Abstract
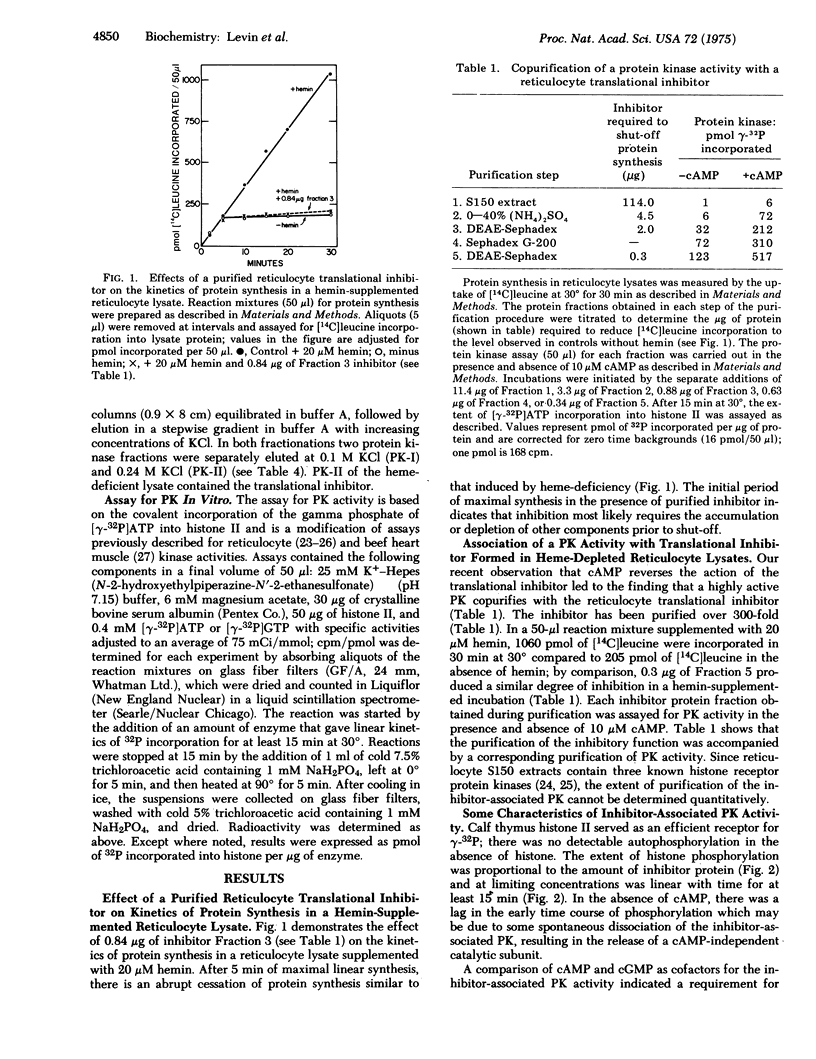
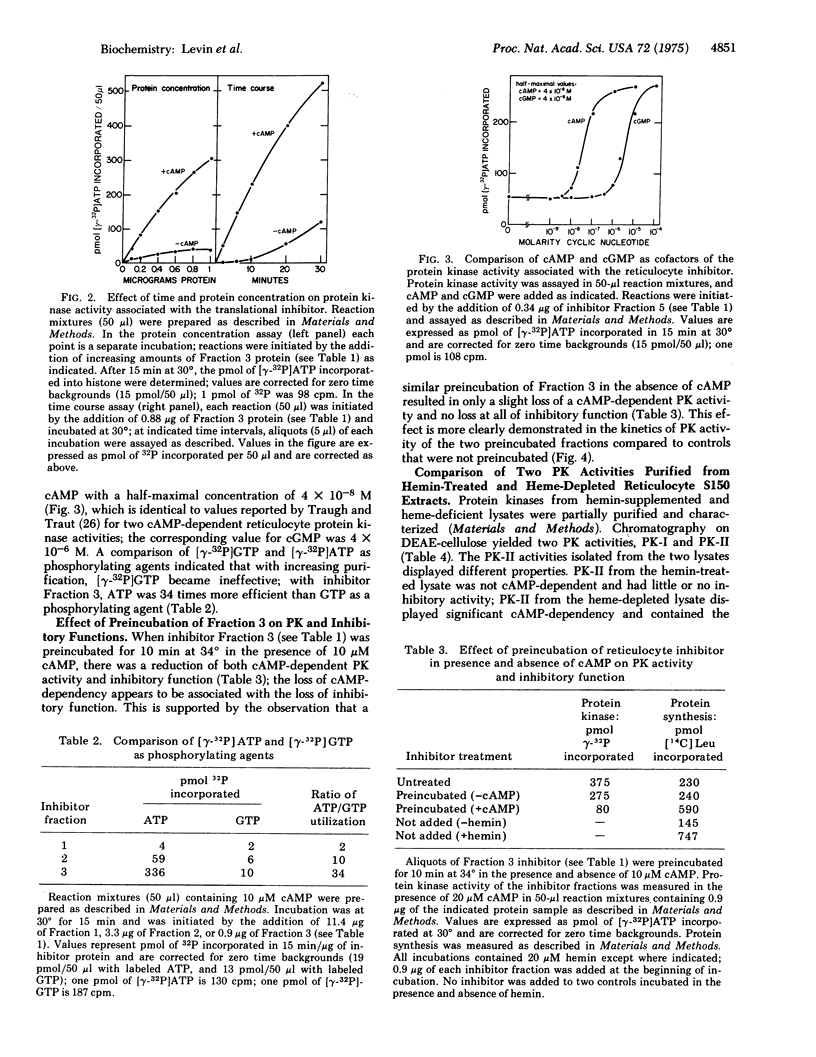
In the absence of added hemin, protein synthesis in rabbit reticulocyte lysates proceeds at maximal linear rates for several minutes and then ceases abruptly. Inhibition involves the action of a translational inhibitor whose formation is regulated by hemin. Addition of the isolated inhibitor to hemin-supplemented lysates produces an inhibition of protein chain initiation similar to that observed in heme-deficiency. The inhibitor has been purified over 300-fold and contains a protein kinase activity that copurifies with the inhibitory function. With calf thymus histone II as the phosphate receptor, the inhibitor-associated protein kinase requires ATP as the phosphorylating agent. Cycle AMP stimulates kinase activity 5- to 8-fold; the concentration of cycle AMP required for halfmaximal activity is 4 X 10-8 M. Preincubation of the inhibitor in the presence of cyclic AMP significantly reduces cyclic AMP-dependent phosphorylation and inhibitory activity. The corresponding protein kinase activity from hemin-supplemented lysates displays reduced cyclic AMP-dependency and little or no inhibitory activity. These findings suggest that the protein kinase activity associated with the purified translational inhibitor is involved in the mechanism of inhibition of initiation observed in hemedeficient reticulocyte lysates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Yau P. M., Herbert E., Zucker W. V. Involvement of hemin, a stimulatory fraction from ribosomes and a protein synthesis inhibitor in the regulation of hemoglobin synthesis. J Mol Biol. 1972 Jan 28;63(2):247–264. doi: 10.1016/0022-2836(72)90373-7. [DOI] [PubMed] [Google Scholar]
- Ashby C. D., Roberts S. Phosphorylation of ribosomal proteins in rat cerebral cortex in vitro. J Biol Chem. 1975 Apr 10;250(7):2546–2555. [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: effect of a translational repressor on Met-tRNAf binding to the small ribosomal subunit and its relation to the activity and alailability of an initiation factor. Biochim Biophys Acta. 1973 Oct 26;324(3):397–409. doi: 10.1016/0005-2787(73)90284-0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Henshaw E. C., Rahamimoff H., London I. M. Met-tRNAfMet binding to 40S ribosomal subunits: a site for the regulation of initiation of protein synthesis by hemin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2946–2950. doi: 10.1073/pnas.71.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Hunt T., Jackson R. J. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hunt T., Ehrenfeld E. Cytoplasm from poliovirus-infected HeLa cells inhibits cell-free haemoglobin synthesis. Nat New Biol. 1971 Mar 17;230(11):91–94. doi: 10.1038/newbio230091a0. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. A cell-free system with ribosomal protein kinase activity. Biochemistry. 1971 Jan 19;10(2):197–203. doi: 10.1021/bi00778a001. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Benerofe B., Hunt T., Kosower E. M. Inhibition of protein synthesis by glutathione disulfide in the presence of glutathione. Biochem Biophys Res Commun. 1971 Nov 5;45(3):816–821. doi: 10.1016/0006-291x(71)90490-6. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Kosower E. M. Glutathione. 8. The effects of glutathione disulfide on initiation of protein synthesis. Biochim Biophys Acta. 1972 Jul 31;272(4):623–637. [PubMed] [Google Scholar]
- Legon S., Brayley A., Hunt T., Jackson R. J. The effect of cyclic AMP and related compounds on the control of protein synthesis in reticulocyte lysates. Biochem Biophys Res Commun. 1974 Feb 4;56(3):745–752. doi: 10.1016/0006-291x(74)90668-8. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969 Apr 10;35(1):79–85. doi: 10.1016/0006-291x(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Tao M., Hackett P. Adenosine cyclic 3':5'-monophosphate-dependent protein kinase from rabbit erythrocytes. Purification and characterization of multiple forms. J Biol Chem. 1973 Aug 10;248(15):5324–5332. [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauch J. A., Mumby M., Traut R. R. Phosphorylation of ribosomal proteins by substrate-specific protein kinases from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):373–376. doi: 10.1073/pnas.70.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Traut R. R. Characterization of protein kinases from rabbit reticulocytes. J Biol Chem. 1974 Feb 25;249(4):1207–1212. [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]


