Abstract
Background. Influenza affects host susceptibility to pneumococcus. We sought to evaluate whether this relationship varies by pneumococcal serotype using a large epidemiological database covering 3 decades.
Methods. Weekly rates of invasive pneumococcal pneumonia (IPP) were obtained from the Danish National Laboratory Surveillance System, and influenza-like illness (ILI) data were collected from Danish sentinel surveillance, Statens Serum Institut, 1977–2007. We fit Poisson regression models for each age and comorbidity group, with predictors for seasonality and secular changes, ILI activity, and serotype.
Results. Among individuals with low levels of comorbidities, influenza had the largest impact on IPP incidence among low-invasiveness serotypes (influenza attributable percent: 17.9%, 95% confidence interval [CI], 13.6–21.9) as compared with high-invasiveness serotypes (6.7%, 95% CI, 3.8%–11.7%). Among those with higher levels of comorbidities, the effect of influenza was smaller, but high-invasiveness serotypes increased more than low-invasiveness serotypes (8.9% [95% CI, 6.6–11.8] vs 1.3% [95% CI, −1.6–5.4].
Conclusions. Influenza was associated with the greatest increases in the incidence of disease caused by serotypes with lower invasive potential and among individuals with low levels of comorbid conditions. The importance of influenza for adult IPP varies by serotype and host comorbidity.
Keywords: pneumococcus, influenza, coinfection, comorbidity, interaction, regression
Pneumococcus is an important cause of pneumonia, meningitis, and septicemia. The most important virulence factor is the polysaccharide capsule. Based on the structure of these capsules, pneumococcus is classified into more than 90 serotypes. These serotypes differ in prevalence among healthy carriers [1], capacity to cause invasive disease [2], disease severity [3, 4], and tendency to cause disease in otherwise healthy or comorbid individuals [5]. Some of these variations between serotypes can be attributed to the capsule itself [6], but noncapsular virulence factors [7], characteristics of the host populations, and interactions with other pathogens [8] might also influence the patterns of virulence.
The synergy between pneumococcus and influenza has been well documented [8–16], with influenza affecting both transmission and host susceptibility to the bacteria [14, 17–23]. However, it is not clear whether this effect varies between serotypes and whether such interactions are related to the virulence characteristics of the strains and the underlying health conditions of the host. Reports from individual outbreaks and pandemic periods suggest that some serotypes increase more than others during periods of intense influenza activity [11, 23–28]. Likewise, experiments in animal models demonstrate that the effect of influenza on pneumococcal virulence varies between strains [18]. Further, there is a relationship between the invasive disease potential of different serotypes and host comorbidities [5], suggesting that underlying chronic conditions may modulate the interaction between influenza and pneumococcus serotypes.
The impact of influenza on the incidence of a particular serotype will likely depend on a balance of many factors including the intrinsic virulence of the serotype and host susceptibility to disease. Because highly virulent serotypes can cause disease in the absence of influenza, we hypothesize that influenza would have a larger effect on the incidence of disease caused by less invasive serotypes. In this study, we tested whether the effect of influenza activity on invasive pneumococcal pneumonia varies by serotype and comorbidity using Danish national surveillance data from a 30-year period.
MATERIALS AND METHODS
Data Sources
Invasive pneumococcal disease (IPD) data were obtained from the Danish National Laboratory Surveillance System at the Statens Serum Institut (SSI) as previously described [3, 29]. This database consists of all IPD cases in Denmark reported to SSI since 1938. We included patients with invasive pneumococcal pneumonia (IPP) from 1977 to 2007, defined as the occurrence of culture confirmed pneumococcal bacteremia in a hospitalized patient who received a World Health Organization International Classification of Disease (ICD)-8 or -10 code at discharge corresponding to pneumonia (073, 471, 480–486 from ICD-8 and J12–J18 from ICD-10). We excluded cases aged <40 years due to small numbers and different seasonal patterns. All cases aged >40 years were analyzed together. Subanalyses of those aged 40–64 and ≥65+ years were also performed but gave comparable results to the combined analysis and are not presented here.
The level of comorbidity of patients was estimated by calculating the Charlson index as previously described [3]. Briefly, a person was considered to have a low level of comorbidity if they had a Charlson score of 0 and medium/high comorbidity if they had a Charlson score of ≥1. Uptake of the 23-valent pneumococcal vaccine in the population aged ≥65 years was very limited in the study population [30].
Weekly incidence of influenza-like illnesses (ILIs), defined as acute onset of fever, myalgia, and respiratory symptoms, was used as a proxy for influenza viral activity [31, 32]. ILI data have been shown to accurately reflect the timing and intensity of influenza virus epidemics [33, 34]. ILI data were collected from 2 sources, both managed by SSI. The Danish sentinel surveillance system, covering the period 1994/1995–2006/2007, is based on voluntary participation of up to 150 nationwide general practitioners. Between week 40 and week 20 of the following calendar year, general practitioners report the weekly number of total and ILI consultations in their practice [35]. For 1982/1983–1990/1991, data were obtained from nationwide reports of general practitioners. Weekly ILI data were not available between 1991/1992 and 1993/1994. The ILI data from both surveillance systems were standardized by dividing weekly incidences by the mean winter activity for the respective surveillance system and then subtracting the mean summer value to anchor the minimum values to 0.
The Danish Data Protection Agency (record number 2007-41-0229) approved the study.
Effect of Influenza on Pneumococcal Pneumonia Incidence
To estimate the impact of influenza on IPP incidence, we fit weekly time series regression models predicting the incidence of IPP as a function of influenza activity, while controlling for seasonal variations in IPP incidence that are unrelated to influenza and allowing for the influenza impact to vary between serotypes and comorbidity groups [13]. We considered detailed differences in influenza effects by serotypes and broader differences by levels of invasiveness (based on Sleeman et al [36]; low invasiveness, serotypes: 3, 6A, 6B, 9N, 19F, 23F; medium, 8, 12F, 19A, 22F; high, 1, 4, 5, 7F, 9 V, 18C). Akaike information criterion (AIC) criteria were used to identify the best fitting model with the most parsimonious seasonal baseline. The best model included 52-week and 26- week harmonic terms and dummy variables for weeks 51–52 and weeks 1–3, reflecting the impact of Christmas holidays. Alternative models included simpler ones that included fewer harmonic terms or more complicated models that allowed the harmonic and dummy variables to vary between serotype and comorbidity strata. The final model was:
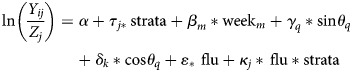 |
where Yij is the weekly number of IPP cases in week i for comorbidity- and serotype- or invasiveness-strata j and Zj is an offset representing the mean number of strata-specific cases during noninfluenza summer weeks (July–November and April–June of each respiratory season). This offset effectively adjusts for changes in detection/reporting, multiyear changes in incidence, and changes in population size. ‘α’ and ‘τj’ are the overall and strata-specific intercepts; ‘weekm’ are indicator variables for weeks of the year 51, 52, 1, 2, 3; ‘sinθq’ and ‘cosθq’ are harmonic terms representing annual and semiannual periodicities (52 week and 26 week) to control for baseline seasonal fluctuations; and ‘flu’ represents weekly ILI activity, lagged by 1 week (lags of 0 and 2 weeks were also evaluated and gave similar results). The coefficient ‘κj’ represents a strata-specific influenza effect, modeled as an interaction between the influenza proxy and a dummy variable for strata. The combination of average influenza effect, ‘ε’, and strata-specific effect, ‘κj’, gives the overall effect of influenza in the stratum. The model was fit separately to data from each age group, and the influenza-attributable fraction of IPP was calculated as described elsewhere [13, 23]. Confidence intervals (CIs) were calculated using seasonal block bootstraps [37] with 1000 replicates.
To compare the attributable percent estimates with different strata, we tested the hypothesis that the difference between the strata was 0. We calculated an approximate variance for each attributable percent estimate from the bootstrap distributions. To compare 2 attributable percent estimates, we took the difference between them and added their approximate variance estimates. If the 95% confidence limit of the difference did not include 0, we considered the difference between the strata to be significant.
Data were analyzed using PROC GENMOD, SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Between 1977 and 2007, there were 9941 cases of IPP in adults aged >40 years that had an ICD-8/10 code of pneumonia and had available pneumococcal serotype data from an invasive isolate; 8308 of these cases occurred during the 22 years with available data on influenza activity. All of the serotypes exhibited a similar seasonal distribution, with the minimum incidence occurring in July and August, a broad increase during the winter months, and a sharp spike in late December that corresponds to the Christmas holiday period (Supplementary Figure 1).
Effect of Influenza Varies With Host Comorbidity and Serotype Invasive Disease Potential
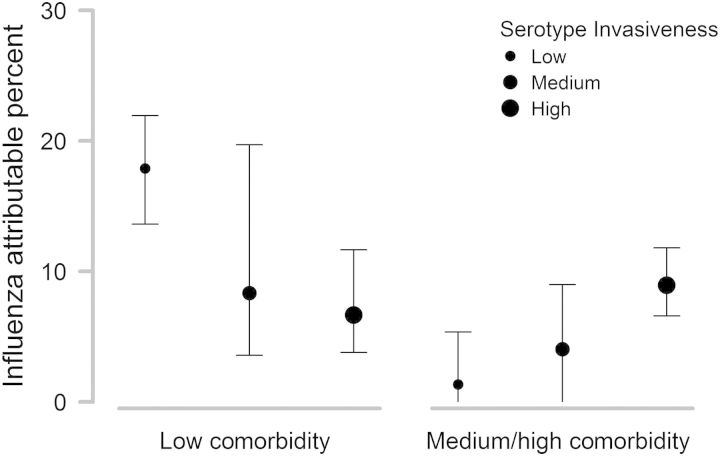
We first estimated the effect of influenza on the incidence of IPP for low-, medium-, and high-invasiveness serotypes and for 2 levels of comorbidities estimated by the Charlson index (Charlson score = 0 vs Charlson score ≥ 1). Among adults with low levels of comorbidities, influenza was associated with a significantly higher percentage of IPP cases caused by low-invasiveness serotypes compared with high-invasiveness serotypes (influenza attributable percent: 17.9%, 95% CI, [13.6–21.9] vs 6.7%, 95% CI, [3.8–11.7]; Figure 1). In contrast, among those with higher levels of comorbidities, the pattern was reversed, with influenza having a significantly larger effect on the incidence of high-invasiveness serotypes (Figure 1).
Figure 1.
Percent of invasive pneumococcal pneumonia cases caused by each serotype category that was attributable to influenza, stratified by Charlson comorbidity level; ±95% confidence intervals.
Next, we compared the effect of influenza between the comorbidity-level groups. For the low-invasiveness serotypes, the effect of influenza was significantly greater among cases with low levels of comorbidities according to the Charlson index (Figure 1). In contrast, for the high-invasiveness serotypes, the effect of influenza was comparable between the high-comorbidity and low-comorbidity patients (Figure 1).
Effect of Influenza on Invasive Pneumococcal Pneumonia Incidence Varies by Serotype
To determine whether specific serotypes were driving the patterns described above, we estimated the effect of influenza on IPP incidence for individual serotypes. For 10 of the serotypes, we had sufficient data to stratify both by serotype and by comorbidity level (Supplementary Table 1). The broad pattern for individual serotypes was the same as in the grouped analysis, that is, among those with low levels of comorbidities (Charlson = 0), influenza was associated with a larger percent of disease caused by low-invasiveness serotypes, but the opposite pattern was seen among patients with higher levels of comorbidities (Figure 2). For serotype 3 (a low-invasiveness serotype), influenza was associated with a particularly large fraction of cases among those with low levels of comorbidities (20.6%, 95% CI, 14.7%–26.1%) but had no detectable effect on the incidence of disease among patients with higher levels of comorbidities (Figure 2). Conversely, for the highly invasive serotype 4, the effect of influenza was substantial among those with known comorbidities but not among those with low levels of comorbidity (Figure 2). There was considerable variability in the point estimates for the effect of influenza within comorbidity and serotype groups, but the CIs were broad.
Figure 2.
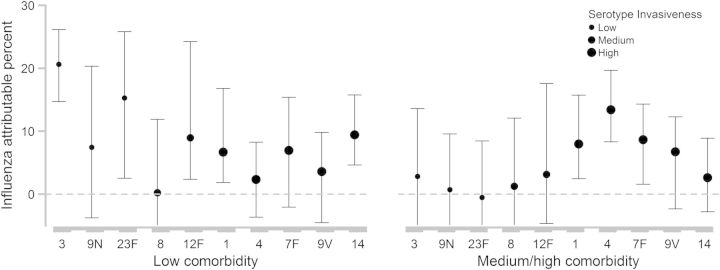
Percent of invasive pneumococcal pneumonia cases caused by each serotype that was attributable to influenza, stratified by Charlson comorbidity level; ±95% confidence intervals.
In absolute terms, influenza was associated with the largest number of excess cases of IPP for high-invasiveness serotypes among individuals with higher levels of comorbidities (Table 1). However, across all serotypes, there were more influenza-associated excess cases of IPP among individuals without or with low levels of comorbidities (Table 1).
Table 1.
Estimated Number of Excess Cases Due to Influenza Among Adults Aged ≥40 Year, Stratified by Comorbidity and Serotype Invasiveness
| Comorbiditya | Serotype Invasivenessb | Total Cases | Excess Cases Due to Influenza (N) |
|---|---|---|---|
| Low | Low | 771 | 138 |
| Medium | 684 | 57 | |
| High | 2807 | 187 | |
| Other | 339 | 24 | |
| Total | 4601 | 406 | |
| Medium/high | Low | 1243 | 17 |
| Medium | 838 | 34 | |
| High | 2516 | 224 | |
| Other | 743 | 32 | |
| Total | 5340 | 307 |
a Charlson comorbidity score.
b Invasiveness groups based on Sleeman [36]. Low invasiveness: 3, 6A, 6B, 9N, 19F, 23F; medium: 8, 12F, 19A, 22F; high: 1, 4, 5, 7F, 9 V, 18C.
Effect of Influenza on PCV13 and Non-PCV13 Serotypes
Finally, since pediatric pneumococcal conjugate vaccines have altered the distribution of serotypes causing disease among adults, we determined whether the effect of influenza is similar among the vaccine serotypes and nonvaccine serotypes and whether the impact of influenza might change following vaccine introduction. There was no significant difference overall in the influenza attributable percent between the PCV13 and the non-PCV13 serotypes among patients with low levels of comorbidity (9.3% [95% CI, 6.6%–13.7%] vs 7.6% [95% CI, 2.9%–14.9%] for vaccine serotypes and nonvaccine serotypes, respectively). Likewise, among patients with moderate to high comorbidity levels, there was no significant difference in the effect of influenza (7.0% [95% CI, 5.4%–9.6%] vs 3.4% [95% CI, −.8%–9.1%]). However, the CIs were broad for the nonvaccine serotypes, limiting our ability to detect a difference.
DISCUSSION
Using data from more than 8000 invasive pneumococcal pneumonia cases among adults collected over a 30-year period, we demonstrated that the effect of influenza varied with pneumococcal serotypes and that this effect was modified by host comorbidity. These results suggest that influenza has the largest impact among individuals with the lowest baseline risk for disease and on the strains that have the lowest intrinsic virulence.
Our findings are consistent with a hospital-based study in Spain that found that viruses were detected more frequently among pediatric IPP cases caused by low-invasiveness serotypes compared with high-invasiveness serotypes [28]. Other influenza-associated variations in the incidence of specific serotypes have been described previously. Serotype 3 was identified as being important during influenza outbreaks in Scotland [27], in a US pediatric population [38], and during the 1918 pandemic [39]. Serotype 1 occurs in outbreak settings [40] and it also undergoes long-term multiyear changes in incidence that tend to coincide with severe influenza seasons. It has been suggested that the serotypes that increase the most during influenza periods are those that are carried the most frequently in the population [11]. However, we could not test this hypothesis since we did not have data on adult carriage in this population.
Our analyses focused on differences between serotypes, with the assumption that all variants within a serotype have similar virulence characteristics. This is a reasonable simplification given that case fatality rates [4] and pediatric invasiveness [2] are somewhat stable in epidemiologically diverse settings. However, noncapsular virulence factors could also influence pneumococcal strains [7, 41] and therefore might influence the magnitude of the effect of influenza. Further population-based studies and animal studies that use epidemiologically relevant clones could help to clarify the importance of bacterial factors in the interaction with influenza.
The risk of developing pneumococcal disease depends on a balance of at least 4 host and bacterial factors that are independent of influenza: comorbidities in the individual, underlying frailty due to age or other conditions, the intrinsic invasiveness of the serotype, and the prevalence of a particular strain among healthy carriers (exposure). We would expect influenza to have the largest relative effect in the groups of individuals with the lowest baseline risk for disease. Low comorbidity, younger age, and lower serotype virulence should all be associated with lower baseline disease risk, and thus influenza should have the largest impact on disease in these groups. Reflecting this principle, influenza had the largest impact on the incidence of low- and medium-invasiveness serotypes among low comorbidity individuals and a relatively small impact among those with high comorbidities.
Among those patients with low levels of comorbidities, the importance of influenza on the incidence of disease caused by high- and low-invasiveness serotypes was reversed. This pattern could be explained based on known serotype-associated patterns [5]. Low-invasiveness serotypes tend to cause disease primarily in individuals with underlying conditions (eg, immune-compromise). However, influenza might increase susceptibility to bacterial infections in otherwise healthy individuals. Therefore, a large percentage of the cases of disease among healthy individuals caused by these low-invasiveness serotypes would be attributed to influenza. In contrast, among those with underlying conditions, the low-invasiveness serotypes could cause disease with or without a viral infection, so the influenza-attributable percent would be negligible. The high-invasiveness serotypes are readily able to cause disease both in those with or without underlying conditions. Influenza could further increase the likelihood of disease caused by these high-invasiveness serotypes either directly (increased susceptibility) or indirectly (increased transmission).
Pneumococcal conjugate vaccines have profoundly altered the distribution of serotypes that cause disease, including invasive pneumonia. Also, since the effect of influenza varies by serotype, the importance of influenza as a risk factor might change as well. PCV13 has been in use among Danish children since 2010. Influenza was associated with a similar effect among PCV13 and non-PCV13 serotypes (across all serotypes included in the vaccines), so our findings support the belief that the effect of influenza is unlikely to change in the postvaccine period. However, the strongest effect of influenza in our study was observed for serotype 3, which is one of the PCV13 serotypes. Therefore, the vaccine might have an added benefit for low-comorbidity individuals who might have been susceptible to influenza-associated serotype 3 disease. Further studies of influenza in the post-PCV13 period would help to determine whether influenza continues to have an important effect despite the changing serotype distribution.
Previous studies in this population have demonstrated differences in case fatality ratios between serotypes and comorbidity levels. We considered whether influenza might contribute to these patterns. During weeks when influenza activity was above a seasonal threshold [42], case fatality ratios were elevated for low- and medium-invasiveness serotypes among individuals without known comorbidities (Supplementary Table 2). These are the groups that had the largest influenza-attributable effects in our analyses. In other serotype and comorbidity groups, there was no detectable difference between influenza and noninfluenza periods. Further studies could focus on the importance of viral coinfections in determining disease outcome among otherwise healthy individuals infected with low-invasiveness serotypes.
The invasiveness index (cases/carriers ratio) that we used to group the serotypes is based on pediatric data [2, 43]. While there is not a similar measure for adults, the invasiveness of a serotype in children is likely a good proxy for the inherent virulence of that strain. Comorbidity levels in children are lower, so patterns in children are more likely to reflect the virulence of the bacteria rather than host characteristics. However, the interplay among host comorbidities, bacterial virulence factors, and viral coinfections is complex.
Our study has strengths and limitations. We used national and sentinel surveillance data from Denmark that were collected in centralized databases for an extended period of time, resulting in relatively large numbers of disease isolates. However, as with all studies of IPP, the case definition is highly specific (with only bacteriologically confirmed patients who were admitted to the hospital and who received a pneumonia diagnosis on the discharge code) but has low sensitivity. While such low sensitivity might affect estimates of influenza-associated disease burden, the temporal patterns are unlikely to be affected. However, it is possible that the effect of influenza on less severe cases of pneumococcal disease might be larger or smaller than the effect on hospitalized bacteremic cases. In addition, the analyses presented here are likely underpowered to detect differences between serotypes. While there were more than 8000 cases of invasive pneumococcal pneumonia in the period that we examined, stratification by serotype, month, and age category resulted in small numbers of cases in each stratum. We also used influenza-like illness as a proxy for influenza activity. While ILI is not as specific an outcome as viral surveillance, it is well established that it provides a reliable proxy for the timing and intensity of influenza activity [33, 34].
In summary, we have shown that the effect of influenza on pneumococcal disease varies significantly between serotypes and that this effect is modified by host comorbidity. Influenza was associated with a larger fraction of disease caused by less invasive serotypes and among those with low levels of comorbidities. Our data suggest that the impact of influenza on IPP incidence in adults is unlikely to change substantially in the post-PCV13 period. However, the patterns might be different in younger age groups, which were not studied here, or during pandemic periods. Future work should consider the importance of influenza in driving the incidence of disease among those without underlying conditions and among younger age groups and should attempt to further disentangle the relationship between influenza, comorbidity, and serotype.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgements. We thank Dr Keith Klugman for helpful discussions about these results.
D. M. W. and Z. B. H. designed the experiments and the study; D. M. W. analyzed the data; Z. B. H., H. B. K., T. G. K., and K. M. collected data and did experiments for the study; D. M. W. and Z. B. H. wrote the first draft of the paper; and D. M. W., Z. B. H., T. G. K., C. V., K. M., M. M., and H. B. K. contributed to the writing of the paper. All authors agree with the manuscript's results and conclusions.
Financial support. This work is conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), with funding from the Office of Global Health Affairs’ International Influenza Unit in the Office of the Secretary of the Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bogaert D, de Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann AB, Peto TEA, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 3.Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6:e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger DM, Harboe ZB, Sanders EAM, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51:692–9. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjostrom K, Spindler C, Ortqvist A, et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis. 2006;42:451–9. doi: 10.1086/499242. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DM, Trzcinski K, Lu Y-J, et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathogens. 2009;5:e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandgren A, Sjostrom K, Olsson-Liljequist B, et al. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis. 2004;189:785–96. doi: 10.1086/381686. [DOI] [PubMed] [Google Scholar]
- 8.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowska K, Hogberg L, Penttinen P, Svensson A, Ekdahl K. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006;6:58. doi: 10.1186/1471-2334-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges R, MacLeod C. Epidemic pneumococcal pneumonia. Am J Epidemiol. 1946;44:231–6. [PubMed] [Google Scholar]
- 11.Klugman KP, Chien Y-W, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27:C9–14. doi: 10.1016/j.vaccine.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Mahdi LK, Ogunniyi AD, LeMessurier KS, Paton JC. Pneumococcal virulence gene expression and host cytokine profiles during pathogenesis of invasive disease. Infect Immun. 2008;76:646–57. doi: 10.1128/IAI.01161-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter ND, Taylor TH, Shay DK, et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–83. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 14.Kuster SP, Tuite AR, Kwong JC, McGeer A, Fisman DN the Toronto Invasive Bacterial Diseases N. Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med. 2011;8:e1001042. doi: 10.1371/journal.pmed.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morens David M, Taubenberger Jeffery K, Fauci Anthony S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Short KR, Habets MN, Hermans PWM, Diavatopoulos DA. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol. 2012;7:609–24. doi: 10.2217/fmb.12.29. [DOI] [PubMed] [Google Scholar]
- 17.Madhi S, Petersen K, Madhi A, Wasas A, Klugman K. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J. 2000;19:1141–7. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 18.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–95. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diavatopoulos DA, Short KR, Price JT, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. The FASEB Journal. 2010;24:1789–98. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 20.Talbot T, Poehling K, Hartert T, et al. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–91. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Techasaensiri B, Techasaensiri C, Mejías A, McCracken GHJ, Ramilo O. Viral coinfections in children with invasive pneumococcal disease. Pediatr Infect Dis J. 2010;29:519–23. doi: 10.1097/INF.0b013e3181cdafc2. [DOI] [PubMed] [Google Scholar]
- 22.Edwards J, Markey P, Cook H, Trauer J, Krause V. The relationship between influenza and invasive pneumococcal disease in the Northern Territory, 2005–2009. Med J Aust. 2011:194–207. doi: 10.5694/j.1326-5377.2011.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller MA, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the US. J Infect Dis. 2012;205:458–65. doi: 10.1093/infdis/jir749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mufson MA, Kruss DM, Wasil RE, Metzger WI. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974;134:505–10. [PubMed] [Google Scholar]
- 25.Gleich S, Morad Y, Echague R, et al. Streptococcus pneumoniae serotype 4 outbreak in a home for the aged: report and review of recent outbreaks. Infect Control and Hospital Epidemiol. 2000;21:711–7. doi: 10.1086/501717. [DOI] [PubMed] [Google Scholar]
- 26.Spooner LH. The specific diagnosis and treatment of acute lobar pneumonia. The Boston Medical and Surgical Journal. 1920;182:224–8. [Google Scholar]
- 27.Christie I. Epidemiological significance of the serological types of pneumococci. Lancet. 1934;224:39–42. [Google Scholar]
- 28.Launes C, de-Sevilla M-F, Selva L, Garcia-Garcia J-J, Pallares R, Muñoz-Almagro C. Viral coinfection in children less than 5 year-old with invasive pneumococcal disease. Pediatr Infect Dis J. 2012;31:650–3. doi: 10.1097/INF.0b013e31824f25b0. Publish Ahead of Print:10.1097/INF.0b013e31824f25b0. [DOI] [PubMed] [Google Scholar]
- 29.Harboe ZB, Benfield Thomas L, Valentiner-Branth P, et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis. 2010;50:329–37. doi: 10.1086/649872. [DOI] [PubMed] [Google Scholar]
- 30.Harboe ZB, Valentiner-Branth P, Benfield TL, et al. Estimated effect of pneumococcal conjugate vaccination on invasive pneumococcal disease and associated mortality, Denmark 2000–2005. Vaccine. 2008;26:3765–71. doi: 10.1016/j.vaccine.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 31.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–6. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen J, Mazick A, Glismann S, Molbak K. Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994–2010. BMC Infect Dis. 2011;11:350. doi: 10.1186/1471-2334-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viboud C, Bjørnstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312:447–51. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz JR, Zhou H, Shay DK, Neuzil KM, Fowlkes AL, Goss CH. Monitoring influenza activity in the United States: a comparison of traditional surveillance systems with google flu trends. PLoS ONE. 2011;6:e18687. doi: 10.1371/journal.pone.0018687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazick A, Christiansen A, Samuelsson S, Mølbak K. Using sentinel surveillance to monitor effectiveness of influenza vaccine is feasible: a pilot study in Denmark. Euro Surveill. 2006;11:254–56. [PubMed] [Google Scholar]
- 36.Sleeman KL, Griffiths D, Shackley F, et al. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis. 2006;194:682–8. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 37.Politis D. Resampling time series with seasonal components. Frontiers in Data Mining and Bioinformatics: Proceedings of the 33rd Symposium on the Interface of Computing Science and Statistics.2001. [Google Scholar]
- 38.O'Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–9. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 39.Sheng Z-M, Chertow DS, Ambroggio X, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci. 2011;108:16416–21. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 41.Sandgren A, Albiger B, Orihuela CJ, Tuomanen E, Normark S, Henriques-Normark B. Virulence in mice of pneumococcal clonal types with known invasive disease potential in humans. J Infect Dis. 2005;192:791–800. doi: 10.1086/432513. [DOI] [PubMed] [Google Scholar]
- 42.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Reports (1896–1970) 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberger D, Harboe Z, Flasche S, Scott J, Lipsitch M. Prediction of serotypes causing invasive pneumococcal disease in unvaccinated and vaccinated populations. Epidemiology. 2011;22:199–207. doi: 10.1097/EDE.0b013e3182087634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.