frequency of 400 MHz with a repetition delay of 3 sec for both 1H and 13C. The 2-D 1H-13C heteronuclear single quantum correlation (HSQC) NMR spectra were acquired on a Bruker Avance 600 MHz NMR spectrometer with a triple resonance inverse (TCI) cryo-probe and recorded as 2048 × 256 complex points with 32 scans and 1 sec repetition time. Newly appearing peaks belonged to the intermediate or final products resulting from 13C3-glycerol fermentation by microorganisms (Chitarra et al. 2000).
Minimal bactericidal concentration (MBC) assays
To determine the MBC values of SCFAs, P. acnes (10 μl; 108 CFU/ml in PBS) was incubated overnight with SCFAs at various concentrations (10 μl; 2.5–100 mM in PBS) as indicated in each individual experiment in media on a 96-well microplate (100 μl per well). The control received only 10 μl PBS. After incubation, the bacteria were diluted 1:10–1:106 with PBS. MBC was defined as a 99.9% killing level and determined by spotting the dilution (5 μl) on an agar plate supplemented with media for CFU counting. To determine the effect of pH on its survival, P. acnes in PBS was incubated overnight with 5 mM succinic acid (10 μl) on a 96-well microplate (100 μl per well) before spotting on an agar plate. As controls, P. acnes was incubated with 10 μl of PBS (pH 7.4) alone, PBS (pH 5.5; a pH value corresponding to the MBC of succinic acid in PBS), or buffered succinic acid (5 mM succinic acid, pH 7.4 buffered with ammonium hydroxide) on a 96-well microplate (100 μl per well). To determine the inhibitory constant (Ki), the growth of P. acnes (ATCC6919) in PBS or succinic acid (1–50 mM) for 0, 6 12, 24, 30 and 36 h was measured by reading OD600. The Ki for succinic acid was calculated using the software Curve Expert 1.4™ via the Haldane equation as previously described (Rigo and Alegre 2004).
Measurement of intracellular pH
Measurement of intracellular pH of P. acnes using a carboxyfluorescein succinimidyl ester (cFSE) florescence probe (Life Technologies, Grand Island, NY) was previously described (Chitarra et al., 2000). Briefly, bacteria were loaded with cFSE (5 μM) for 30 min at 37°C in 50 mM 4-(2-hydroxyethyl)-1 -piperazineethanesulfonic acid (HEPES) and 5 mM ethylenediaminetetraacetic acid (EDTA). To eliminate unbound probe, bacteria were incubated with glucose (10 mM) for an additional 30 min, washed twice in PBS with 10 mM MgCl2, pH 7.0, and then re-suspended in PBS. The cFSE-loaded bacteria (3 × 104 CFU) were dispensed on a 96-well microplate containing 100 μl/well of PBS or succinic acid (5 mM). Fluorescence intensities were measured immediately and every min for 5 min using an excitation wavelength of 490 nm and emission wavelength of 520 nm. A drop in relative fluorescence indicates a decrease in intracellular pH. Fluorescence of the bacteria-free filtrate (background fluorescence) was measured after the 5-min assay. In this case, treated suspensions were centrifuged at 5,000 g for 5 min. The fluorescence of the bacteria-free supernatant was measured and deducted from values for the treated suspensions. Calibration curves were obtained by incubation of un-treated, cFSE-loaded bacteria in buffers of various pHs. The buffer containing glycine (50 mM), citric acid (50 mM), Na2HPO4.2H2O (50 mM), and KCl (50 mM) was adjusted to various pH values ranging from 4 to 10. Equilibration of the intracellular and extracellular pH was conducted by addition of 1 μM valinomycin and nigericin (Sigma, St. Louis, MO).
In vivo effects of succinic acid on P. acnes colonization and P. acnes-induced inflammation
The Institute for Cancer Research (ICR) mice (2–3 month-old females; Harlan Labs, Placentia, CA, USA) were anesthetized by isoflurane. Five mice per group were used in each experiment. The ears of ICR mice were injected intradermally with P. acnes (107 CFU in 10 μl PBS) or PBS (10 μl) using a 28-gauge needle. One day after injection, succinic acid (5 mM; 10 μl) or PBS was injected into inflamed lesions for an additional two days. For topical application, succinic acid (100 mM; 10 μl) or PBS was applied topically on the surface of inflamed lesions once per day every day for three days. Succinic acid or PBS was applied topically to mouse ears away from the needle injection sites to avoid it entering the dermis via a hole created by the needle injection. Ears were excised, weighed and homogenized for cytokine detection and bacterial counts. The macrophage-inflammatory protein-2 (MIP-2) in supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) kit as directed by the manufacturer (BD Biosciences, San Diego, CA). The level of MIP-2 was normalized to the total amount of protein per gram of excised ear. To determine the bacterial counts in P. acnes-inoculated ears, mouse ears were excised and homogenized in 200 μl of sterile PBS with a tissue grinder. Bacterial CFUs in the mouse ears were enumerated by plating serial dilutions (1:101 –1:106) of the homogenate on Brucella broth agar plates (BD, Sparks, MD) supplemented with 5% (v/v) defibrinated sheep blood (LAMPIRE Biological Laboratories, Pipersville, PA), vitamin K (5 mg/ml, Remel, Lenexa, KS), and hemin (50 mg/ml, Remel, Lenexa, KS). The plates were incubated for 3 days at 37oC under anaerobic conditions using Gas-Pak (BD, Sparks, MD) to count colonies. The bacterial numbers (CFUs) per gram of excised ear were calculated. A Student’s t-test was used to determine the significance of the differences between groups. Data represent a 95% confidence interval (95% CI) of the mean from three independent experiments. All experiments using mice were conducted in a biosafety level 2 (BSL-2) facility and in accordance with institutional guidelines for animal experiments.
Statistical analysis
To determine significances between groups, comparisons were made using the two-tailed t-test. For all statistical tests, the P-values of <0.05 (*), <0.01 (**), and <0.001 (***) were accepted for statistical significance.
GenBank accession numbers of the 16S rRNA gene sequences
Nine colonies of skin microorganisms that created inhibition zones at the boundary of a P. acnes were selected for 16S rRNA gene sequencing. The 16S rRNA genes derived from eight of these colonies, namely EH-1, EH-2, EH-3, EH-4, EH-5, EH-6, EH-7, and EH-8, shared 97–99% identity with the 16S rRNA genes in S. epidermidis ATCC12228 or S. epidermidis RP62A. The 16S rRNA genes derived from one colony, namely EH-9, had 96% homology to the 16S rRNA genes in Paenibacillus sp. Y412MC10. The bankit numbers, sequenceID, and GenBank accession numbers of 16S rRNA sequences of nine bacterial colonies (EH-1 to EH-9) are: BankIt1661928, Seq1, KF683955; BankIt1661902, Seq2, KF683947; BankIt1661903, Seq3, KF683948; BankIt1661906, Seq4 , KF683949; BankIt1661907, Seq5, KF683950; BankIt1661908, Seq6 , KF683951; BankIt1661908, Seq6, KF683951; BankIt1661908, Seq6, KF683951; BankIt1661909, Seq7, KF683952; BankIt1661910, Seq8, KF683953; BankIt1661911, Seq9, KF683954. To obtain the phylogenetic relationships of EH-1 to EH-9, multiple sequence alignment was performed by ClustalX (ver 1.83) using the default parameters. The neighbor-joining (NJ) phylogenetic tree was constructed using Kimura’s 2-parameter model (Kimura 1980).
RESULTS
Bacterial interference enhanced by fermentation
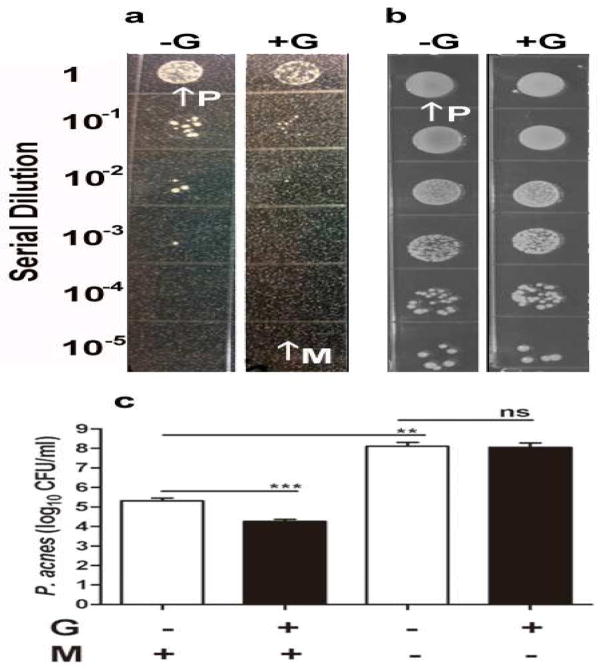
Serial dilutions of P. acnes were spotted on the top of a homogeneous lawn of skin microorganisms. As shown in Figure 1a, the colonies of P. acnes were significantly reduced (2.1 ± 0.8 × 105 CFU) (without glycerol) and 1.7 ± 0.3 × 104 CFU (with glycerol)] when they were grown on the top of a microbial lawn in the presence of glycerol under anaerobic conditions for 3 days (Figure 1a, c). Glycerol was specifically chosen for fermentation over other carbon sources because it is a naturally produced metabolite found in human skin (Fluhr et al. 2008) and has been approved by FDA for skin care products. Although P. acnes is an obligate anaerobic organism, it is capable of growing under both aerobic and anaerobic conditions (Cove et al. 1983; Uckay et al. 2010). The reduction in the colonies of P. acnes was not observed when P. acnes/skin microorganisms were grown on an agar plate and incubated under aerobic conditions (data not shown). There was no significant difference in the number of P. acnes colonies grown on regular agar plates (without a microbial lawn on the bottom) in the absence (1.2 ± 0.2 × 108 CFU) or presence (1.0 ± 0.5 × 108 CFU) of glycerol (Figure 1b, c). However, in the absence of glycerol, the number of P. acnes colonies that grew with microorganisms on a microbial lawn was more than three logs lower than the number of P. acnes colonies that grew without microorganisms (Figure 1a, b, c). The results suggest that bacterial interference occurred between P. acnes and skin microorganisms, and interference of P. acnes growth by skin microorganisms was enhanced by glycerol fermentation.
Figure 1. Inhibition of the growth of P. acnes by skin microorganisms in a homogeneous microbial lawn.
(a) A homogeneous lawn of microbes was created by pouring the skin microorganisms (M, arrow; 105 CFU) that were pre-mixed with 1% agar with/without glycerol (+G/-G; 20 g/l) in TSB. P. acnes (P, arrow) bacteria with a serial dilution (5 × 106- 5 × 101 CFU in 5 μl PBS) were spotted on the top of microbial lawn for three days for CFU counts. (b) The serially diluted P. acnes was spotted on the regular plates (without pouring skin microorganisms) with/without glycerol. (c) The CFU counts of P. acnes were presented as 95% CI of the means of three independent experiments. **P<0.01; ***P<0.001 was obtained by two-tailed t-tests. ns, not significant.
Bacterial interference in the fermented skin fingerprints
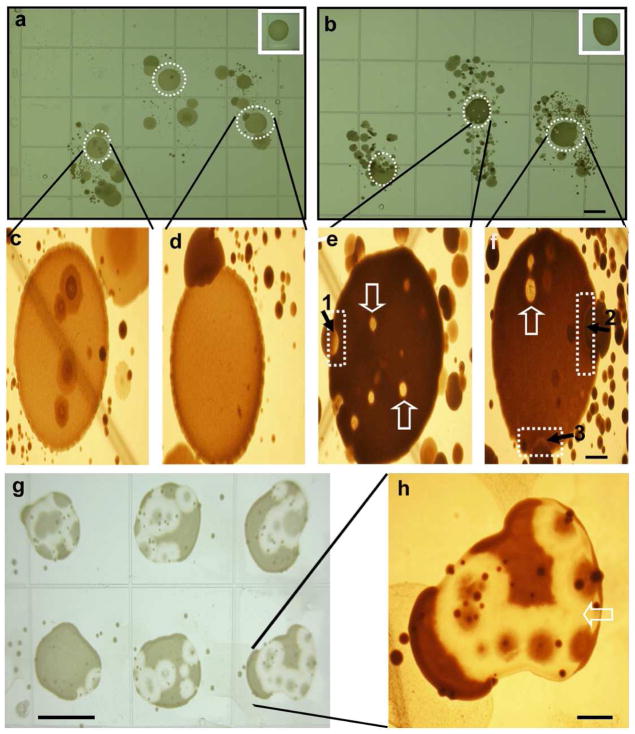
Although the isolated skin microorganisms (Figure 1) exerted anti-P. acnes action, these microorganisms may not fully represent the diversity of microbial populations on human skin since some skin bacteria are not cultivable. In addition, it is possible that only a few dominant microorganisms in a mixture of microorganisms may survive after multiple passages. To obtain skin microorganisms without multiple passages, fingerprints of index, middle, and ring fingers were pressed onto the surface of agar plates supplemented with or without glycerol. Since the overgrowth of P. acnes has been linked to acne vulgaris, we dropped a high dose of P. acnes (ATCC6919; 107 CFU) on the central portion of the fingerprints. The interaction between P. acnes and skin microorganisms on the fingerprints was observed daily. Under anaerobic conditions, P. acnes grew into a larger colony and was surrounded by skin microorganisms six days after incubation (Figure 2). On the glycerol-free agar plates, the colonies of P. acnes and skin microorganisms grew close to each other without developing inhibition zones (Figure 2a, c, d). Some microorganisms can grow within a large P. acnes colony. However, on the glycerol-containing agar plates, inhibition zones, the areas on the agar plate that remain free from microbial growth, were detected at the boundary of the P. acnes colonies and the skin microorganisms (Figure 2b, e, f). Some skin microorganisms created bubble-like competition territories within a colony of P. acnes. The bubble-like competition territories were not due to the gas production during fermentation because they were not formed within a large P. acnes colony that grew on the same agar plate, but instead formed far away from skin microorganisms (Figure 2b, insert panel). These results suggest that skin microorganisms can interfere with the growth of P. acnes through glycerol fermentation.
Figure 2. Skin fingerprint analysis of glycerol fermentation of skin microorganisms against P. acnes.
The fingerprints of index, middle, and ring fingers were pressed onto the surface of rich medium agar plates in the absence (a) or presence (b) of 20 g/l glycerol at 30°C unde r anaerobic conditions using Gas-Paks. P. acnes (107 CFU in 5 μl PBS) was spotted on the central portion of each fingerprint. Spotting P. acnes away from fingerprints served as controls (inserts). The high magnitude photos of (a) and (b) were displayed in (c, d) and (e, f), respectively. The inhibition zones (dash squares in e and f) were detected at the boundary between colonies of P. acnes and skin microorganisms. The bubble-like territories of competition (open arrows) were found within P. acnes colonies. In a representative plate, single colonies labeled 1 and 2 (solid arrows) were identified as S. epidermidis. A single colony labeled 3 was identified as Paenibacillus sp. Y412MC1. Six additional colonies from fingerprint bacteria of two different subjects were identified as S. epidermidis. S. epidermidis (105 CFU in 100μl PBS) from colony 1 was re-streaked on an agar plate containing glycerol followed by spotting six separate drops of P. acnes (107 CFU in 5 μl PBS) on the top of a S. epidermidis streak (g). A high magnitude photo of one of P. acnes colonies (g) was displayed in (h). Bars (a-b, g)=0.5 cm; (c-f, h)=0.1 cm.
Identification of S. epidermidis as a skin probiotic microorganism against P. acnes
The sequence analysis of 16S rRNA genes (Lindh et al. 2005) was performed to identify skin microorganisms. Single colonies of nine skin microorganisms that created inhibition zones at the boundary of a P. acnes colony, were picked up for 16S rRNA gene sequencing. The combination of PCR using isolated DNA with 16S rRNA 27F and 534R primers with DNA sequencing was conducted as previously described (Lindh et al. 2005). The 16S rRNA genes derived from eight of these colonies shared 97–99% identity with the 16S rRNA genes in S. epidermidis ATCC12228 or S. epidermidis RP62A (data not shown). Phylogenetic analysis revealed that most bacterial colonies were clustered together with S. epidermidis RP62A and S. epidermidis ATCC12228 (Supplementary Figure S1). The 16S rRNA genes derived from one of the colonies had 96% homology to the 16S rRNA genes in Paenibacillus sp. Y412MC10. S. epidermidis is a common skin bacterium (Cogen et al. 2010). While S. epidermidis is a facultative bacterium, it has been reported that it can undergo fermentation under anaerobic conditions (Sivakanesan and Dawes 1980). Paenibacillus is a genus of facultative anaerobic, Gram-positive bacteria and can be detected in a variety of environments including soil and water as well as in clinical samples (Chow et al. 2012). Although Paenibacillus sp. is not a skin permanent bacterium, it has been reported that the strain of Paenibacillus sp. JDR-2 can ferment pyruvate to propionic acid (Chow et al. 2012). Taken together, these results demonstrate that 1) the human skin microbiome contains both short-term and long-term resident microorganisms (Lemon et al. 2012), and 2) S. epidermidis may control overgrowth of P. acnes via fermentation. To validate the anti-P. acnes activity of fermenting S. epidermidis, one of the colonies identified as S. epidermidis (105 CFU in 100 μl) was re-streaked on new plates containing glycerol. A high dose of P. acnes (ATCC6919; 107 CFU) was then spotted on the plate. Many inhibition zones developed within a large P. acnes colony (Fig. 2g, f), demonstrating that S. epidermidis exerts probiotic activity against P. acnes. Although selected colonies shared 97–99% identity with S. epidermidis, an ATCC (12228) S. epidermidis strain was chosen to confirm its inhibitory activity against P. acnes (Supplementary Figure S2). P. acnes was spotted on the top of a homogeneous lawn of S. epidermdis in the absence or presence of glycerol under anaerobic conditions for 3 days. A zone of inhibition between P. acnes and S. epidermidis colonies was observed only on the S. epidermdis lawn containing glycerol. This result indicates that glycerol fermentation is indispensable for S. epidermidis to repel P. acnes.
SCFAs in fermented media of skin microorganisms
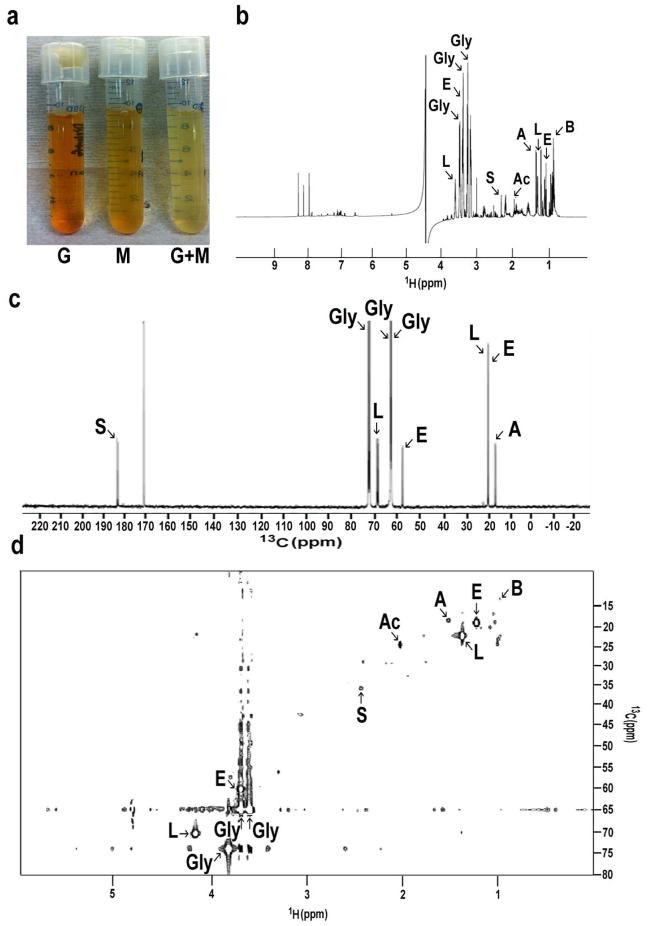
To examine their fermentation activity, skin microorganisms were incubated in rich medium under anaerobic conditions in the presence of glycerol. Rich media plus either glycerol or skin microorganisms were used as controls. To monitor the fermentation process, cultures were tested with phenol red, a fermentation indicator, to assess SCFA production as a result of glycerol fermentation. Only media in the culture of skin microorganisms with glycerol turned yellow (more acidic) after six days of incubation (Figure 3a), indicating fermentation of skin microorganisms. This finding was further validated quantitatively by measuring the pH values of rich media. The pH values of rich media containing glycerol, microorganisms and glycerol plus microorganisms were 6.5, 6.4, and 6.0, respectively, following 6 days of incubation. To identify the SCFAs in the ferments, the skin microorganisms were incubated in rich medium under anaerobic conditions in the presence of 13C3-glycerol (20 g/l) for six days. Supernatants of microbial fermentation in 10% deuterium oxide (D2O) were subjected to 1-D (Figure 3b, c) and 2-D (Figure 3d) 13C and 1H NMR analysis. In addition to ethanol and alanine, four SCFAs [acetic acid, butyric acid, lactic acid, and succinic acid] were detected in the fermented media of skin microorganisms. These four SCFAs, but not ethanol or alanine, were also detectable in the 13C3-glycerol fermented media of a selected re-streaked colony (Fig. 2g, f) of S. epidermidis (data not shown). These results demonstrate that skin microorganisms including S. epidermidis fermentatively metabolized 13C3-glycerol into SCFAs.
Figure 3. Identification of SCFAs in the fermented media of skin microorganisms.
(a) Skin microorganisms (105 CFU/ml) were incubated in rich medium in the absence (M) and presence (G+M) of glycerol for six days under anaerobic conditions. Rich medium plus glycerol without skin microorganisms (G) was included as a control. Fermented media of skin microorganisms were centrifuged and passed through a 0.2 μm filter. Supernatants were then mixed with 10% D2O and analyzed by NMR spectrometers. Representative 1-D 1H- (b) and 13C- (c) NMR spectra (400 MHz) that reveal the principal SCFAs in the fermented media six days after addition of 13C3-glycerol. (d) A 2-D 1H-13C HSQC NMR spectrum (600 MHz) was displayed. In addition to glycerol (Gly), ethanol (E), alanine (A), four SCFAs [acetic acid (Ac), butyric acid (B), lactic acid (L), and succinic acid (S)] were detected in the ferments of skin microorganisms.
Succinic acid decreases the survival of P. acnes via reduction of intracellular pH of P. acnes
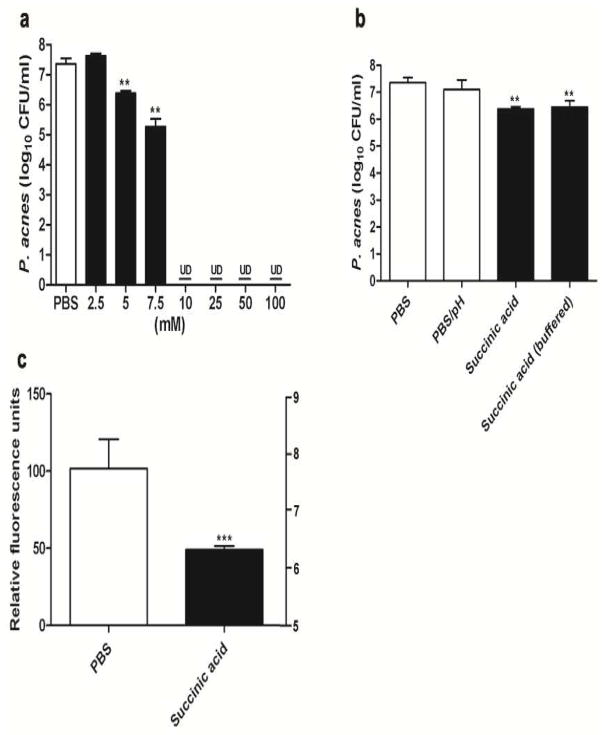
MBC assays were performed to determine if SCFAs exert antimicrobial activities against P. acnes. Bacteria were incubated with acetic acid, butyric acid, lactic acid, and succinic acid at various concentrations in media for 24 h. After incubation, the bacteria were diluted with PBS and spotted on an agar plate to count CFUs. The MBC values of acetic acid, butyric acid, lactic acid, and succinic acid against P. acnes were 7.5, 10, 10, and 5 mM, respectively (Figure 4a and Supplementary Figure S3). Since succinic acid had the lowest MBC value, we determined the Ki of the growth of P. acnes for 0–36 h in the presence of succinic acid. The Ki for succinic acid was 0.97 mM (Supplementary Figure S4). This acid was selected for evaluation of its anti-P. acnes activity in vivo (Figure 5). Succinic acid effectively suppressed the survival of P. acnes at concentrations ≥5 and 7.5 mM, and completely killed P. acnes at a concentration ≥10 mM (Figure 4a). To assess the acidity (pH 5.5) of 5 mM succinic acid in affecting survival of P. acnes, bacteria were incubated with PBS (pH 5.5) or ammonium hydroxide-buffered succinic acid (pH 7.4). Incubation of P. acnes with PBS (pH 5.5) did not alter the survival of P. acnes. The antimicrobial activity of succinic acid persisted even after buffering 5 mM succinic acid with ammonium hydroxide (Figure 4b), suggesting that the ability to suppress the survival of P. acnes by succinic acid was unrelated to direct killing by extracellular acidification.
Figure 4. The MBC of succinic acid against P. acnes, the effect of pH on the anti-P. acnes activity of succinic acid, and the decrease in intracellular pH of P. acnes by succinic acid.
(a) P. acnes (108 CFU/ml) was incubated with succinic acid (2.5–100 mM in PBS) on a 96-well microplate overnight. Bacteria incubated with PBS alone as a control. (b) P. acnes was incubated with PBS (pH 7.4), PBS (PBS/pH; pH 5.5), 5 mM succinic acid (pH 5.5) or ammonium hydroxide-buffered succinic acid (pH 7.4) to determine if the acidity of 5 mM succinic acid affects the survival of P. acnes. After incubation, P. acnes was diluted 1:10–1:106 with PBS, and 5 μl of the dilutions were spotted on an agar plate for CFU counts. (c) The cFSE-loaded P. acnes (3 × 104 CFU) was treated with 5 mM succinic acid or PBS. The change in the relative fluorescence units corresponding to intracellular pH of P. acnes was measured 5 min after treatment. **P<0.01; ***P<0.001 (two-tailed t-tests). Data are 95% CI of the means of three individual experiments. UD, undetectable.
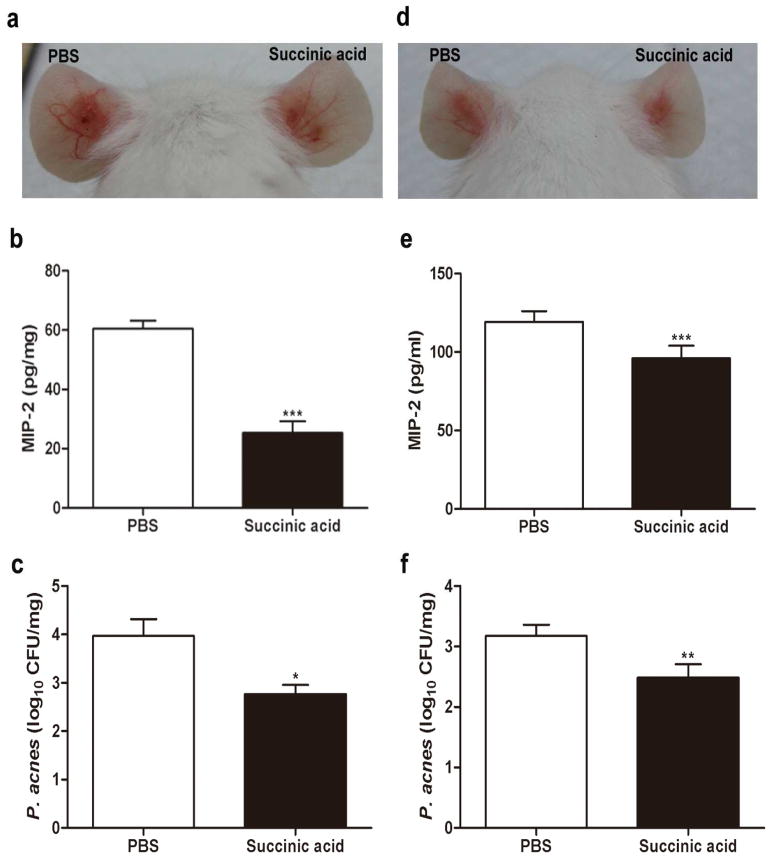
Figure 5. Succinic acid suppresses P. acnes-induced inflammation and decreases bacterial colonization in vivo.
The ears of ICR mice were injected intradermally with P. acnes (107 CFU in 10 μl PBS) or PBS (10 μl). One day after injection of P. acnes or PBS, succinic acid or PBS was intralesionally injected into inflamed lesions (a-c) or topically applied on the surface of inflamed lesions (d-f). Photos of ear inflammation were taken three (a) or four (d) days after P. acnes injection. The levels of MIP-2 cytokines (b, e) in the homogenates of succinic acid- or PBS-treated ears were measured by an ELISA kit. The CFUs (c, f) in the ears treated with succinic acid or PBS were enumerated by plating serial dilutions (1:101–1:106) of the homogenate on an agar plate. ***P<0.001; ***P<0.01; *P<0.05. P-values were evaluated using two-tailed t-tests. Data are 95% CI of the means of three separate experiments using five mice per group.
The antimicrobial effects of SCFAs are caused mainly by the undissociated form of SCFAs (Ostling and Lindgren 1993; Ricke 2003). Non-dissociated SCFAs can passively diffuse through the cell wall of microorganisms and, once internalized into the neutral pH of the cell cytoplasm, can dissociate into anions and protons. Generation of both anions and protons presents potential problems for microorganisms that must maintain their cytoplasm at a near-neutral pH in order to sustain functional macromolecules. Export of excess protons requires consumption of cellular adenosine triphosphate (ATP) and may result in depletion of cellular energy (Ostling and Lindgren 1993). To determine the mechanism of action of succinic acid against P. acnes, we loaded P. acnes with cFSE, an internally conjugated fluorescent pH probe. As shown in Figure 4c, succinic acid significantly lowered the intracellular pH of P. acnes, in agreement with previous findings that a lowered intracellular pH of microbe is a lethal mechanism of SCFA (Ricke 2003).
In vivo efficacy of succinic acid against P. acnes
To examine the effectiveness of succinic acid as an intralesional injection therapy against P. acnes, mouse ears were injected with a single intradermal injection of P. acnes. An intradermal injection was used as an animal model for the granulomatous type of acne inflammation that follows follicular rupture based on previous publications demonstrating that intradermal injection of P. acnes into mouse ears induces a remarkable granulomatous response (Liu et al. 2011; Nakatsuji et al. 2008a; Nakatsuji et al. 2008b), as well as the fact that P. acnes can enter the dermis after follicular wall rupture in severe acne (Kligman 1974; Nakatsuji et al. 2008a; Nakatsuji et al. 2008b). Furthermore, the outbred ICR mice were used for this study because they are polymorphic at a significant number of loci and have a complex genetic history similar to a human population, potentially making these results more applicable to the human population. After the intradermal injection of P. acnes, succinic acid (10 μl; 5 mM, a MBC concentration) or PBS control was injected into the same sites previously injected with P. acnes (Figure 5). Injection of succinic acid reduced P. acnes-induced redness compared with injection of an equal amount of PBS (Figure 5a). It has been reported that P. acnes can induce the production of interleukin (IL)-8 via activation of toll-like receptor 2 (TLR-2) (Kim 2005; Nagy et al. 2005). To determine whether succinic acid can reduce the production of P. acnes-induced inflammation, ears were homogenized two days after injection with succinic acid or PBS. The level of MIP-2, a murine counterpart of IL-8, was measured by an ELISA. MIP-2 production in the ear injected with succinic acid was approximately 50% less than that detected in the ear injected with PBS (Figure 5b). To determine the intensity of bacterial colonization, ears injected with succinic acid or PBS were homogenized to estimate the CFU. The P. acnes numbers in ears injected with PBS and succinic acid were 4.7 ± 1.3 × 105 and 2.9 ± 1.3 × 104 CFU, respectively, suggesting that succinic acid considerably decreased the growth of P. acnes in the lesions (Figure 5c).
SCFAs can penetrate human skin and have even been used as skin penetration enhancers (Kanikkannan et al. 2000). Since topical anti-acne agents can be designed as both over-the-counter and prescription medications, the potency of topical application of succinic acid against P. acnes was evaluated. One day after P. acnes injection, the surface of the P. acnes-inoculated mouse ear was treated with 100 mM topical succinic acid or PBS once per day. Both P. acnes-induced redness (Figure 5d) and MIP-2 production (Figure 5e) were significantly attenuated in succinic acid-treated ears compared to PBS-treated ears. The P. acnes numbers in succinic acid- and PBS-treated ears were 7.5 ± 1.5 × 104 and 1.5 ± 0.4 × 104 CFU, respectively (Figure 5f). Results in Figure 5 demonstrate the effective use of intralesional and topical succinic acid for the suppression of inflammation and P. acnes growth in vivo.
DISCUSSION
The human body is home to ten times more bacteria than human cells (Fujimura et al. 2010). The skin is the human body's largest organ, colonized by a diverse milieu of microorganisms (the skin microbiome), most of which are commensal organisms since they are harmless or sometimes even beneficial to their host (Grice and Segre 2011). SCFAs in the skin play a key role in influencing the composition of bacteria on normal human skin (Ushijima et al. 1984). It has been documented that P. acnes can undergo glycerol fermentation to produce SCFAs (Moss et al. 1967). Thus, application of glycerol on acne lesions may trigger the fermentation of P. acnes, which influences the growth of other skin commensals. As shown in Supplementary Figure S5, P. acnes suspension/agar was poured into agar plates to produce a homogeneous P. acnes lawn. Glycerol was used to trigger the fermentation of P. acnes. Serial dilutions of skin microorganisms were spotted on the top of a homogeneous P. acnes lawn. The colony numbers of skin microorganisms were no different on agar plates with/without glycerol, suggesting that the fermentation of P. acnes did not significantly disrupt the growth of skin microorganisms.
Bacterial interference in which friendly bacteria are used to prevent colonization of the host by pathogens has been shown to be a promising modality for preventing and/or treating infections (Frank et al. 2010; Ji et al. 1997; Nicoll and Jensen 1987; Otto 2009; Wei et al. 2006; Whitehead et al. 1993; Wilkinson and Jensen 1987). Therapeutic application of bacterial interference by active colonization using a human commensal bacterial strain, S. epidermidis, was successful in counteracting the infection of Staphylococcus aureus (S. aureus) (Iwase et al. 2010; Shinefield et al. 1971). Results from a previous study have shown that S. epidermidis secretes a serine protease to inhibit the colonization of S. aureus (Iwase et al. 2010). A previous publication from our laboratory has demonstrated that P. acnes can exploit glycerol fermentation to suppress the growth of pathogenic USA300, the most prevalent community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) (Shu et al. 2013). All of the above studies demonstrated the ability of commensal bacteria to prevent colonization by pathogens. Little is known about the interactions among commensal bacteria. A condition with microbial imbalances on or inside the human body has been termed dysbiosis (Scanlan et al. 2012). Our results indicate that S. epidermidis is a probiotic bacterium that may employ glycerol fermentation to rein in the overgrowth of P. acnes and therefore control the dysbiosis that manifests as acne vulgaris.
As shown in Figure 1, we demonstrated that skin microorganisms inhibited the growth of P. acnes. Although this inhibition may result from antimicrobial proteins/peptides and/or nutrient competition, it was significantly augmented by glycerol fermentation. Eight colonies selected from skin microorganisms that were identified as S. epidermidis developed inhibition zones when they were co-cultured with P. acnes (Figure 2). We speculate that, under normal physiologic conditions, S. epidermidis and P. acnes may co-exist on the skin surface without counteracting each other. S. epidermidis may later enter acne lesions when acne comedones are created by the overgrowth of P. acnes. Human hosts may take advantage of S. epidermidis fermentation within an anaerobic acne lesion to combat the overgrowth of P. acnes. Thus, future studies will include detecting bacterial survival after co-injection of S. epidermidis and P. acnes with glycerol in mice. In addition, given that some papers report that the two bacteria do not coexist to a great extent (Fitz-Gibbon et al. 2013; Moon et al. 2012), it is possible that the abundance of P. acnes and S. epidermidis in an acne lesion may depend on the stage of acne vulgaris. If glycerol fermentation of S. epidermidis against P. acnes occurs in an acne lesion, it is worth determining if the ratio of P. acnes to S. epidermidis correlates with the severity of acne vulgaris.
The bacterial interference defined by the formation of inhibition zones and bubble-like territories of competition between P. acnes and other skin microorganisms was detectable in the microbiome of fingerprints. Although the composition of the human skin microbiome varies from individual to individual and is dynamic over time in every individual (Grice and Segre 2011), S. epidermidis, a long-term resident microorganism in the skin, appeared to strongly compete against P. acnes in three different subjects. Paenibacillus sp. EH-9 are mainly found in the environment and thus recognized as a short-term skin resident microorganism. Our observation above supports an ecological theory that each person can be viewed as an island-like “patch” of habitat occupied by both long-term and short-term microbial assemblages (Costello et al. 2012). Skin microorganisms collected from both the surface of the nose (Figure 1) and the fingertips (Figure 2) can mediate glycerol fermentation to interfere with the growth of P. acnes. Due to different locations and culture media, the composition of skin microorganisms cultured from fingertips may be distinct from that of skin microorganisms cultured from the surface of the nose. Although S. epidermdis on the fingertips was identified as a fermenting bacterium against P. acnes, it is unclear whether S. epidermidis contributes to the bacterial interference between P. acnes and skin microorganisms cultured from the surface of the nose. To address this issue, skin microorganisms cultured from nasal surface were streaked on agar plates supplemented with or without glycerol followed by spotting P. acnes on the top of bacterial streaks. A zone of inhibition of bacterial growth developed exclusively on the agar plates containing glycerol (Supplementary Figure S6). A colony of skin microorganisms that created an inhibition zone within a P. acnes colony was identified as S. epidermdis by 16S rRNA gene sequencing, demonstrating that glycerol fermentation of S. epidermidis in skin microorganisms cultured from the surface of the nose played a role in outcompeting P. acnes.
Both intralesional and topical application of succinic acid significantly neutralize P. acnes-induced inflammation (Figure 5). The P. acnes-induced MIP-2 production in the ear injected intralesionally with PBS or succinic acid (Figure 5b) was markedly higher than that in the ear treated with topical PBS or succinic acid (Figure 5d). The higher production of MIP-2 may result from the two consecutive needle injections with P. acnes or PBS/succinic acid. It has been reported that SCFAs have anti-inflammatory activities (Vinolo et al. 2011). Succinic acid can activate a G-protein coupled receptor (GPR) to prevent inflammation (Karaki et al. 2006). SCFAs can regulate several leukocyte functions including production of cytokines [tumor necrosis factor alpha (TNF)-α, IL-2, IL-6 and IL-10]. The ability of leukocytes to migrate to foci of inflammation and destroy microbial pathogens can be affected by the SCFAs (Vinolo et al. 2011). In addition, SCFAs, most notably butyrate, significantly reduced expression of lipopolysaccharide (LPS)-induced interferon (IFN)-γ, TNF-α, and IL-12 (Chakravortty et al. 2000), and S. aureus-induced IL-2 and IFN-γ (Park et al. 2007). Aquaporin-3 functions as a glycerol transporter in mammalian skin (Zheng and Bollinger Bollag 2003). It has been known that glycerol helps maintain healthy skin integrity (Fluhr et al. 2008). Aquaporin 3-deficient mice exhibit skin defects, including impairment of water holding capacity, barrier recovery, and wound healing (Zheng and Bollinger Bollag 2003). The above results suggest that acne probiotics containing SCFAs and glycerol may be bi-functional therapeutics targeting both P. acnes and skin cells.
Results in Figure 2 and Supplementary Figure S1 indicated that glycerol fermentation of S. epidermidis was essential in counteracting P. acnes. Succinic acid exerted efficient effects against P. acnes (Figure 5), however, it still remains unclear which SCFA in the products of S. epidermidis glycerol fermentation primarily contributes to the anti-P. acnes effect. It is also undetermined whether SCFAs act together with other antimicrobial molecules in fermentation products to display their anti-P. acnes activities. Our recent results have demonstrated that the fermented media of S. epidermidis ATCC12228 significantly suppressed the growth of P. acnes (Supplementary Figure S7). The anti-P. acnes activity of the fermented media persisted after boiling the fermented media, suggesting that the antimicrobial proteins/peptides may be not the major contributors to the anti-P. acnes activity of fermented media. A higher dose of SCFAs may be required to achieve in vivo efficacy due to its rapid metabolism by skin cells (Schroder et al. 2000; Stein et al. 2000). The pro-drugs of SCFAs such as pivaloylomethyl butyrate (AN-9) (Blank-Porat et al. 2007)have been developed to achieve pharmacologic concentrations of SCFAs in vivo. S. epidermidis grown on rich medium agar plates without glycerol failed to develop inhibition zones against P. acnes (Figure 2 and Supplementary Figure S1). In fact, TSB in rich media contains 2.5 g/l glucose. Thus, in the absence of 20 g/l glycerol, S. epidermidis may produce insufficient amounts of SCFAs via glucose fermentation.
Although ferments (SCFAs) were used in this study as anti-P. acnes agents, live S. epidermidis can potentially be used as an active component in acne probiotics for bacteriotherapy against acne vulgaris. Future studies will include an injection of S. epidermidis along with P. acnes into mouse ears in the absence or presence of glycerol. Ear homogenates will be spotted on an agar plate supplemented with furazolidone (furoxone), a culture medium selective for P. acnes to determine the presence of the organism in individuals with and without acne vulgaris (Marino and Stoughton 1982). Since S. epidermidis does not grow on this medium, only the colonies of P. acnes can be seen on a plate spotted with ear homogenates containing both S. epidermidis and P. acnes. The interference of S. epidermidis with P. acnes in vivo can be thus quantified using furazolidone-supplemented agar plates. SCFAs are normal human metabolites and theoretically less toxic, but SCFAs at high doses may create an extremely acidic solution that may be toxic to skin cells. Thus, buffered SCFAs or pro-drugs of SCFAs may serve as alternative anti-P. acnes agents. Application of succinic acid notably, but not completely, suppressed the P. acnes-induced inflammation (Figure 5). Thus, application with an acne probiotic composed of more than one SCFA (Martin-Pelaez et al. 2010) or multiple beneficial microorganisms may be needed for full potency. S. epidermidis that interferes with the growth of P. acnes via fermentation was isolated from the human skin microbiome in an attempt to develop acne probiotics. We believe that various skin microorganisms have the specific ability to antagonize different (non-)pathogens using fermentation. Thus, besides acne probiotics, other “skin probiotics” using fermentation of skin microorganisms to treat various skin conditions can potentially be achieved.
Supplementary Material
Acknowledgments
This work was supported by NIH grants (1R41AR064046-01 and 1R21AI088147). We thank Dr. Teruaki Nakatsuji for assistance for the 16S rRNA gene sequencing.
References
- US Food and Drug Administration. Listing of Food Additives Status Part II. 2011 Oct 27; PUblisher. http://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/ucm191033.htm#ftn.
- Blank-Porat D, Gruss-Fischer T, Tarasenko N, Malik Z, Nudelman A, Rephaeli A. The anticancer prodrugs of butyric acid AN-7 and AN-9, possess antiangiogenic properties. Cancer Lett. 2007;256(1):39–48. doi: 10.1016/j.canlet.2007.05.011. S0304-3835(07)00248-0 [pii] [DOI] [PubMed] [Google Scholar]
- Burtenshaw JM. The mechanism of self-disinfection of the human skin and its appendages. J Hyg (Lond) 1942;42(2):184–210. doi: 10.1017/s0022172400035373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravortty D, Koide N, Kato Y, Sugiyama T, Mu MM, Yoshida T, Yokochi T. The inhibitory action of butyrate on lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophage cells. J Endotoxin Res. 2000;6(3):243–7. [PubMed] [Google Scholar]
- Chitarra LG, Breeuwer P, Van Den Bulk RW, Abee T. Rapid fluorescence assessment of intracellular pH as a viability indicator of Clavibacter michiganensis subsp. michiganensis. J Appl Microbiol. 2000;88(5):809–16. doi: 10.1046/j.1365-2672.2000.01014.x. [pii] [DOI] [PubMed] [Google Scholar]
- Chow V, Nong G, St John FJ, Rice JD, Dickstein E, Chertkov O, Bruce D, Detter C, Brettin T, Han J, Woyke T, Pitluck S, Nolan M, Pati A, Martin J, Copeland A, Land ML, Goodwin L, Jones JB, Ingram LO, Shanmugam KT, Preston JF. Complete genome sequence of Paenibacillus sp. strain JDR-2. Stand Genomic Sci. 2012;6(1):1–10. doi: 10.4056/sigs.2374349. sigs.2374349 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130(1):192–200. doi: 10.1038/jid.2009.243. jid2009243 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–62. doi: 10.1126/science.1224203. science.1224203 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove JH, Holland KT, Cunliffe WJ. Effects of oxygen concentration on biomass production, maximum specific growth rate and extracellular enzyme production by three species of cutaneous propionibacteria grown in continuous culture. J Gen Microbiol. 1983;129(11):3327–34. doi: 10.1099/00221287-129-11-3327. [DOI] [PubMed] [Google Scholar]
- Demaerel P, Van Hecke P, Van Oostende S, Baert AL, Jaeken J, Declercq PE, Eggermont E, Plets C. Bacterial metabolism shown by magnetic resonance spectroscopy. Lancet. 1994;344(8931):1234–5. doi: 10.1016/s0140-6736(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr Comp Biol. 2004;44(4):315–23. doi: 10.1093/icb/44.4.315. 44/4/315 [pii] [DOI] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, Modlin RL, Miller JF, Sodergren E, Craft N, Weinstock GM, Li H. Propionibacterium acnes Strain Populations in the Human Skin Microbiome Associated with Acne. J Invest Dermatol. 2013;133(9):2152–60. doi: 10.1038/jid.2013.21jid201321. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159(1):23–34. doi: 10.1111/j.1365-2133.2008.08643.x. BJD8643 [pii] [DOI] [PubMed] [Google Scholar]
- Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5(5):e10598 d. doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435–54. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach SL, Mayhew JW, Bartlett JG, Thadepalli H, Onderdonk AB. Rapid diagnosis of anaerobic infections by direct gas-liquid chromatography of clinical speciments. J Clin Invest. 1976;57(2):478–84. doi: 10.1172/JCI108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–53. doi: 10.1038/nrmicro2537. nrmicro2537 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004;292(6):726–35. doi: 10.1001/jama.292.6.726. 292/6/726 [pii] [DOI] [PubMed] [Google Scholar]
- Huang CP, Liu YT, Nakatsuji T, Shi Y, Gallo RR, Lin SB, Huang CM. Proteomics integrated with Escherichia coli vector-based vaccines and antigen microarrays reveals the immunogenicity of a surface sialidase-like protein of Propionibacterium acnes. Proteomics Clin Appl. 2008;2(9):1234–45. doi: 10.1002/prca.200780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahiyerobo-Ip JI, Dinulos JG. Changing the topography of acne with topical medications. Curr Opin Pediatr. 2011;23(1):121–5. doi: 10.1097/MOP.0b013e3283425457. [DOI] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465(7296):346–9. doi: 10.1038/nature09074. nature09074 [pii] [DOI] [PubMed] [Google Scholar]
- Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276(5321):2027–30. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem. 2000;7(6):593–608. doi: 10.2174/0929867003374840. [DOI] [PubMed] [Google Scholar]
- Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353–60. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–8. doi: 10.1159/000087011. 87011 [pii] [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kligman AM. An overview of acne. J Invest Dermatol. 1974;62(3):268–87. doi: 10.1111/1523-1747.ep12676801. [DOI] [PubMed] [Google Scholar]
- Layton AM, Dreno B, Gollnick HP, Zouboulis CC. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20(7):773–6. doi: 10.1111/j.1468-3083.2006.01671.x. JDV1671 [pii] [DOI] [PubMed] [Google Scholar]
- Lemon KP, Armitage GC, Relman DA, Fischbach MA. Microbiota-targeted therapies: an ecological perspective. Sci Transl Med. 2012;4(137):137rv5. doi: 10.1126/scitranslmed.3004183. 4/137/137rv5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RM, Rasmussen JE. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch Dermatol. 1983;119(6):480–1. [PubMed] [Google Scholar]
- Lindh JM, Terenius O, Faye I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl Environ Microbiol. 2005;71(11):7217–23. doi: 10.1128/AEM.71.11.7217-7223.2005. 71/11/7217 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Nakatsuji T, Zhu W, Gallo RL, Huang CM. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine. 2011;29(17):3230–8. doi: 10.1016/j.vaccine.2011.02.036. S0264-410X(11)00249-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CW, Lai YK, Liu YT, Gallo RL, Huang CM. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting beta-hemolysin and CAMP factor. J Invest Dermatol. 2011;131(2):401–9. doi: 10.1038/jid.2010.319. jid2010319 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino C, Stoughton RB. Clinical use of a selective culture medium for wild and antibiotic-resistant Propionibacterium acnes. J Am Acad Dermatol. 1982;6(5):902–8. doi: 10.1016/S0190-9622(82)80124-2. [DOI] [PubMed] [Google Scholar]
- Martin-Pelaez S, Costabile A, Hoyles L, Rastall RA, Gibson GR, La Ragione RM, Woodward MJ, Mateu E, Martin-Orue SM. Evaluation of the inclusion of a mixture of organic acids or lactulose into the feed of pigs experimentally challenged with Salmonella Typhimurium. Vet Microbiol. 2010;142(3–4):337–45. doi: 10.1016/j.vetmic.2009.09.061. S0378-1135(09)00497-0 [pii] [DOI] [PubMed] [Google Scholar]
- Menon S, Bharadwaj R, Chowdhary AS, Kaundinya DV, Palande DA. Rapid identification of non-sporing anaerobes using nuclear magnetic resonance spectroscopy and an identification strategy. Indian J Med Microbiol. 2007;25(4):330–5. doi: 10.4103/0255-0857.37334. [DOI] [PubMed] [Google Scholar]
- Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39(10):833–7. doi: 10.1111/j.1346-8138.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- Moss CW, Dowell VR, Jr, Lewis VJ, Schekter MA. Cultural characteristics and fatty acid composition of Corynebacterium acnes. J Bacteriol. 1967;94(5):1300–5. doi: 10.1128/jb.94.5.1300-1305.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124(5):931–8. doi: 10.1111/j.0022-202X.2005.23705.x. JID23705 [pii] [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Liu YT, Huang CP, Zoubouis CC, Gallo RL, Huang CM. Antibodies elicited by inactivated propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008a;128(10):2451–7. doi: 10.1038/jid.2008.117. jid2008117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Liu YT, Huang CP, Zouboulis CC, Gallo RL, Huang CM. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS One. 2008b;3(2):e1551 d. doi: 10.1371/journal.pone.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Shi Y, Zhu W, Huang CP, Chen YR, Lee DY, Smith JW, Zouboulis CC, Gallo RL, Huang CM. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008c;8(16):3406–15. doi: 10.1002/pmic.200800044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797 d. doi: 10.1371/journal.pone.0014797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll TR, Jensen MM. Staphylococcosis of turkeys. 5. Large-scale control programs using bacterial interference. Avian Dis. 1987;31(1):85–8. [PubMed] [Google Scholar]
- Nishijima S, Kurokawa I, Katoh N, Watanabe K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions. J Dermatol. 2000;27(5):318–23. doi: 10.1111/j.1346-8138.2000.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Ostling CE, Lindgren SE. Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol. 1993;75(1):18–24. doi: 10.1111/j.1365-2672.1993.tb03402.x. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis--the 'accidental' pathogen. Nat Rev Microbiol. 2009;7(8):555–67. doi: 10.1038/nrmicro2182. nrmicro2182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7(1):70–7. doi: 10.1016/j.intimp.2006.08.015. S1567-5769(06)00261-X [pii] [DOI] [PubMed] [Google Scholar]
- Ricke SC. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult Sci. 2003;82(4):632–9. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- Rigo M, Alegre RM. Isolation and selection of phenol-degrading microorganisms from industrial wastewaters and kinetics of the biodegradation. Folia Microbiol (Praha) 2004;49(1):41–5. doi: 10.1007/BF02931644. [DOI] [PubMed] [Google Scholar]
- Ryssel H, Kloeters O, Germann G, Schafer T, Wiedemann G, Oehlbauer M. The antimicrobial effect of acetic acid--an alternative to common local antiseptics? Burns. 2009;35(5):695–700. doi: 10.1016/j.burns.2008.11.009. S0305-4179(08)00361-6 [pii] [DOI] [PubMed] [Google Scholar]
- Scanlan PD, Buckling A, Kong W, Wild Y, Lynch SV, Harrison F. Gut dysbiosis in cystic fibrosis. J Cyst Fibros. 2012;11(5):454–5. doi: 10.1016/j.jcf.2012.03.007. S1569-1993(12)00046-X [pii] [DOI] [PubMed] [Google Scholar]
- Schroder O, Opritz J, Stein J. Substrate and inhibitor specificity of butyrate uptake in apical membrane vesicles of the rat distal colon. Digestion. 2000;62(2–3):152–8. doi: 10.1159/000007807. 7807 [pii] 7807. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Phillip LE, Fellner V, Idziak ES. Comparative assessment of bacterial inoculation and propionic acid treatment of aerobic stability and microbial populations of ensiled high-moisture ear corn. J Anim Sci. 1996;74(2):447–56. doi: 10.2527/1996.742447x. [DOI] [PubMed] [Google Scholar]
- Shinefield HR, Ribble JC, Boris M. Bacterial interference between strains of Staphylococcus aureus, 1960 to 1970. Am J Dis Child. 1971;121(2):148–52. doi: 10.1001/archpedi.1971.02100130102013. [DOI] [PubMed] [Google Scholar]
- Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, Gallo RL, Huang CM. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One. 2013;8(2):e55380. doi: 10.1371/journal.pone.0055380. PONE-D-12-30971 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakanesan R, Dawes EA. Anaerobic glucose and serine metabolism in Staphylococcus epidermidis. J Gen Microbiol. 1980;118(1):143–57. doi: 10.1099/00221287-118-1-143. [DOI] [PubMed] [Google Scholar]
- Stein J, Zores M, Schroder O. Short-chain fatty acid (SCFA) uptake into Caco-2 cells by a pH-dependent and carrier mediated transport mechanism. Eur J Nutr. 2000;39(3):121–5. doi: 10.1007/s003940070028. [DOI] [PubMed] [Google Scholar]
- Uckay I, Dinh A, Vauthey L, Asseray N, Passuti N, Rottman M, Biziragusenyuka J, Riche A, Rohner P, Wendling D, Mammou S, Stern R, Hoffmeyer P, Bernard L. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect. 2010;16(4):353–8. doi: 10.1111/j.1469-0691.2009.02801.x. CLM2801 [pii] [DOI] [PubMed] [Google Scholar]
- Ushijima T, Takahashi M, Ozaki Y. Acetic, propionic, and oleic acid as the possible factors influencing the predominant residence of some species of Propionibacterium and coagulase-negative Staphylococcus on normal human skin. Can J Microbiol. 1984;30(5):647–52. doi: 10.1139/m84-096. [DOI] [PubMed] [Google Scholar]
- Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–76. doi: 10.3390/nu3100858. nutrients-03-00858 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Cao Z, Zhu YL, Wang X, Ding G, Xu H, Jia P, Qu D, Danchin A, Li Y. Conserved genes in a path from commensalism to pathogenicity: comparative phylogenetic profiles of Staphylococcus epidermidis RP62A and ATCC12228. BMC Genomics. 2006;7:112. doi: 10.1186/1471-2164-7-112. 1471-2164-7-112 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Leavitt RW, Jensen MM. Staphylococcosis of turkeys. 6. Development of penicillin resistance in an interfering strain of Staphylococcus epidermidis. Avian Dis. 1993;37(2):536–41. [PubMed] [Google Scholar]
- Wilkinson DM, Jensen MM. Staphylococcosis of turkeys. 4. Characterization of a bacteriocin produced by an interfering Staphylococcus. Avian Dis. 1987;31(1):80–4. [PubMed] [Google Scholar]
- Zheng X, Bollinger Bollag W. Aquaporin 3 colocates with phospholipase d2 in caveolin-rich membrane microdomains and is downregulated upon keratinocyte differentiation. J Invest Dermatol. 2003;121(6):1487–95. doi: 10.1111/j.1523-1747.2003.12614.x. 12614 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.