Abstract
Formalin-fixed, paraffin-embedded tumors (FFPETs) are a valuable source of DNA for genotype association studies and are often the only germline DNA resource from cancer clinical trials. The anti-estrogen tamoxifen is metabolized into endoxifen by CYP2D6, leading to the hypothesis that patients with certain CYP2D6 genotypes may not receive benefit because of their inability to activate the drug. Studies testing this hypothesis using FFPETs have provided conflicting results. It has been postulated that CYP2D6 genotype determined using FFPET may not be accurate because of somatic tumor alterations. In this study, we determined the concordance between CYP2D6 genotypes generated using 3 tissue sources (FFPETs; formalin-fixed, paraffin-embedded unaffected lymph nodes [FFPELNs]; and whole blood cells [WBCs]) from 122 breast cancer patients. Compared with WBCs, FFPET and FFPELN genotypes were highly concordant (>94%), as were the predicted CYP2D6 metabolic phenotypes (>97%). We conclude that CYP2D6 genotypes obtained from FFPETs accurately represent the patient’s CYP2D6 metabolic phenotype.
DNA from formalin-fixed, paraffin-embedded tumors (FFPETs) has been used for “prospective–retrospective” (1) studies testing for associations between gene variants with cancer outcomes. Several studies have used FFPETs for germline CYP2D6 genotyping to test for associations with response to tamoxifen therapy (2–8). Tamoxifen is metabolized into a more potent anti-estrogenic metabolite (endoxifen) by CYP2D6 (9). Patients receiving tamoxifen who are homozygous for CYP2D6 null alleles and classified as poor metabolizers (PMs) have substantially lower serum endoxifen concentrations than patients with heterozygous null (intermediate metabolizers [IMs]) and homozygous wild-type (extensive metabolizers [EMs]) alleles (10). Although it has been hypothesized that CYP2D6 PMs do not benefit from tamoxifen therapy, studies testing this hypothesis have provided conflicting results [reviewed in (11)].
Results from two phase III studies (ATAC and BIG1-98), in which FFPETs were used for genotyping, failed to detect an association between CYP2D6 and tamoxifen efficacy in postmenopausal, early-stage, estrogen receptor–positive breast cancer patients (7,8). Subsequently, three letters in the Journal of the National Cancer Institute suggested the CYP2D6 genotypes from the BIG1-98 study might not reflect patients’ germline CYP2D6 genotypes because of a possible misclassification effect of somatic tumor alterations (12–14). Nakamura et al. suggested many (33%) breast tumors exhibit loss of one CYP2D6 allele because of loss of heterozygosity (LOH) within the chromosomal region containing CYP2D6, resulting in the excess of CYP2D6 homozygote genotypes, and deviations from Hardy–Weinberg equilibrium in the BIG1-98 trial (14). Previously, we showed that CYP2D6 genotypes in a small sample set (n = 10) were 100% concordant between FFPETs and whole blood cells (WBCs) (15). This study used whole tissue sections from FFPETs, which contain both tumor and normal cells, with the latter providing germline DNA that has not undergone somatic alterations. In contrast, the BIG1-98 study used DNA extracted from 1-mm diameter cores taken from malignant areas within the FFPETs. Given the clinical significance of the BIG1-98 investigation and other studies that have used FFPETs for CYP2D6 genotyping (2–8), we investigated the concordance of CYP2D6 genotypes from FFPETs and germline DNA derived from WBCs.
To assess CYP2D6 genotypes from different tissue sources, DNA from FFPETs, formalin-fixed, paraffin-embedded unaffected lymph nodes (FFPELNs), and WBCs were analyzed from 122 breast cancer patients who provided written informed consent (Supplementary Table 1, available online). Three 0.6-mm diameter cores were taken from areas of high tumor burden within the FFPETs as identified by hematoxylin and eosin staining of the donor block. Three cores were also obtained from FFPELNs. Phlebotomy yielded WBC specimens. DNA was extracted from the 122 matched FFPETs, FFPELNs, and WBCs and genotyped for six CYP2D6 single nucleotide polymorphismss using the Taqman allelic discrimination assays (Applied Biosystems, Foster City, CA) as previously described (8). In combination, these single nucleotide polymorphisms determine CYP2D6*1, *2, *3, *4, *6, *10, and *41 genotypes. In addition, CYP2D6 copy number variants (CNVs) were determined using DNA obtained from WBCs, as described previously (16) (Supplementary Material and Methods, available online).
CYP2D6 allelic variants and genotype frequencies and tests for Hardy–Weinberg equilibrium deviation from the 122 WBCs are given (Supplementary Table 2, available online). We were unable to genotype one FFPET and five FFPELNs because of low DNA quality/concentration and one FFPELN for unknown reasons. Using CYP2D6 genotypes from WBCs as our reference, concordance among formalin-fixed, paraffin-embedded samples providing quality DNA for CYP2D6 genotyping was 94.2% (n = 114 of 121; 95% confidence interval [CI] = 88.4% to 97.6%) and 97.4% (n = 114 of 117; 95% CI = 92.7% to 99.5%) for FFPETs and FFPELNs, respectively. Based on CYP2D6 genotypes, samples were assigned CYP2D6 phenotype scores of 0 (PM); 0.5, 1.0, and 1.5 (IM); and 2 (EM) (8). The concordance among formalin-fixed, paraffin-embedded samples providing quality DNA for CYP2D6 score determination was 98.3% (n = 119 of 121; 95% CI = 94.2% to 99.8%) and 97.4% (n = 114 of 117; 95% CI = 92.7% to 99.5%) for FFPETs and FFPELNs, respectively (Figure 1).
Figure 1.
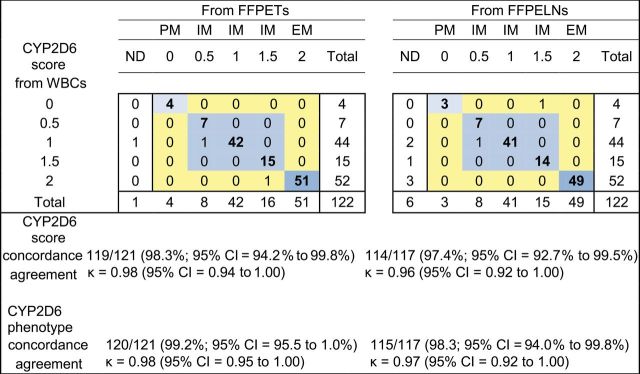
Concordance and agreement of CYP2D6 scores and predicted phenotypes of 122 breast cancer patients’ matched DNA samples from white blood cells (WBCs), formalin-fixed, paraffin-embedded tumors (FFPETs), and formalin-fixed, paraffin-embedded unaffected lymph nodes (FFPELNs). Genotypes could not be determined for one FFPET and five FFPELNs because of low DNA quality/concentration and for one FFPELN for unknown reasons. Concordance and agreement exclude the one nondetermined (ND) FFPET and five/six ND FFPELN samples without adequate DNA quality/concentration for genotyping. EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer.
Germline CYP2D6 CNVs, both deletions and amplifications, have been identified (17). Currently, it is not feasible to test CYP2D6 CNVs from formalin-fixed, paraffin embedded samples because the quality and quantity of the extracted DNA is below that required for quantitative polymerase chain reaction or direct sequencing of known splice sites. We determined the potential impact of the missing CYP2D6 CNV information from FFPETs on concordance between formalin-fixed, paraffin-embedded samples and WBCs with known CNVs. In the 122 WBC DNA samples, we observed nine (7.4%; 95% CI = 3.4% to 13.5%) deletions and six (4.9%; 95% CI = 1.8% to 10.4%) amplifications in CYP2D6. All deletions and amplifications were in samples having 100% concordant genotypes between WBCs, FFPETs, and FFPELNs. Adjusting CYP2D6 scores to account for deletions, eight patients scored as 2 (EM) became 1 (IM), and one scored as 1 (IM) became 0.5 (IM). The six gene amplifications had only minimal impact on CYP2D6 scores (Supplementary Table 3, available online). The missing CNV information from FFPETs and FFPELNs had no impact on predicting CYP2D6 PM phenotypes.
Our data show that CYP2D6 genotypes obtained from FFPETs and FFPELNs are more than 94% concordant with WBC genotypes. Furthermore, predicted CYP2D6 metabolic phenotypes based on genotypes obtained from FFPETs and FFPELNs were more than 97% concordant with WBCs. Our data are consistent with an earlier study showing strong agreement between CYP2D6 genotypes using DNA extracted from archived breast tumors and normal lymph nodes (18). These data refute the concern that breast tumor LOH causes patients to be misclassified when using FFPETs for CYP2D6 genotyping. Of note, we observed similar rates of discordance between FFPETs and FFPELNs versus WBCs. Given that these study lymph nodes contain solely normal tissue, we conclude that the minor genotype disagreements observed in our study are due to the technical challenges of CYP2D6 genotyping using DNA of low quality or quantity of DNA obtained from formalin-fixed, paraffin-embedded tissues or both.
Although earlier studies have suggested that the chromosomal region containing the CYP2D6 gene (22q13) undergoes LOH in breast tumors, these studies analyzed only a small number of tumors and failed to conduct the genomic fine mapping required to identify the specific genes exhibiting LOH (19,20). Recently, large fine gene mapping studies generating very high resolution data failed to show substantial LOH in CYP2D6 in the majority of breast cancer tumor types (21,22).
These data, along with those from the large breast cancer genomic studies that failed to detect LOH in the CYP2D6 locus, demonstrate that CYP2D6 genotypes obtained from FFPETs are suitable for CYP2D6 germline genotype determinations. With less than 10% estimated misclassification of predicted CYP2D6 IM and EM phenotypes using FFPETs, resulting mostly from CYP2D6 deletions that cannot be determined from FFPETs, such misclassification could not alter the conclusions of the BIG1-98 investigation. In that investigation, in which the observed hazard ratio of recurrence was near 1.0 for CYP2D6 PM and IM phenotypes vs EM phenotype, upwards of 75% misclassification would be necessary to have obscured a true hazard ratio of 1.5 or greater. Although it is preferable to use DNA from WBCs for germline pharmacogenetic studies, in situations where the only source of DNA is FFPETs, these specimens are an appropriate alternative for CYP2D6 genotyping and their corresponding CYP2D6 phenotype determination.
Funding
This work was supported in part by the Breast Cancer Research Foundation (BCRF) (N003173 to JMR and DFH; 1RO1GM099143 to JMR; 5K23DE020197 and UL1RR024986 to CVP; KG080081 to GV and MMR; and 5U24CA075362 to MMR).
Supplementary Material
References
- 1. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101(21):1446–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318 [DOI] [PubMed] [Google Scholar]
- 3. Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101(1):113–121 [DOI] [PubMed] [Google Scholar]
- 4. Schroth W, Antoniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25(33):5187–5193 [DOI] [PubMed] [Google Scholar]
- 5. Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lash TL, Cronin-Fenton D, Ahern TP, et al. CYP2D6 inhibition and breast cancer recurrence in a population-based study in Denmark. J Natl Cancer Inst. 2011;103(6):489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104(6):441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764 [DOI] [PubMed] [Google Scholar]
- 10. Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74 [DOI] [PubMed] [Google Scholar]
- 11. Hertz DL, McLeod HL, Irvin WJ., Jr Tamoxifen and CYP2D6: a contradiction of data. Oncologist. 2012;17(5):620–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pharoah PD, Abraham J, Caldas C. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(16):1263–1264 [DOI] [PubMed] [Google Scholar]
- 13. Stanton V., Jr Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104(16):1265–1266 [DOI] [PubMed] [Google Scholar]
- 14. Nakamura Y, Ratain MJ, Cox NJ, McLeod HL, Kroetz DL, Flockhart DA. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104(16):1264 [DOI] [PubMed] [Google Scholar]
- 15. Rae JM, Cordero KE, Scheys JO, Lippman ME, Flockhart DA, Johnson MD. Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics. 2003;13(8):501–507 [DOI] [PubMed] [Google Scholar]
- 16. Ramamoorthy A, Flockhart DA, Hosono N, Kubo M, Nakamura Y, Skaar TC. Differential quantification of CYP2D6 gene copy number by four different quantitative real-time PCR assays. Pharmacogenet Genomics. 2010;20(7):451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–243 [DOI] [PubMed] [Google Scholar]
- 18. Ahern TP, Christensen M, Cronin-Fenton DP, et al. Concordance of metabolic enzyme genotypes assayed from paraffin-embedded, formalin-fixed breast tumors and normal lymphatic tissue. Clin Epidemiol. 2010;2(22)241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benetkiewicz M, Piotrowski A, Díaz De Ståhl T, et al. Chromosome 22 array-CGH profiling of breast cancer delimited minimal common regions of genomic imbalances and revealed frequent intra-tumoral genetic heterogeneity. Int J Oncol. 2006;29(4):935–945 [PubMed] [Google Scholar]
- 20. Hirano A, Emi M, Tsuneizumi M, et al. Allelic losses of loci at 3p25.1, 8p22, 13q12, 17p13.3, and 22q13 correlate with postoperative recurrence in breast cancer. Clin Cancer Res. 2001;7(4):876–882 [PubMed] [Google Scholar]
- 21. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis MJ, Ding L, Shen D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


