Abstract
Researchers have theorized that social and psychosocial factors increase vulnerability to the deleterious health effects of environmental hazards. We used baseline examination data (2000–2002) from the Multi-Ethnic Study of Atherosclerosis. Participants were 45–84 years of age and free of clinical cardiovascular disease at enrollment (n = 6814). The modifying role of social and psychosocial factors on the association between exposure to air pollution comprising particulate matter less than 2.5 µm in aerodynamic diameter (PM2.5) and blood pressure measures were examined using linear regression models. There was no evidence of synergistic effects of higher PM2.5 and adverse social/psychosocial factors on blood pressure. In contrast, there was weak evidence of stronger associations of PM2.5 with blood pressure in higher socioeconomic status groups. For example, those in the 10th percentile of the income distribution (i.e., low income) showed no association between PM2.5 and diastolic blood pressure (b = −0.41 mmHg; 95% confidence interval: −1.40, 0.61), whereas those in the 90th percentile of the income distribution (i.e., high income) showed a 1.52-mmHg increase in diastolic blood pressure for each 10-µg/m3 increase in PM2.5 (95% confidence interval: 0.22, 2.83). Our results are not consistent with the hypothesis that there are stronger associations between PM2.5 exposures and blood pressure in persons of lower socioeconomic status or those with greater psychosocial adversity.
Keywords: air pollution, blood pressure, population groups, social environment, social medicine, social psychology
A large body of work has assessed the health impacts of social factors and environmental hazards, although this work has generally appeared in separate disciplinary literatures. Consensus is growing on the need to investigate their joint effects, as they are often spatially correlated, may operate through common biological mechanisms, and may act synergistically to affect health (1–6). Indeed, the United States Air Pollution Prevention and Control Act (also known as the Clean Air Act) requires that the National Ambient Air Quality Standards protect populations that may be particularly vulnerable to the health effects of air pollution (7). It is therefore a public health imperative to understand the factors that increase vulnerability to the health effects of air pollution.
There is some evidence that social and psychosocial factors may be important modifiers of the associations between air pollution and health. Socially and economically disadvantaged groups, such as blacks and those of low socioeconomic status (SES), exhibit stronger associations between air pollution and health than do whites or persons in higher SES groups, respectively (8–11), although this finding has not been consistent across all studies (12–14). Evidence has also suggested that psychosocial stress increases the harmful effects of air pollution on asthma (4, 15, 16) and other respiratory diseases (17).
Although several studies have focused on respiratory or mortality outcomes, a growing body of work has documented positive associations between particulate matter less than 2.5 µm in aerodynamic diameter (PM2.5) and cardiovascular disease (CVD) (5, 18–20) and blood pressure (21, 22). A parallel body of work has documented associations of social disadvantage and psychosocial factors with CVD (23–25), including hypertension (26–29). The impact of air pollution on cardiovascular outcomes may be modified by social disadvantage or psychosocial factors. Limited evidence has suggested that social disadvantage enhances the unhealthy associations between PM2.5 and health (9, 30, 31), although results have not always been consistent (32), and the research on the interactive associations between air pollution and social or psychosocial factors and blood pressure is scant.
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA) and the MESA and Air Pollution (MESA Air) Study to investigate the extent to which short-term exposures to PM2.5 and social and psychosocial exposures may act together to affect blood pressure. This work builds on findings of a cross-sectional association between PM2.5 and baseline blood pressure in MESA (21) by exploring the modifying role of social and psychosocial factors. Specifically, we examined 1) whether SES or race/ethnicity modified the association between PM2.5 and blood pressure, and 2) whether psychosocial factors previously linked to changes in blood pressure modified the association between PM2.5 and blood pressure. We hypothesized that associations of PM2.5 with blood pressure would be stronger in socially disadvantaged groups and in persons with greater levels of psychosocial adversity.
MATERIALS AND METHODS
Study population and data
Data came from the baseline examination of MESA, a longitudinal study in 6 sites (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St. Paul, Minnesota). Details of the MESA cohort have been published elsewhere (33). Briefly, 6,814 adults of white or black race or Hispanic or Chinese ethnicity who were between the ages of 45–85 years and free of clinical CVD were recruited for the study in 2000–2002 through various population-based approaches. Although MESA is currently a longitudinal study, the present study builds on prior work using baseline data only (21).
On the basis of prior work (21), systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP), and mean arterial pressure (MAP) were the outcomes examined. Blood pressure was measured using the Critikon Dinamap Pro 100 (Critikon, Tampa, Florida). The average of the second and third of 3 seated blood pressure readings taken 2 minutes apart were used in the analyses. PP was calculated as SBP minus DBP. MAP was calculated as follows: (((2 × DBP) + SBP)/3).
To focus analyses on temporal rather than spatial variation in exposure, daily PM2.5 estimates were calculated for each MESA site using the average of all United States Environmental Protection Agency Air Quality System monitoring stations within each MESA site with complete time series during the 2000–2002 study period. Data from all such monitors within the boundaries of the following areas were included in the site-wide averages: Baltimore County, Maryland; Cook County, Illinois; Los Angeles County, California; Bronx County, New York; Ramsey and Hennepin Counties, Minnesota; and Forsyth County, North Carolina. Each participant was assigned the PM2.5 concentration for that site for the month prior to their examination visit. On the basis previous work that showed that the most consistent associations of PM2.5 with blood pressure were observed for the average exposure for the month prior to the examination (21), we used a prior 1-month average PM2.5 as the key measure, with other averaging periods examined in sensitivity analyses.
Social disadvantage was measured using race/ethnicity and SES information that were collected via questionnaire. Race/ethnicity was categorized as: non-Hispanic white, Chinese, non-Hispanic black, and Hispanic. Educational level was examined as a continuous variable representing the number of years of education. Family income for the previous 12 months was reported at each examination and divided into 13 categories. Income was operationalized as continuous by using the midpoint of the reported category. Median household income in the census tract of the respondent was collected from the 2000 Census.
The psychosocial factors examined included chronic stress, depressive symptoms, trait anger, trait anxiety, and lack of emotional support. The measure of chronic stress was a chronic burden of stress scale collected via questionnaire (34). Chronic stress was measured as the sum of the number of times the participant affirmed the current presence of a moderately or very stressful ongoing problem in any of 5 domains (own health, close person's health, job, finances, or relationship) that lasted more than 6 months. The range of the scale was 0 to 5. Depressive symptoms were assessed via responses to 20 questions from the Center for Epidemiologic Studies Depression Scale (35) about the frequency of depressive feelings and behaviors over the previous week. Responses were on a Likert-like scale ranging from 1 (<1 day) to 4 (5–7 days). The range of the scale was 20 to 80. Trait anger and trait anxiety scores were determined as the sum of the responses to 10 questions each from the Spielberger Trait Anger Expression Inventory and Spielberger Trait Anxiety Inventory about the current frequency with which the participant had anger-related or anxiety-related feelings and behaviors, respectively (36). The responses on were on a Likert-like scale ranging from 1 (almost never) to 4 (almost always), with the range of the scale 10 to 40. Emotional support was measured as the sum of the responses to 6 questions from the Emotional Social Support Index about the frequency of the respondent's perceived availability of current emotional support. Responses were on Likert-like scale ranging from 1 (all of the time) to 5 (none of the time). The items were reverse-coded so that greater values represented lack of emotional support, which is a more adverse psychosocial situation,. The range of the scale was 6 to 30.
Additional covariates included sociodemographic and health-related measures. Data on sex, age (in years), current alcohol use (yes or no), current diabetes status (yes or no, using American Diabetes Association 2003 criteria (37)), and antihypertensive medication use were collected via questionnaire. Body mass index was calculated from measured height and weight. A categorical variable to capture the combined role of smoking and exposure to second-hand smoke (ETS) was created from reports of smoking and ETS. The categories were current smoker, former smoker with less than 1 hour/week of ETS, former smoker with 1 or more hours/week of ETS, (d) never smoker with less than 1 hour/week of ETS, and never smoker with 1 or more hours/week of ETS. Apparent temperature for the month prior to the examination was calculated using relative humidity, temperature, and an equation from the National Oceanic and Atmospheric Administration (38). Because apparent temperature is related to blood pressure in a nonlinear fashion, splines were created with a knot at 11.4°C based on exploratory analyses. The month of each examination was noted, and variables to capture season (January–March, April–June, July–September, and October–December) were created as was done in a previous study (21).
The number of participants at the baseline examination was 6,814. No information on 1-month average PM2.5 concentration was available for 811 persons, and information on blood pressure was lacking from an additional 3 persons. The following key information was missing for an additional 539 persons: annual income (n = 210), smoking/ETS (n = 149), census tract median household income (n = 72), educational level (n = 19), diabetes status (n = 17), and alcohol use (n = 16). This yielded a final analytic sample size of 5,570 persons. The demographic characteristics of this subset were similar to those of the entire MESA cohort (results not shown).
Statistical analysis
First, descriptive analyses of the sample were conducted, and the distributions of potential modifying factors and covariates were summarized across quartiles of PM2.5 exposure.
Then, to examine the independent associations between blood pressure and each of the focal independent variables (PM2.5 exposure and social and psychosocial factors), linear regression models were estimated, with a separate model for each blood pressure outcome. Model 1 included the social disadvantage measures: race/ethnicity, educational level, income, and census tract median household income. Model 2 further included the 5 psychosocial measures. Model 3 included further adjustment for additional risk factors for hypertension: body mass index, smoking/ETS, diabetes, and current alcohol use. Model 3 was investigated in order to determine whether any observed associations were confounded and/or mediated by hypertension risk factors that have been linked to social disadvantage or psychosocial factors. All models included adjustment for age, sex, apparent temperature, season, antihypertensive medication use, MESA site, and the interaction between season and MESA site. This interaction was included because of the seasonal characteristics that may confound the association between PM2.5 and blood pressure differ by site.
Because social disadvantage and the psychosocial measures were included in the same models, correlations among these variables were computed and variance inflation factors examined for each of these factors as in model 2, which was the base model for all interactions presented in the figures (described below). High multicollinearity was defined as an average variance inflation factor greater than or equal to 10 (39). The average variance inflation factor was 4.49, with the individual variance inflation factors of the PM2.5, social, and psychosocial measures ranging from 1.28 (trait anger) to 2.87 (PM2.5).
Finally, to examine the presence of additive interactions between PM2.5 and each of the social disadvantage and psychosocial factors, the interaction between PM2.5 and each of these factors was added to model 2. In order to maximize power and utilize all available data, interactions were tested using continuous predictors. Graphical displays show predicted associations of PM2.5 with blood pressure for each category of race/ethnicity and at the 10th (i.e., low) and 90th (i.e., high) percentiles of continuous SES and psychosocial measure distribution (Figures 1–4).
Figure 1.
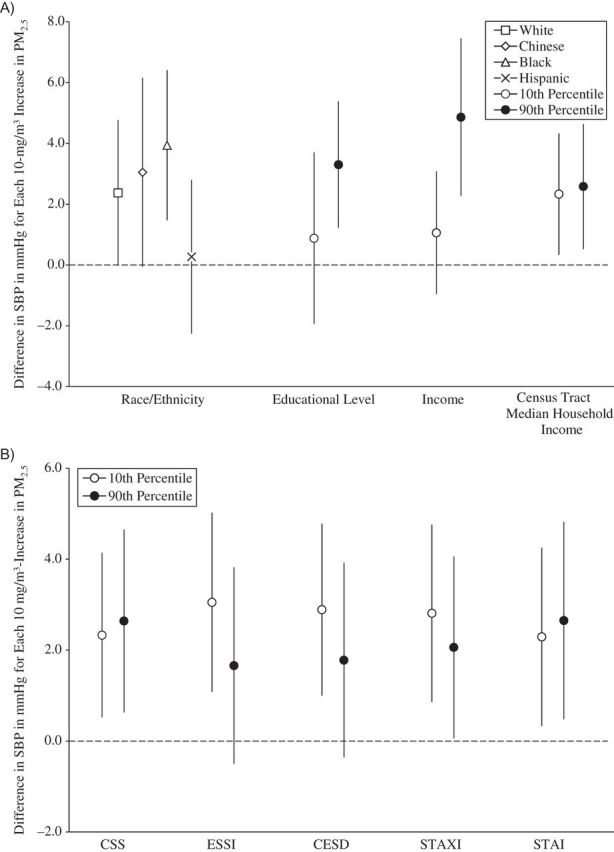
Associations between concentrations of particulate matter less than 2.5μm in aerodynamic diameter (PM2.5) and systolic blood pressure (SBP), by social disadvantage and psychosocial level or category, Multi-Ethnic Study of Atherosclerosis, 2000–2002. A) Associations by social disadvantage level or category. B) Associations by psychosocial level. All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity. The scale of the psychosocial measures is per 1 unit. Models were adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, and all other socioeconomic and psychosocial variables in the figure. Results are the difference in SBP per 10-μg/m3 increase in PM2.5 exposure averaged over the month prior to the examination. Income and census tract median household income values are per $10,000. CESD, Center for Epidemiologic Studies Depression Scale; CSS, Chronic Stress Scale; ESSI, Emotional Social Support Index (reverse-coded); STAI, Spielberger Trait Anxiety Inventory; STAXI, Spielberger Trait Anger Expression Inventory. Bars, 95% confidence intervals.
Figure 2.
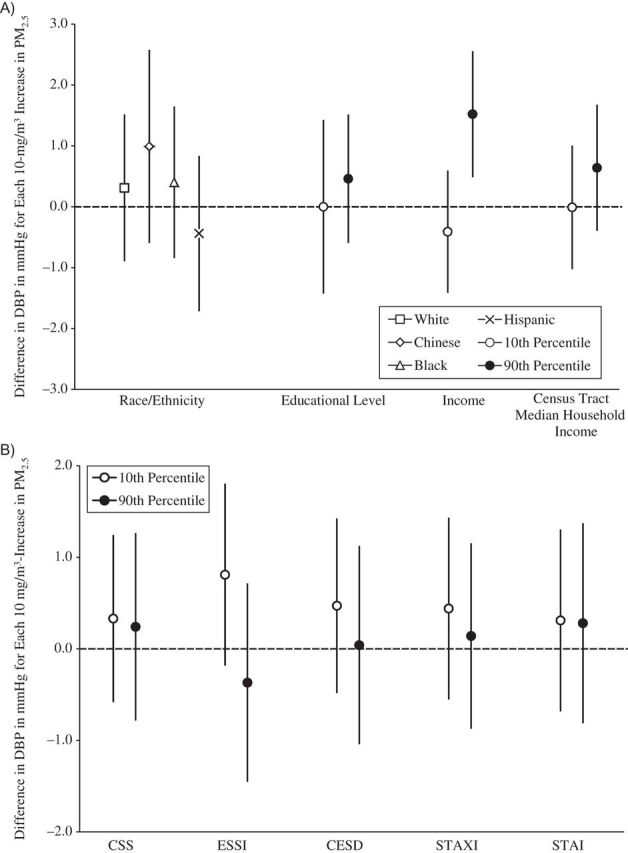
Associations between concentrations of particulate matter less than 2.5μm in aerodynamic diameter (PM2.5) and diastolic blood pressure (DBP), by social disadvantage and psychosocial level or category, Multi-Ethnic Study of Atherosclerosis, 2000–2002. A) Associations by social disadvantage level or category. B) Associations by psychosocial level. All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity. The scale of the psychosocial measures is per 1 unit. Models were adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, and all other socioeconomic and psychosocial variables in the figure. Results are the difference in DBP per 10-μg/m3 increase in PM2.5 exposure averaged over the month prior to the examination. Income and census tract median household income values are per $10,000. CESD, Center for Epidemiologic Studies Depression Scale; CSS, Chronic Stress Scale; ESSI, Emotional Social Support Index (reverse-coded); STAI, Spielberger Trait Anxiety Inventory; STAXI, Spielberger Trait Anger Expression Inventory. Bars, 95% confidence intervals.
Figure 3.
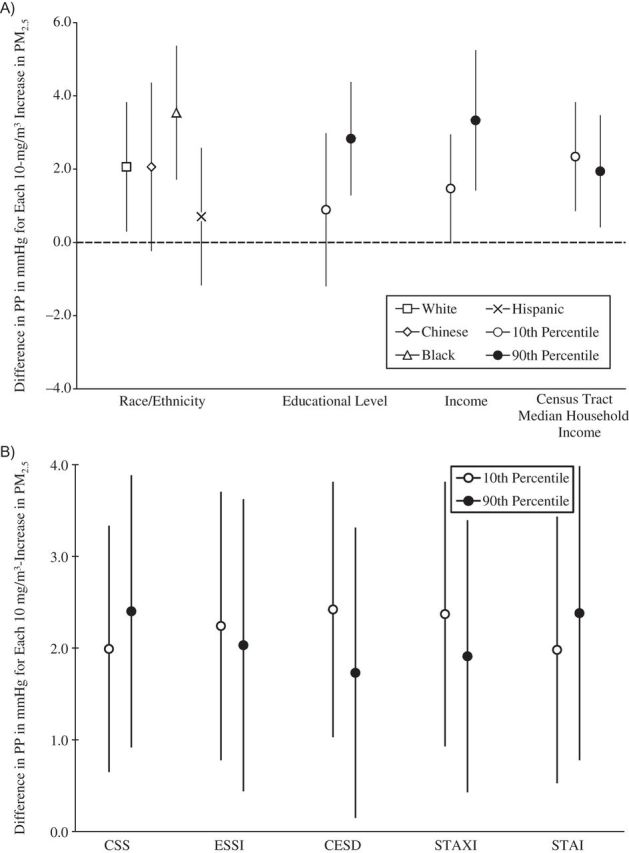
Associations between concentrations of particulate matter less than 2.5μm in aerodynamic diameter (PM2.5) and pulse pressure (PP), by social disadvantage and psychosocial level or category, Multi-Ethnic Study of Atherosclerosis, 2000–2002. A) Associations by social disadvantage level or category. B) Associations by psychosocial level. All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity. The scale of the psychosocial measures is per 1 unit. Models were adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, and all other socioeconomic and psychosocial variables in the figure. Results are the difference in PP per 10-μg/m3 increase in PM2.5 exposure averaged over the month prior to the examination. Income and census tract median household income values are per $10,000. CESD, Center for Epidemiologic Studies Depression Scale; CSS, Chronic Stress Scale; ESSI, Emotional Social Support Index (reverse-coded); STAI, Spielberger Trait Anxiety Inventory; STAXI, Spielberger Trait Anger Expression Inventory. Bars, 95% confidence intervals.
Figure 4.
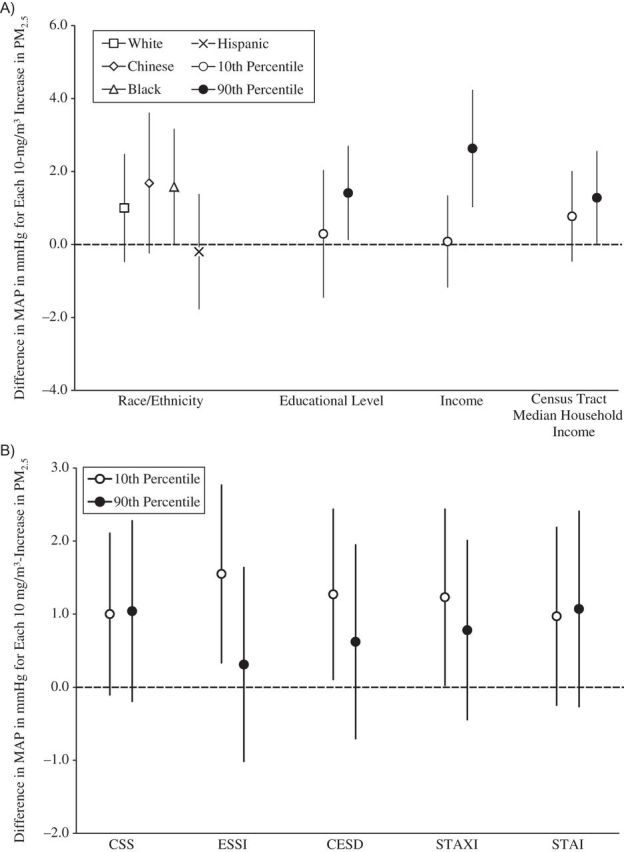
Associations between concentrations of particulate matter less than 2.5μm in aerodynamic diameter (PM2.5) and mean arterial pressure (MAP), by social disadvantage and psychosocial level or category, Multi-Ethnic Study of Atherosclerosis, 2000–2002. A) Associations by social disadvantage level or category. B) Associations by psychosocial level. All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity. The scale of the psychosocial measures is per 1 unit. Models were adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, and all other socioeconomic and psychosocial variables in the figure. Results are the difference in MAP per 10-μg/m3 increase in PM2.5 exposure averaged over the month prior to the examination. Income and census tract median household income values are per $10,000. CESD, Center for Epidemiologic Studies Depression Scale; CSS, Chronic Stress Scale; ESSI, Emotional Social Support Index; STAI, Spielberger Trait Anxiety Inventory; STAXI, Spielberger Trait Anger Expression Inventory. Bars, 95% confidence intervals.
Several sets of sensitivity analyses were performed. First, the impact of different approaches to account for use of antihypertensive medications was examined. These included 1) excluding those who reported antihypertensive medication use (n = 2536) and 2) adding 10 mmHg and 3) 15 mmHg to the SBPs of those who reported medication use. This latter approach has previously been shown to be as valid as more complex imputation approaches (40), and it may produce less bias than adjusting for medication use, which is a consequence of the outcome (41).
Second, other acute PM2.5 exposures, including 1-, 3-, and 7-day and 2-month averages, were examined. Finally, because PM2.5 exposure may vary within a site, all analyses were performed using predicted individual-level PM2.5 exposures at each participant's residence that accounted for both time and space (42, 43). These PM2.5 exposures were predicted from a combination of measurements from the Environmental Protection Agency Air Quality System and MESA Air monitoring stations and spatial covariates, such as proximity to roadways and local land use. These measures were available only for the 1-month and 2-month averaged time periods.
Institutional review board approval was granted at each study site, and written informed consent was obtained from all participants. All statistical tests for significance were 2-sided. Analyses were conducted in STATA MP, version 11.0 (StataCorp LP, College Station, Texas).
RESULTS
The distributions of covariates and outcomes across quartiles of PM2.5 exposure for the month prior to the visit are shown in Table 1. In general, these characteristics were similar across levels of air pollution, although air pollution and some covariates varied substantially among sites (data not shown), as has been documented in other work based on these data (32, 44). There were modest positive associations between SES and air pollution and modest inverse associations between psychosocial adversity and air pollution. There were no associations between any of the blood pressure outcomes and air pollution.
Table 1.
Sociodemographic Characteristics, Psychosocial Factors, and Blood Pressure Outcomes by Levels of Exposure to Particulate Matter Less Than 2.5 µm in Aerodynamic Diameter Averaged Over the Month Before the Examination, The Multi-Ethnic Study of Atherosclerosis, 2000–2002
| Variable | Quartile of PM2.5 Exposurea |
P for trendb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
||||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Sociodemographic characteristics | |||||||||
| Age, years | 62.5 (10.6) | 61.9 (9.9) | 61.5 (10.1) | 61.1 (9.8) | <0.001 | ||||
| Female sex | 52 | 55 | 54 | 50 | <0.05 | ||||
| Race/ethnicity | |||||||||
| White | 50 | 39 | 39 | 29 | <0.001 | ||||
| Chinese | 3 | 7 | 16 | 25 | |||||
| Black | 22 | 30 | 26 | 25 | |||||
| Hispanic | 25 | 23 | 18 | 21 | |||||
| Educational level, years | 13.2 (3.9) | 13.0 (3.9) | 13.5 (3.9) | 13.1 (4.4) | |||||
| Income/$10,000 | 4.8 (3.3) | 4.7 (3.2) | 5.3 (3.5) | 5.1 (3.7) | <0.001 | ||||
| Census tract median household income/ $10,000 | 4.4 (1.2) | 4.2 (2.1) | 4.6 (2.2) | 4.5 (2.4) | <0.001 | ||||
| Site | |||||||||
| Forsyth County, North Carolina | 21 | 15 | 15 | 11 | <0.001 | ||||
| New York, New York | 14 | 29 | 13 | 5 | |||||
| Baltimore, Maryland | 10 | 24 | 14 | 10 | |||||
| St. Paul, Minnesota | 44 | 13 | 6 | 0 | |||||
| Chicago, Illinois | 11 | 12 | 26 | 26 | |||||
| Los Angeles County, California | 1 | 7 | 27 | 49 | |||||
| Psychosocial characteristics | |||||||||
| Chronic stressors | 0.9 (1.1) | 0.9 (1.1) | 0.9 (1.1) | 0.8 (1.1) | <0.05 | ||||
| Lack of emotional support | 12.1 (5.3) | 11.8 (5.3) | 11.5 (5.1) | 11.5 (5.2) | <0.05 | ||||
| Depressive symptoms | 7.8 (7.3) | 7.8 (7.6) | 7.0 (6.9) | 6.9 (7.8) | <0.001 | ||||
| Trait anger | 14.7 (3.6) | 14.8 (3.7) | 14.7 (3.4) | 14.8 (3.6) | |||||
| Trait anxiety | 16.0 (4.4) | 15.9 (4.5) | 15.7 (4.3) | 15.7 (4.6) | <0.05 | ||||
| Hypertension risk factors and other confounders | |||||||||
| Smoking/ETS status | |||||||||
| Never smoker/ETS <1 hour/week | 26 | 31 | 34 | 37 | <0.001 | ||||
| Never smoker/ETS ≥1 hour/week | 20 | 18 | 20 | 16 | |||||
| Past smoker/ETS <1 hour/week | 18 | 20 | 19 | 22 | |||||
| Past smoker/ ETS ≥1 hour/week | 20 | 17 | 16 | 13 | |||||
| Current smoker | 14 | 13 | 12 | 12 | |||||
| Current alcohol use | 61 | 57 | 56 | 52 | <0.001 | ||||
| Body mass indexc | 28.8 (5.3) | 28.7 (5.6) | 27.8 (5.3) | 27.5 (5.3) | <0.001 | ||||
| Diabetes | 25 | 27 | 23 | 26 | |||||
| Antihypertensive medication use | 34 | 41 | 33 | 35 | <0.001 | ||||
| 1-month average apparent temperature, °C | 10.1 (8.3) | 8.0 (8.5) | 12.1 (9.8) | 12.9 (10.8) | <0.001 | ||||
| Blood pressure measures, mm Hg | |||||||||
| Systolic blood pressure | 126.0 (21.2) | 126.6 (20.7) | 124.5 (20.6) | 126.3 (21.4) | |||||
| Diastolic blood pressure | 71.5 (10.2) | 72.2 (10.1) | 71.4 (10.1) | 72.1 (10.3) | |||||
| Pulse pressure | 54.5 (17.1) | 54.3 (16.6) | 53.1 (16.2) | 54.2 (17.3) | |||||
| Mean arterial pressure | 89.6 (12.4) | 90.3 (12.2) | 89.1 (12.3) | 90.1 (12.5) | |||||
Abbreviations: ETS, exposure to second hand smoke; PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter; SD, standard deviation.
a Averaged over the month before the examination. Quartile 1, <13.2 μg/m3; quartile 2, 13.2−15.69 μg/m3; quartile 3, 15.7−19.19 μg/m3; and quartile 4, ≥19.2 μg/m3.
b P values (2-sided) by χ2 for trend for categorical variables and by regression for continuous variables.
c Weight (kg)/height (m)2.
After adjusting for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, and the SES measures, higher PM2.5 exposure during the month before the examination was associated with higher SBP (mean difference per 10-μg/m3 increase in PM2.5 = 2.49 mmHg; 95% confidence interval: 0.82, 4.15; SBP model 1 in Table 2). This association did not change after adjustment for psychosocial factors (SBP model 2) and was slightly attenuated after further adjustment for health-related factors (SBP model 3). PM2.5 was not associated with DBP (Table 2, DBP models 1–3). PM2.5 was positively associated with PP (mean difference per 10-μg/m3 increase in PM2.5 = 2.17 mmHg; 95% confidence interval: 0.94, 3.40; Table 3, PP Model 1). The association did not change after adjustment for psychosocial measures (PP model 2) and was slightly attenuated after adjustment for the health-related factors (PP model 3). There was also a positive association between PM2.5 and MAP (mean difference per 10-μg/m3 increase in PM2.5 = 1.04 mmHg; 95% confidence interval: 0.01, 2.07; MAP model 1 in Table 3). The association did not change with the addition of psychosocial (MAP model 2) or health-related (MAP model 3) factors. (Differences between these results and previously published results (21) are due to adjustment for the season-by-site interaction).
Table 2.
Mean Difference in Systolic and Diastolic Blood Pressure Associated With Exposure to Particulate Matter Less Than 2.5 µm in Diameter in the Previous Month and Covariates in Sequentially Adjusted Models, The Multi-Ethnic Study of Atherosclerosis, 2000–2002a
| Variable | Systolic Blood Pressure, mm Hg |
Diastolic Blood Pressure, mm Hg |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1b |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|||||||
| b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | |
| 1-month average PM2.5 /10 μg/m3 | 2.49** | 0.82,4.15 | 2.45** | 0.78,4.11 | 2.39** | 0.74,4.03 | 0.32 | −0.52, 1.16 | 0.30 | −0.54, 1.14 | 0.31 | −0.53, 1.15 |
| Social disadvantage | ||||||||||||
| Race/ethnicity | ||||||||||||
| White | Referent | Referent | Referent | Referent | Referent | Referent | ||||||
| Chinese | 1.60 | −0.36, 3.56 | 1.11 | −1.18, 3.39 | 2.87** | 0.56, 5.19 | 2.08*** | 1.09, 3.06 | 2.04*** | 1.04, 3.04 | 2.47*** | 1.43, 3.52 |
| Black | 6.47*** | 5.06, 7.88 | 9.51*** | 7.88, 11.14 | 6.81*** | 5.20, 8.43 | 3.97*** | 3.26, 4.67 | 3.91*** | 3.20, 4.63 | 3.80*** | 3.07, 4.53 |
| Hispanic | 4.40*** | 2.72, 6.09 | 4.31*** | 2.36, 6.27 | 2.33*** | 0.42, 4.24 | 1.26** | 0.42, 2.11 | 1.21** | 0.36, 2.06 | 1.10* | 0.24, 1.96 |
| Educational level (per year)c | −0.38*** | −0.54, −0.22 | −0.39*** | −0.55, −0.23 | −0.36*** | −0.52, −0.20 | −0.14*** | −0.22, 0.06 | −0.14*** | −0.22, 0.06 | −0.15*** | −0.23, 0.07 |
| Income (per $10,000)c | −0.23* | −0.42, −0.03 | −0.27** | −0.47, −0.08 | −0.27** | −0.47, −0.08 | 0.01 | −0.09, 0.10 | −0.02 | −0.11, −0.08 | −0.03 | −0.13, 0.07 |
| Census tract median household income/ $10,000c | 0.00 | −0.28, 0.29 | 0.00 | −0.28, 0.29 | 0.02 | −0.26, 0.30 | 0.04 | −0.11, 0.18 | 0.04 | −0.11, 0.18 | 0.03 | −0.11, 0.17 |
| Psychosocial adversityd | ||||||||||||
| Chronic stressors | 0.39 | −0.13, 0.90 | 0.15 | −0.37, 0.66 | 0.04 | −0.23, 0.30 | 0.00 | −0.27, 0.26 | ||||
| Lack of emotional support | −0.09 | −0.20, 0.01 | −0.08 | −0.19, 0.03 | −0.03 | −0.08, 0.03 | −0.02 | −0.08, 0.03 | ||||
| Depressive symptoms | −0.03 | −0.13, 0.06 | −0.05 | −0.14, 0.04 | 0.00 | −0.05, 0.04 | 0.00 | −0.05, 0.04 | ||||
| Trait anger | 0.10 | −0.05, 0.26 | 0.06 | −0.09, 0.21 | 0.05 | −0.03, 0.13 | 0.04 | −0.04, 0.12 | ||||
| Trait anxiety | −0.08 | −0.23, 0.07 | −0.03 | −0.18, 0.12 | −0.05 | −0.13, 0.03 | −0.04 | −0.12, 0.04 | ||||
Abbreviations: CI, confidence interval; PM2.5, particulate matter less than 2.5μm in aerodynamic diameter.
* P < 0.05, **P < 0.01, ***P < 0.001 by χ2 for trend for categorical variables and by regression for continuous variables.
a Results from main effect models (n = 5570).
b Model 1 was adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, race/ethnicity, educational level, income, and census tract median household income. Model 2 was adjusted for the factors in model 1 as well as ongoing burdens, depressive symptoms, trait anger, trait anxiety, and lack of emotional support. Model 3 was adjusted for the factors in model 2 as well as current alcohol use, smoking/exposure to tobacco smoke, body mass index, and diabetes.
c All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity.
d The scales of the psychosocial measures are per 1 unit.
Table 3.
Mean Difference in Pulse Pressure and Mean Arterial Pressure Associated With Exposure to Particulate Matter Less Than 2.5 µm in Diameter in the Previous Month and Covariates in Sequentially Adjusted Models, The Multi-Ethnic Study of Atherosclerosis, 2000–2002a
| Variable | Pulse Pressure, mm Hg |
Mean Arterial Pressure, mm Hg |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1b |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|||||||
| b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI | |
| 1-month average PM2.5 /10 μg/m3 | 2.17*** | 0.94, 3.40 | 2.15*** | 0.92, 3.38 | 2.08*** | 0.87, 3.29 | 1.04* | 0.01, 2.07 | 1.01 | 0.00, 2.04 | 1.00 | 0.00, 2.03 |
| Social disadvantage | ||||||||||||
| Race/ethnicity | ||||||||||||
| White | Referent | Referent | Referent | Referent | Referent | Referent | ||||||
| Chinese | −0.48 | −1.93, 0.96 | −0.40 | −1.87, 1.06 | 0.63 | −0.88, 2.14 | 1.92** | 0.71, 3.13 | 1.91** | 0.68, 3.13 | 2.68*** | 1.41, 3.96 |
| Black | 2.50*** | 1.46, 3.54 | 2.45*** | 1.40, 3.50 | 1.32* | 0.26, 2.38 | 4.80*** | 3.93, 5.67 | 4.73*** | 3.85, 5.61 | 4.24*** | 3.35, 5.13 |
| Hispanic | 3.14*** | 1.90, 4.38 | 3.14*** | 1.89, 4.39 | 2.27*** | 1.02, 3.51 | 2.31*** | 1.27, 3.35 | 2.26*** | 1.21, 3.30 | 1.86*** | 0.80, 2.91 |
| Educational level (per year)c | −0.24*** | −0.36,−0.13 | −0.25*** | −0.37, −0.13 | −0.22*** | −0.33, −0.10 | −0.22*** | −0.32, −0.12 | −0.22*** | −0.32, −0.13 | −0.22*** | −0.31, −0.13 |
| Income (per $10,000)c | −0.23*** | −0.37, −0.09 | −0.26*** | −0.40, −0.11 | −0.24*** | −0.39, −0.10 | −0.07 | -0.19, −0.05 | −0.10 | −0.22, 0.02 | −0.11 | −0.23, 0.01 |
| Census tract median household income/ $10,000c | −0.03 | −0.24, 0.17 | −0.03 | −0.24, 0.17 | −0.02 | −0.22, 0.19 | 0.03 | −0.15, 0.20 | 0.03 | −0.15, 0.20 | 0.03 | −0.15, 0.20 |
| Psychosocial adversityd | ||||||||||||
| Chronic stressors | 0.35 | −0.03, 0.73 | 0.15 | −0.23, 0.53 | 0.15 | −0.17, 0.47 | 0.05 | −0.27, 0.37 | ||||
| Lack of emotional support | −0.07 | −0.15, 0.01 | −0.06 | −0.14, 0.02 | −0.05 | −0.12, 0.02 | −0.04 | −0.11, 0.02 | ||||
| Depressive symptoms | −0.03 | −0.10, 0.04 | −0.05 | −0.11, 0.02 | −0.01 | −0.07, 0.05 | −0.02 | −0.08, 0.04 | ||||
| Trait anger | 0.05 | −0.06, 0.17 | 0.02 | −0.09, 0.14 | 0.07 | −0.03, 0.16 | 0.05 | −0.05, 0.14 | ||||
| Trait anxiety | −0.03 | −0.14, 0.08 | 0.01 | −0.10, 0.12 | −0.06 | −0.16, 0.03 | −0.04 | −0.13, 0.06 | ||||
Abbreviations: CI, confidence interval; PM2.5, particulate matter less than 2.5μm in aerodynamic diameter.
* P < 0.05, **P < 0.01, ***P < 0.001 by χ2 for trend for categorical variables and by regression for continuous variables.
a Results from main effect models (n = 5570).
b Model 1 was adjusted for age, sex, season, site, season-by-site interaction, antihypertensive medication use, apparent temperature, race/ethnicity, educational level, income, and census tract median household income. Model 2 was adjusted for the factors in model 1 as well as ongoing burdens, depressive symptoms, trait anger, trait anxiety, and lack of emotional support. Model 3 was adjusted for the factors in model 2 as well as current alcohol use, smoking/exposure to tobacco smoke, body mass index, and diabetes.
c All socioeconomic measures were operationalized so that higher values represented a greater socioeconomic resource. All psychosocial measures were operationalized so that higher values represent greater adversity.
d The scales of the psychosocial measures are per 1 unit.
Socially disadvantaged groups generally had higher SBP, PP, and MAP, but not necessarily DBP, compared with more socially advantaged groups. For example, higher SBP was found for blacks compared with whites, and higher SES was associated with lower SBP. Psychosocial adversity was not associated with any of the blood pressure outcomes.
Figures 1–4 show the associations of PM2.5 with SBP, DBP, PP, and MAP, respectively, for different levels of social disadvantage (Figures 1A, 2A, 3A, and 4A) and psychosocial factors (Figures 1B, 2B, 3B, and 4B) as predicted from regression models that included an additive interaction between each social/psychosocial measure and 1-month prior PM2.5 exposure, setting other covariates to their mean. In general, the patterns of results were similar across blood pressure outcomes. There was no evidence of a modifying role for race/ethnicity, educational level, or Census tract median household income in the association between PM2.5 and blood pressure. However, there was evidence of a modifying role for income in the associations between PM2.5 and SBP, DBP, and MAP. In general, greater levels of income were associated with stronger relationships between PM2.5 and blood pressure, and although the differences were small, the interactions reached statistical significance at the P < 0.05 level (for SBP and income, interaction term = 0.37, 95% confidence interval: 0.06, 0.68; for DBP and income, interaction term = 0.19, 95% confidence interval: 0.03, 0.34; for PP and income, interaction term = 0.18, 95% confidence interval: −0.04, 0.41; and for MAP and income, interaction term = 0.25, 95% confidence interval: 0.06,0.44). There was no evidence of a modifying role for any of the psychosocial measures.
Results from sensitivity analyses using individual-level PM2.5 exposures that accounted for space yielded similar results. PM2.5 was not associated with blood pressure outcomes when using the 1-, 3-, or 7-day averaged exposures, as was found in previous work (21). Results using 2-month average exposures were similar to the associations found when using the 1-month averaged exposure period. Results when using alternative adjustments for antihypertensive medication use yielded results similar to those presented.
DISCUSSION
We examined the associations between exposure to PM2.5 and blood pressure by levels of social disadvantage and psychosocial factors in a large, population-based cohort of adults without a history of CVD. These associations did not differ significantly by most measures of social disadvantage and or psychosocial adversity. Contrary to the study hypotheses, higher levels of income were associated with stronger associations between PM2.5 and blood pressure.
The pathways through which PM2.5 exposure could affect blood pressure have been hypothesized to include oxidative stress and inflammatory processes, as well as alterations in cardiovascular reactivity and autonomic function (5, 45). Both of these pathways may also be affected by social and psychosocial factors (25, 46, 47), which suggests that both types of exposures may act synergistically. Moreover, some of the behavioral correlates of social disadvantage (e.g., sedentary lifestyles, unhealthy diets) could also enhance the adverse cardiovascular effects of environmental exposures. However, few studies have had information on PM2.5 exposures together with the social and psychosocial factors necessary to investigate their interactive effects.
Although few studies have evaluated the modifying role of social disadvantage on the association between PM2.5 and blood pressure, researchers have examined other cardiovascular outcomes, with mixed results. For example, contrary to our results, Ostro et al. (9) reported that, compared with persons of higher SES, those of lower SES showed a stronger association between exposure to PM2.5 and cardiovascular mortality. However, others reported that the association between PM2.5 and self-reported hypertension was stronger in whites than in nonwhites (30) and that the association between PM2.5 and aortic calcification was stronger in persons with higher incomes compared with those with lower incomes (31). Finally, others reported no racial/ethnic differences in the association between PM2.5 and microvasculature narrowing (32).
Only a few studies have examined heterogeneity in the air pollution–health association by level of psychosocial factors, and those studies only examined respiratory outcomes (4, 15). In all of the studies, persons who reported greater levels of stressors or strain showed a stronger association between air pollution and asthma risk (15, 48) or clinical asthma symptoms (16) than did those who reported lower levels of stressors or strain. To our knowledge, there have been no studies on the modifying role of psychosocial adversity on the association between air pollution and cardiovascular outcomes.
A number of methodological factors may have contributed to the absence of synergies between PM2.5 exposures and social disadvantage or psychosocial factors. First, the MESA sample excluded persons with clinical CVD symptoms at enrollment. Because of this, participants may be healthier than the general United States population and perhaps less vulnerable to the influence of social and psychosocial factors (as main effects or as modifiers of the effects of other exposures such as air pollution).
In addition, single measures of social disadvantage or psychosocial conditions may not adequately reflect the causal factors that increase vulnerability to the hypertensive effects of PM2.5. Researchers have argued that multiple social and psychosocial factors operate cumulatively to increase vulnerability to the health effects of environmental hazards (1, 6, 49, 50). In the future, researchers should examine measures of social disadvantage or psychosocial stress that capture the multiple and co-occurring factors that could increase vulnerability to the health effects of environmental hazards, such as residential segregation, composite individual-level social factors (e.g., social participation, unfair treatment, exposure to violence), or composite neighborhood-level social factors (e.g., crime, unemployment, green spaces).
Undoubtedly, the psychosocial factors were measured with error. Furthermore, the measures themselves may have been limited in their validity and reliability. They may not have captured the long-term exposure to psychosocial stress that is likely more relevant as an effect modifier of the hypertensive effects of air pollution compared with a more contemporaneous measure. These factors could have severely limited the ability to detect interactions.
An unexpected finding was that the associations of PM2.5 exposures with blood pressure were actually stronger among persons with higher incomes. CVD prevalence is higher in persons of lower SES compared with those of higher SES (51, 52). Persons who were of low SES and still free from CVD (a requirement for inclusion in MESA) may have other factors that buffer them against the PM2.5–blood pressure association. Adjustment for standard hypertension risk factors did not alter the interaction patterns, and it is difficult to pinpoint any specific omitted factors correlated with SES exposures that explain the interaction. Environmental exposures may have a weaker effect in the presence of blood pressure that is already elevated due to other important risk factors, as may be the case in persons with low SES. This may make it difficult to detect the added association of another risk factor, such as PM2.5 exposure, with its relatively weak associations with blood pressure. Although some of these unexpected interactions were statistically significant, chance remains a possible explanation for the results.
Other health outcomes that are more strongly linked to inflammation and oxidative stress may be more appropriate in the study of the synergistic effects between PM2.5 and social disadvantage/psychosocial factors. Environmental researchers have theorized that inflammation and oxidative stress are major pathways that link PM2.5 and cardiovascular health (18). Researchers have also linked stress and cardiovascular health through these pathways (25).
The lack of associations between shorter-term PM2.5 exposures and blood pressure were consistent with results from prior work (21). The authors of previous work had posited that accumulated exposures may have stronger impacts on health or that smoothing across the wide shorter-term fluctuations in exposures may reduce error, allowing for the detection of any PM2.5–blood pressure associations (21). Overall, the results of the present study do not support the hypothesis that social disadvantage or psychosocial adversity confers increased vulnerability to the hypertensive effects of PM2.5. Unexpectedly, higher SES appeared to be associated with a slightly stronger relationship between PM2.5 and blood pressure than did lower SES. This is the first examination of the possible modifying role of race/ethnicity, socioeconomic factors, and psychosocial factors on the association between PM2.5 and blood pressure. Further investigation using improved social disadvantage and psychosocial measures in population-representative samples is necessary to replicate these findings.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Margaret T. Hicken, Sara D. Adar, Ana V. Diez Roux); Department of Environmental Health Sciences, School of Public Health University of Michigan, Ann Arbor, Michigan (Marie S. O'Neill); Department of Environmental and Radiological Health Sciences, College of Veterinary Medicine and Biological Science, Colorado State University, Fort Collins, Colorado (Sheryl Magzamen); Department of Epidemiology and Biostatistics, School of Public Health, Drexel University, Philadelphia, Pennsylvania (Amy H. Auchincloss); and Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Joel D. Kaufman).
The Multi-Ethnic Study of Atherosclerosis (MESA) was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air) awarded by the U.S Environmental protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The authors acknowledge the other investigators and staff of MESA and MESA Air.
We thank the Robert Wood Johnson Foundation Health and Society Scholars Foundation for its support.
A full list of MESA investigators and institutions is available at www.mesa-nhlbi.org.
Conflict of interest: none declared.
REFERENCES
- 1.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morello-Frosch R, Lopez R. The riskscape and the color line: examining the role of segregation in environmental health disparities. Environ Res. 2006;102(2):181–196. doi: 10.1016/j.envres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the Healthy Environments Partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clougherty JE, Kubzansky LD. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 6.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 7.Environmental Protection Agency. Air Pollution Prevention and Control Act. 42 U.S.C. §§7401–7671q. 1970.
- 8.Gwynn RC, Thurston GD. The burden of air pollution: impacts among racial minorities. Environ Health Perspect. 2001;109(suppl 4):501–506. doi: 10.1289/ehp.01109s4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostro BD, Feng W-Y, Broadwin R, et al. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med. 2008;65(11):750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- 10.Bell ML, Dominici F. Effect modification by community characteristics on the short-term effects of ozone exposure and mortality in 98 US communities. Am J Epidemiol. 2008;167(8):986–997. doi: 10.1093/aje/kwm396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou CQ, Hedley AJ, Chung RY, et al. Socioeconomic disparities in air pollution-associated mortality. Environ Res. 2008;107(2):237–244. doi: 10.1016/j.envres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Laurent O, Bard D, Filleul L, et al. Effect of socioeconomic status on the relationship between atmospheric pollution and mortality. J Epidemiol Community Health. 2007;61(8):665–675. doi: 10.1136/jech.2006.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanobetti A, Schwartz J. Race, gender, and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med. 2000;42(5):469–474. doi: 10.1097/00043764-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Zeka A, Zanobetti A, Schwartz J. Individual-Level Modifiers of the Effects of Particulate Matter on Daily Mortality. Am J Epidemiol. 2006;163(9):849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- 15.Clougherty JE, Levy JI, Kubzansky LD, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen E, Schreier HM, Strunk RC, et al. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clougherty JE, Rossi CA, Lawrence J, et al. Chronic Social Stress and Susceptibility to Concentrated Ambient Fine Particles in Rats. Environ Health Perspect. 2010;118(6):769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115(5-6):175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 19.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121(25):2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auchincloss AH, Roux AVD, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116(4):486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvonch JT, Kannan S, Schulz AJ, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53(5):853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 24.Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Ann N Y Acad Sci. 1999;896:262–277. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- 25.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geronimus AT, Bound J, Keene D, et al. Black-white differences in age trajectories of hypertension prevalence among adult women and men, 1999–2002. Ethn Dis. 2007;17(1):40–48. [PubMed] [Google Scholar]
- 27.Kaplan MS, Nunes A. The psychosocial determinants of hypertension. Nutr Metab Cardiovasc Dis. 2003;13(1):52–59. doi: 10.1016/s0939-4753(03)80168-0. [DOI] [PubMed] [Google Scholar]
- 28.Morenoff JD, House JS, Hansen BB, et al. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007;65(9):1853–1866. doi: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19(4):590–598. doi: 10.1097/EDE.0b013e3181772cb2. [DOI] [PubMed] [Google Scholar]
- 30.Johnson D, Parker JD. Air pollution exposure and self-reported cardiovascular disease. Environ Res. 2009;109(5):582–589. doi: 10.1016/j.envres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20(2):254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adar SD, Klein R, Klein BE, et al. Air Pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA) PLoS Med. 2010;7(11):e1000372. doi: 10.1371/journal.pmed.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 34.Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging. 1996;11(2):207–213. doi: 10.1037//0882-7974.11.2.207. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 36.Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI) In: Mariush ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 993–1021. [Google Scholar]
- 37.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35(suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Weather Service. Silver Spring, MD: National Weather Service; 2013. Apparent temperature definition http://graphical.weather.gov/definitions/defineApparentT.html. ). (Accessed July 15, 2013) [Google Scholar]
- 39.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Hoboken, NJ: Wiley-Interscience; 2005. [Google Scholar]
- 40.Tobin MD, Sheehan NA, Scurrah KJ, et al. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 41.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 42.Sampson PD, Szpiro AA, Sheppard L, et al. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45(36):6593–6606. [Google Scholar]
- 43.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43(13):4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10(4):e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3(5):332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 46.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- 48.Shankardass K, McConnell R, Jerrett M, et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morello-Frosch R, Zuk M, Jerrett M, et al. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff (Millwood) 2011;30(5):879–887. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- 50.Hicken M, Gragg R, Hu H. How cumulative risks warrant a shift in our approach to racial health disparities: the case of lead, stress, and hypertension. Health Aff (Millwood) 2011;30(10):1895–1901. doi: 10.1377/hlthaff.2010.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiscella K, Tancredi D. Socioeconomic status and coronary heart disease risk prediction. JAMA. 2008;300(22):2666–2668. doi: 10.1001/jama.2008.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark AM, DesMeules M, Luo W, et al. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6(11):712–722. doi: 10.1038/nrcardio.2009.163. [DOI] [PubMed] [Google Scholar]


