Key Points
Constitutive PI3K activity is associated with less accurate neutrophil migration in healthy aged adults.
This is associated with increased primary granule release and neutrophil elastase activity and may contribute to inflammation and infection.
Abstract
Immunosenescence is the functional deterioration of the immune system during natural aging. Despite increased susceptibility to bacterial infections in older adults, age-associated changes to neutrophil responses are only partially understood, and neutrophil migration has not been characterized in detail. Here we describe reduced chemotaxis but preserved chemokinesis toward a range of inflammatory stimuli in migrating neutrophils isolated from healthy older subjects. Cross-sectional data indicate that migratory behavior changes in the sixth decade of life. Crucially, aberrant migration may increase “bystander” tissue damage and heighten inflammation as a result of excess proteinase release during inaccurate chemotaxis, as well as reducing pathogen clearance. We show evidence of increased neutrophil proteinase activity in older adults, namely, raised levels of neutrophil proteinase substrate-derived peptides and evidence of primary granule release, associated with increased systemic inflammation. Inaccurate migration was causally associated with increased constitutive phosphoinositide 3-kinase (PI3K) signaling; untreated neutrophils from old donors demonstrated significant PI3K activation compared with cells from young donors. PI3K-blocking strategies, specifically inhibition of PI3Kγ or PI3Kδ, restored neutrophil migratory accuracy, whereas SHIP1 inhibition worsened migratory flaws. Targeting PI3K signaling may therefore offer a new strategy in improving neutrophil functions during infections and reduce inappropriate inflammation in older patients.
Introduction
The efficiency of the immune system declines with age. Termed “immunosenescence,” this has been demonstrated in cellular studies1 and by the increased risk of infection-associated mortality, morbidity, increased tissue damage, and physical frailty experienced by the elderly.2,3 The high incidence of bacterial infections in older adults suggests a suboptimal neutrophil response, and in vitro studies support this, demonstrating that bactericidal (superoxide generation and degranulation) and phagocytic function are reduced in neutrophils isolated from older subjects.4,5
In contrast, the effects of aging on neutrophil migration are poorly defined. Neutrophil adherence to endothelium is unaltered,6 but migration appears reduced in some studies.5,7,8 The assays used in these studies comment on overall patterns of cell aggregation (essentially cell accumulation) and not migratory parameters, such as speed or accuracy of movement. They were also unable to address how and why neutrophils from older adults differed during migration from those isolated from younger subjects. The latter is crucial if any age-related changes are to be reversed.
Reduced migratory accuracy could result in reduced bacterial clearance, contributing to poorer responses to bacterial infections. However, the potential negative effects of inaccurate migration are not limited to reduced bacterial killing. Neutrophils use proteinases such as neutrophil elastase (NE) to facilitate migration through complex tissue matrices9 and organs during inflammatory challenge.10 Imprecise migration could lead to excessive elastase release as neutrophils meander inaccurately through tissues, resulting in more widespread tissue injury and increased systemic inflammation. Neutrophils appear to be mediators of tissue damage in chronic diseases,11 many of which are age related, and dysregulated neutrophil functions could be pathologically implicated in disease development.
Directional neutrophil migration requires environmental sampling, cell polarization, and propulsion, initiated by chemoattractant ligands binding to corresponding G-protein–coupled receptors on neutrophils. Class 1 phosphoinositide 3-kinase (PI3K) activity is central to these processes, by directing phosphoinositol 3,4,5-trisphosphate (PIP3) accumulation to the leading edge of the cell,12,13 initiating a signaling cascade that localizes elements required for locomotion.12 Thus, either depletion or overexpression of PI3K will negatively affect cell migration.14 Advancing age is associated with systemic inflammation, with increased concentrations of tumor necrosis factor α (TNF-α) systemically.15 TNF-α alters the neutrophil migratory phenotype with reduced chemotaxis in a manner that is dependent on mitogen-activated protein kinase (MAPK), specifically p38 kinase.16 Both PI3K and p38 are therefore attractive targets for modifying neutrophil cellular functions.
The aims of the present study were to examine migration in quantitative detail in cells isolated from healthy older and younger subjects across numerous inflammatory signals, including biological secretions; to determine when migratory behavior alters with increasing age; to identify underlying mechanisms for differences in migratory behavior, focusing on PI3K and p38 signaling activity; and to assess if older age is associated with increased neutrophil proteinase release and activity, consistent with theories of neutrophil-mediated tissue injury and raised systemic inflammation in older adults.
Materials and methods
Study subjects
Healthy volunteers had never smoked, had no evidence of acute or chronic disease or illness, had normal lung function,17 and were medication free. Older subjects were aged over 65 years, and younger adults were under 35. Cross-sectional data were collected from volunteers meeting the same entry criteria, aged 21 to 89 years (n = 70). All experiments included gender-matched subjects. All subjects gave their informed written consent in accordance with the Declaration of Helsinki and following approval from and in accordance with the Local Research Ethics Committee.
Isolation of blood neutrophils
Neutrophils were isolated from whole blood as described.18 The neutrophils (>95% pure, >97% viable, by exclusion of trypan blue) were resuspended in buffer (RPMI 1640 medium; Flow Laboratories, Rickmansworth, United Kingdom) containing 0.15% bovine serum albumin (Sigma-Aldrich, St. Louis, MO).
Neutrophil chemotaxis
Migration was assessed using an Insall chamber (Weber Scientific International Ltd., Teddington, United Kingdom) as described previously.19 Coverslips were coated with 7.5% culture-tested bovine serum albumin (Sigma-Aldrich), and neutrophils (suspended at 2 × 106/mL) adhered to this surface for 20 minutes at 37°C. The coverslip was inverted on the Insall chamber, and the chamber was filled with buffer (as described previously) or buffer containing the chemoattractant being assessed (100 nM interleukin 8 [CXCL8], 10 nM growth-related oncogene α [CXCL1], 10 nM leukotriene B4 [LTB4], 1 nM complement component 5a [C5a; all R&D Systems, Abbingdon, United Kingdom], or 100 nM formyl-methionyl-leucyl-phenylalanine [fMLP; Sigma-Aldrich]). Neutrophils were also studied when migrating toward pooled soluble sputum samples collected from 5 patients with confirmed Streptococcus pneumoniae–associated pneumonia.
To replicate the systemic inflammatory environment, cells were also incubated with TNF-α 1 pM (R&D Systems) or plasma from old or young subjects for 45 minutes. For inhibitor studies, cells were incubated with the nonselective PI3K inhibitor LY294002 (1 μM) (Selleck, Houston, TX); PI3K isoform selective inhibitors of class 1 α (PI3K-75 7.8 nM; Chemietek, Indianapolis, IN), β (TGX-221 10 nM; Selleck), δ (Cal-101 75 nM; Selleck), or γ (AS-252424 33 nM; Selleck); the p38 kinase inhibitors VX745 and SCIO468 (10 nM and 9 nM, respectively; R&D Systems); and the SHIP1 inhibitor 3AC (10 uM; Merck Millipore, United Kingdom) for 45 minutes prior to migration. Conditions were chosen following appropriate time-course and dose-response experiments. Concentrations of TNF-α were chosen to replicate plasma concentrations measured in older healthy subjects.
Video microscopy
All time-lapse recordings were made using a Zeiss Axiovert 100 inverted microscope fitted with a Fast Mono 12-bit QICAM digital camera. Recordings lasted 20 minutes per experiment, with 20 slides captured using Improvision OpenLab software per film. The Java software ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD) was used to analyze cell tracks. All analysis was carried out by a single analyst, blinded to subject group and cell condition.
Migration was assessed using 3 parameters: Average cell speed of movement (micrometers per minute) was measured from the distance traveled between frames in any direction over time (termed chemokinesis); average cell velocity (speed in a consistent direction toward the chemoattractant, termed chemotaxis) was measured in micrometers per minute. The Insall chamber allows the formation of stable chemoattractant gradients, with defined, consistent direction in the y direction for each experiment (as described previously19). Only distance traveled in the y direction over time was included in calculations of chemotaxis. Accuracy (termed chemotactic index) was calculated by the cosine of the angle between the cell’s direction and the orientation of the chemoattractant gradient at each time frame forming a vector analysis of movement, expressed in a comparative scale (cs) ranging from −1 to 1, where 1 represents movement directly toward the chemoattractant, and −1 represents movement directly away from the chemoattractant source in all frames.
Pooled sputum concentrations of CXCL8, CXCL1, and C5a were assessed by enzyme-linked immunosorbent assay (all R&D Systems), and LTB4 concentrations by parameter assay (R&D Systems) according to manufacturer’s instructions, in triplicate, with an average result given. Aα-VAL360 was measured using methods described previously.20 For methods pertaining to the collection and preparation of sputum samples, and isolation of bacteria in sputum, see the supplemental Methods and supplemental Table 1 (available on the Blood Web site).
Surface receptor expression
Surface expression of chemoattractant receptors (CXCR1 [CXCL8 receptor], CXCR2 [CXCL8 and CXCL1 receptor], FPR1 [fMLP receptor], C5aR [C5a receptor], and BLT1 [LTB4 receptor]) was measured on freshly isolated peripheral blood neutrophils as described in the supplemental Methods. Surface expression of primary granule marker CD63 was measured on neutrophils stained in whole blood following red cell lysis using mouse anti-human CD63-phycoerythrin 1:20 dilution (clone# CLB-gran/12 [CLB-180]; Invitrogen/Life Technologies) and isotype control IgG1-phycoerythrin (clone# MOPC-21; BioLegend). Appropriate antibody dilutions were determined by individual titrations (data not shown).
PI3K expression
PI3K expression was assessed by western blotting as described in the supplemental Methods, with neutrophils incubated with CXCL8 (100 nM; R&D Systems) for 0, 1, 2, or 5 minutes. Primary antibodies used were rabbit anti-human phospho-PI3K p85 (Tyr485)/p55 (Tyr199) (Cell Signaling Technology) and mouse anti-human β-actin (Sigma-Aldrich).
Statistics
PASW Statistics v.18.0 (SPSS Inc., Chicago, IL) was used to analyze data. Data normality was assessed using the Kolmogorov-Smirnov test. Analyses of variance with Bonferroni correction, independent or paired Student t tests, and the Kruskall Wallis test were performed depending on data distribution. Statistical significance was accepted as P < .05. All experiments were preceded by studies of intrasubject and intersubject variance to allow appropriate power calculations to be performed. For the Insall chamber, the inclusion of 10 subjects per group provided 80% power to detect a 25% difference in mean chemokinesis and a 35% difference in mean chemotaxis. For surface receptor expression, the inclusion of 20 subjects per group provided 80% power to detecting a 25% change in mean expression, all at the 5% level of significance.
Results
Neutrophil migration to CXCL8 is less accurate in healthy older subjects
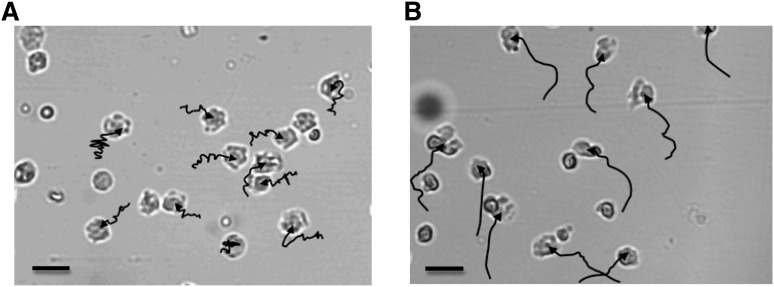
Migratory pathways from individual cells suggested that neutrophils from older subjects migrated with less accuracy than those from younger subjects toward all studied chemoattractants. Figure 1 shows representative neutrophil migratory pathways as tracked in 1 old and 1 young subject.
Figure 1.
Tracks of neutrophil migration from old and young healthy subjects. The images show the final neutrophil positions within the Insall chemotaxis chamber following 20 minutes of time-lapse recording of neutrophils from an old (A) or a young (B) donor. The chemoattractant source (CXCL8) was added at the top of the viewing field, creating a gradient diffusing down to the bottom of the viewing field. The tracks (shown in black) indicate the path of migration for each cell analyzed: each track begins where the cell was positioned at the start of recording and ends where the cell was positioned at the end of recording. Each image is representative of all recordings taken of neutrophils from each group for all chemoattractants tested. Bars represent 10 μm (A-B). Images were taken using a Zeiss Axiovert 100 microscope, magnification ×20 (A-B). Migration pathway arrows were generated using ImageJ software on manual tracking.
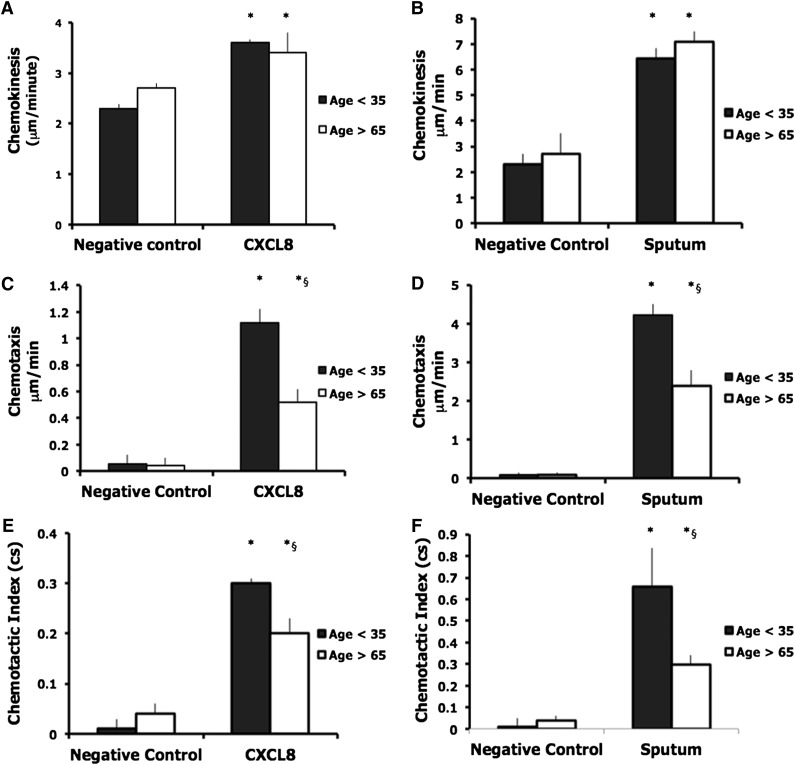
Neutrophils from both young and older subjects demonstrated increased chemokinesis in CXCL8 compared with negative control (mean speed with standard deviation [SD]: young, negative control 2.3 + 0.08 μm/min, CXCL8 3.6 + 0.07 μm/min, P = .0001; old, negative control 2.7 + 0.1 μm/min, CXCL8 3.4 + 0.4 μm/min, P = .0001). There were no differences in chemokinesis between groups (Figure 2A). Neutrophils from both groups demonstrated increased chemotaxis in the presence of CXCL8 compared with the negative control (mean velocity of migration + SD: young, negative control 0.05 + 0.07 μm/min, CXCL8 1.12 + 0.1 μm/min, P < .0001; old, negative control 0.04 + 0.06 μm/min, CXCL8 0.52 + 0.1 μm/min, P = .001). Neutrophils from older subjects were less chemotactic toward CXCL8 (mean difference [MD] 0.73 μm/min, P < .0001) (Figure 2C).
Figure 2.
Chemokinesis, chemotaxis, and chemotactic index (accuracy) in neutrophils from younger and older subjects toward CXCL8 (A,C,E) and sputum (B,D,F). Neutrophils from healthy subjects (aged <35 or >65 years of age) migrated toward CXCL8 (100 nM) or pooled sputum collected from patients admitted to the hospital with an acute lower respiratory tract infection attributable to S. pneumoniae. Measurements were taken from 10 randomly selected cells from each individual, with 10 subjects in each group. The average results for each subject were calculated, and an overall average was used for comparisons between groups. Bars represent the mean migratory parameter with SD shown as the error line (A-F). Chemokinesis and chemotaxis were measured in micrometers per minute. Accuracy is a measure of the cell’s directional orientation and is expressed in a cs ranging from −1 to 1. * indicates significant difference in migratory parameter from negative control data (P < .05); §, significant difference in migratory parameter between young and old samples.
Neutrophils from both young and older subjects demonstrated more chemotactic accuracy when migrating toward CXCL8 compared with the negative control (mean chemotactic index + SD: young, negative control 0.01 + 0.02, CXCL8 0.3 + 0.01, P < .0001; old, negative control 0.04 + 0.02, CXCL8 0.2 + 0.03, P = .001). Neutrophils from old donors migrated with less accuracy toward CXCL8 (MD 0.23, P < .0001; see Figure 2E).
Aberrant neutrophil migration with increasing age is not CXCL8 specific
Studies were repeated in the presence of fMLP, which was chosen because of its relevance in infective processes. Similar migratory patterns were seen, with isolated neutrophils from older subjects maintaining chemokinesis (mean chemokinesis + SD: young, 4.0 μm/min + 0.3 vs old, 4.4 μm/min + 0.3) but displaying reduced chemotaxis (mean chemotaxis + SD: young, 1.0 μm/min + 0.2 vs old, 0.4 μm/min + 0.1, P < .05) and reduced chemotactic accuracy (mean chemotactic index + SD: young, 0.2 + 0.03 vs old, 0.04 + 0.01, P < .05). This “old” migratory phenotype was also seen during migration toward LTB4, C5a, and CXCL1 (see supplemental Table 1) at physiological concentrations (confirmed by measurements from the pooled sputum; see supplemental Table 2), confirming that the altered migratory behavior was not driven by a single chemoattractant or associated receptor.
Biological samples comprise a complex mixture of pro- and anti-inflammatory proteins, and neutrophil responses may vary in this physiological environment. Sputum collected during S pneumoniae–associated pneumonia induced more neutrophil migration than single receptors (eg, old, chemokinesis to CXCL8 100 nM vs sputum, MD 3.4 μm/min + 1.6, P < .0001). Once again, cells from healthy older subjects displayed preserved chemokinesis (old, 7.1 μm/min + 0.4; young, 6.4 μm/min + 0.4; P = not significant [ns]), but reduced chemotaxis (old, 2.4 μm/min + 0.5; young, 4.2 μm/min + 0.7; P = .001) and chemotactic accuracy (chemotactic index: old, 0.3 + 0.1; young, 0.65 + 0.2; P < .0001) when migrating toward pooled sputum (Figure 2B,D,F), containing quantified concentrations of bacteria, CXCL8, LTB4, C5a, and CXCL1 (see supplemental Table 2).
In summary, migrating neutrophils from older subjects demonstrated preserved speed (chemokinesis), but reduced directional speed (chemotaxis) and accuracy toward a panel of inflammatory mediators and complex inflammatory secretions and infection.
Inaccurate neutrophil migration is associated with increasing age
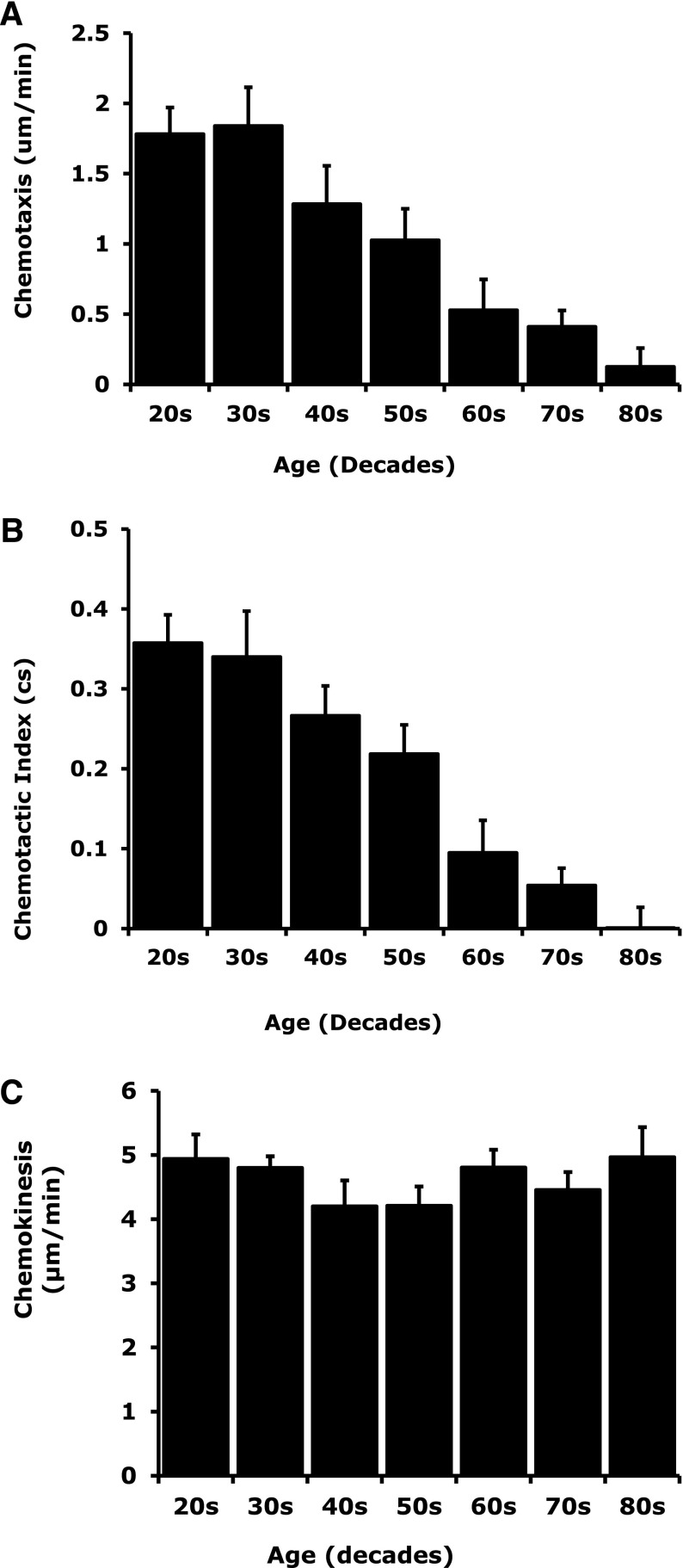
Neutrophil migration was assessed in 70 healthy subjects aged 21 to 89 years. There was an age-associated reduction in neutrophil chemotaxis (R2 = –0.4750, F = 56.99, P < .0001) and chemotactic index (R2 = –0.5399, F = 73.94, P < .0001) with increasing age (Figure 3A-B). Age did not affect neutrophil chemokinesis (R2 = 0.004596, F = 0.2909, P = .5915) (Figure 3C). Chemotaxis and chemotactic efficiency of neutrophils decreased gradually from 30 years of age but became significantly lower after 60 years of age (comparing ages 21-29 with ages 60-69: chemotaxis, MD 1.25 μm/min, confidence interval [CI] 0.3-2.2, P = .02; chemotactic index, MD 0.26, CI 0.1-0.4, P < .001).
Figure 3.
Association between reduced chemotaxis and chemotactic accuracy with increasing age with preserved chemokinesis. Migratory chemotaxis, chemotactic index, and chemokinesis were measured in healthy subjects aged 21 to 89 years of age (n = 70). Linear regression identified an age-associated reduction in (A) neutrophil chemotaxis (R2 = –0.4750, F = 56.99, P < .0001) and (B) chemotactic index (R2 = –0.5399, F = 73.94, P < .0001) with increasing age. Age did not affect (C) neutrophil chemokinesis (R2 = 0.004596, F = 0.2909, P = .5915).
Aberrant migration cannot be induced by exposure to inflammatory signals
Heightened systemic inflammation with age21 might alter neutrophil functions. Systemic concentrations of TNF-α were raised in healthy older subjects (median concentration with range: older donors, 0.21 pM [0.09-0.28]; young donors, 0.08 pM [0.04-0.11]; P = .02). These TNF-α concentrations informed priming experiments in which neutrophils from young and older donors were incubated with TNF-α, buffer (negative control), or pooled plasma isolated from young and old donors and migratory assays were repeated. Incubation with TNF-α or “young donor” or “old donor” pooled plasma increased young donor neutrophil chemokinesis and chemotaxis migrating toward CXCL8 and fMLP (n = 6). Incubation of “old” neutrophils in “young donor” plasma or buffer did not improve chemotaxis. This suggests that removing “old” neutrophils from a proinflammatory environment does not restore accurate migration and that an “old” migratory phenotype cannot be induced by inflammation exposure in “young cells” (see supplemental Figure 1) and therefore may reflect intrinsic age-associated processes.
Surface expression of chemokine receptors was not altered with age
There were no differences in median cell surface expression of chemokine receptors on quiescent or activated neutrophils isolated from either age group over time (Table 1). There was also no difference in surface expression of receptors for LTB4 and C5a (data in supplemental Table 3).
Table 1.
CXCR1, CXCR2, and FPR1 surface expression in neutrophils from healthy young and older donors following activation over time
| Chemokine receptor | Time 0 | 10 min | 30 min | 1 h | ||||
|---|---|---|---|---|---|---|---|---|
| Age <35 | Age >65 | Age <35 | Age >65 | Age <35 | Age >65 | Age <35 | Age >65 | |
| CXCR1 | 102 (12) | 82 (10) | 68 (3) | 61 (4) | 88 (6) | 82 (4) | 54 (9) | 46 (8) |
| CXCR2 | 84 (8) | 81 (6) | 61 (6) | 53 (11) | 73 (6) | 69 (10) | 48 (7) | 41 (9) |
| FPR1 | 45 (5) | 39 (9) | 20 (7) | 16 (4) | 38 (21) | 31 (7) | 24 (6) | 18 (3) |
Each value is the median fluorescence intensity (MFI) for each group (young or older subjects; n = 20 each group) with the SD in parentheses. MFI was calculated by subtracting the MFI for neutrophils incubated with anti-human CXCR1, CXCR2, and FPR1 for each subject from the MFI for neutrophils from the same subject incubated with an isotype-matched irrelevant fluorescein isothiocyanate–labeled IgG2A. All data sets follow normal distribution (Kolmogorov-Smirnov test). Neutrophils were incubated with CXCL8 (100 nM) or fMLP (100 nM), and an aliquot of cells had surface receptor expression semiquantified by immunostaining and flow cytometry.
Neutrophils from older subjects demonstrate constitutive PI3K activation
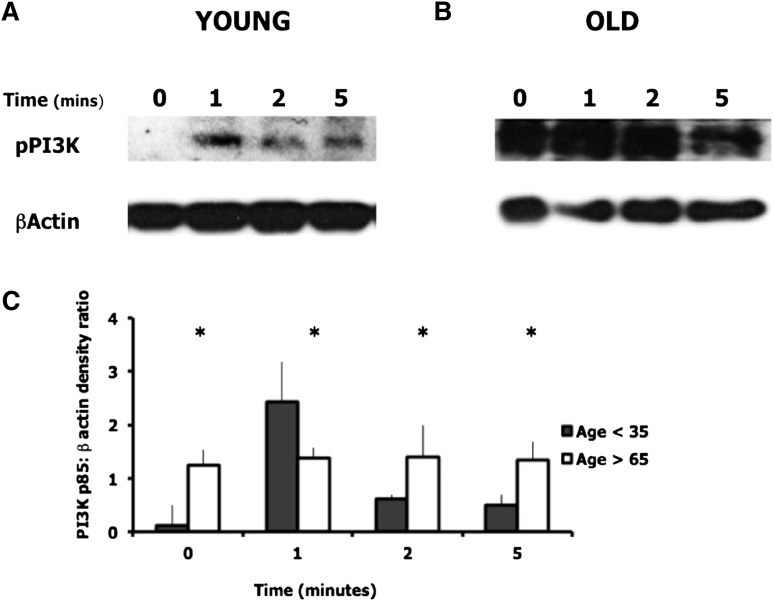
PI3K activity was measured by western blotting to assess levels of phosphorylated PI3K at baseline and following stimulation with CXCL8. Neutrophils from older adults demonstrated basal levels of PI3K activation that did not further increase after exposure to CXCL8, suggesting constitutive activation. In comparison, cells from young adults demonstrated little basal PI3K activity followed by a transient increase in p85 phosphorylation in response to CXCL8, peaking at 1 minute (Figure 4).
Figure 4.
PI3K activation in neutrophils from young and old healthy donors. PI3K activity was assessed by western blotting using an antibody to the phosphorylated p85 regulatory subunit of PI3K. β-actin was assessed as the loading control. Cells were unstimulated or incubated with CXCL8 (100 nM) for the times shown. Blots were run in duplicate. (A-B) Representative western blots for 1 young and 1 older donor. (C) The densitometric ratio of phospho-p85:β-actin for young and old adults (n = 5 each group). There was a significant difference in PI3K:β-actin densitometric ratio between young and old neutrophils across all time points. * indicates significant difference between young and old ratios (P < .05).
Increased PI3K activity influences aberrant neutrophil migration
To determine whether increased PI3K activation was related to aberrant migration, migratory studies were repeated following incubation with 1 μM LY294002, a reversible, isoform nonselective PI3K inhibitor. LY294002 significantly reduced neutrophil chemokinesis (MD –1.6 μm/min, CI −0.8 to −3.1, P = .002), chemotaxis (MD –1.1 μm/min, CI −0.6 to −1.9, P = .001), and chemotactic accuracy (chemotactic index MD –0.3, CI −0.1 to −0.5, P = .008) of “young” cells but improved chemotaxis (MD 0.58 μm/min, CI 0.1-1.2, P = .01) and chemotactic accuracy (chemotactic index MD 0.19, CI 0.02-0.45, P = .03) of “old” neutrophils, restoring parameters to levels seen in younger subjects (data in supplemental Table 4).
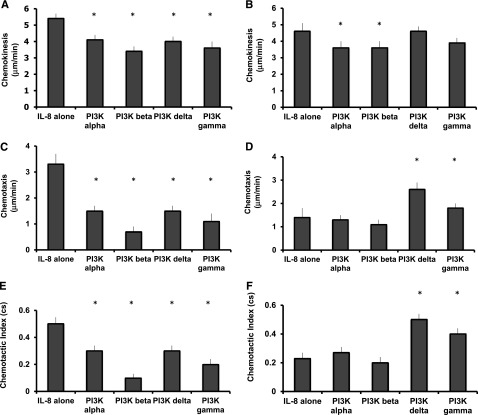
Blockade of PI3K class 1 δ and γ isoforms improves migratory accuracy in cells from old donors
To determine which class 1 PI3K isoforms (α, β, γ, and δ) were involved in the regulation of neutrophil migration, experiments were repeated exposing cells to selective PI3K class 1 isoform inhibitors. The class 1 α and β PI3K inhibitors decreased migratory speed (chemokinesis) in cells from older donors, without affecting chemotaxis or migratory accuracy (untreated migration toward CXCL8 compared with inhibitor-treated neutrophils migrating toward CXCL8: α inhibitor, chemokinesis MD –1.0 μm/min, CI −0.1 to −2.0, P = .03; chemotaxis MD −0.1μm/min, CI −0.4 to −0.6, P = .03; chemotactic index MD 0.0, CI −0.1 to 0.0, P = ns. β inhibitor, chemokinesis MD –1.0 μm/min, CI −0.1 to −2.0, P = .04; chemotaxis, MD −0.3 μm/min, CI −0.4 to 1.3, P = ns; chemotactic index MD 0.0 cs, CI −0.1 to 0.3, P = ns).
PI3K class 1 δ and γ inhibitors improved accuracy (chemotaxis and chemotactic index) without affecting speed (chemokinesis) in “old” neutrophils (Figure 5) (untreated migration compared with inhibitor-treated neutrophils migrating [both toward CXCL8]: δ inhibitor, chemokinesis MD −0.2 μm/min, CI −1.0 to 0.6, P = ns; chemotaxis MD 1.5 μm/min, CI 0.8-2.1, P = .001; chemotactic index MD 0.3, CI 0.2-0.3, P < .001. γ inhibitor, chemokinesis MD −0.5 μm/min, CI −0.5 to 1.5, P = ns; chemotaxis, MD 0.6 μm/min, CI 0.1 to 1.2, P = .06 (ns); chemotactic index MD 0.2, CI 0.1 to 0.3, P < .001).
Figure 5.
Chemokinesis, chemotaxis, and chemotactic index (accuracy) in neutrophils from young and old subjects toward CXCL8 in the presence of PI3K isoform selective inhibitors. Neutrophils from healthy subjects (aged <35 or >65 years of age) (n = 10 each group) migrated toward CXCL8 (100 nM) following incubation with carrier control or the denoted PI3K isoform selective inhibitor for 45 minutes. Measurements were taken from 10 randomly selected cells from each individual: from young donors (A,C,E) and from old donors (B,D,F). The average results for each subject were calculated, and an overall average was used for comparisons between groups. Bars represent the mean migratory parameter with SD shown as the error line (A-F). * indicates significant difference in migratory parameter from carrier control data (P < .05).
These results contrasted with those from young donors. The class 1 α, β, δ, and γ inhibitors decreased all neutrophil migratory parameters, including chemokinesis, chemotaxis, and accuracy of migration (apart from the γ inhibitor, for which there was a nonsignificant trend toward decreasing speed) in “young” cells (untreated migration compared with inhibitor-treated neutrophils migrating toward CXCL8: α inhibitor, chemokinesis MD −1.3 μm/min, CI −0.7 to −2.0, P = .002; chemotaxis MD −1.8 μm/min, CI −1.1 to −2.6, P < .001; chemotactic index MD −0.2, CI −0.1 to −0.3, P = .01. β inhibitor, chemokinesis MD −2.0 μm/min, CI −1.3 to −2.6, P < .001; chemotaxis, MD −2.6 μm/min, CI −1.8 to −3.4, P < .001; chemotactic index MD −0.3, CI −0.2 to −0.5, P = .001. δ inhibitor, chemokinesis MD −0.8 μm/min, CI −0.1 to −1.5, P = .003; chemotaxis MD −1.4 μm/min, CI −0.6 to −2.3, P = .005; chemotactic index MD −0.2, CI −0.1 to −0.4, P = .01. γ inhibitor, chemokinesis MD −1.2 μm/min, CI −0.1 to −2.6, P = .06; chemotaxis MD −1.9 μm/min, CI −0.9 to −2.8, P = .002; chemotactic index MD −0.3, CI −0.2 to −0.4, P = .001) (see Figure 5).
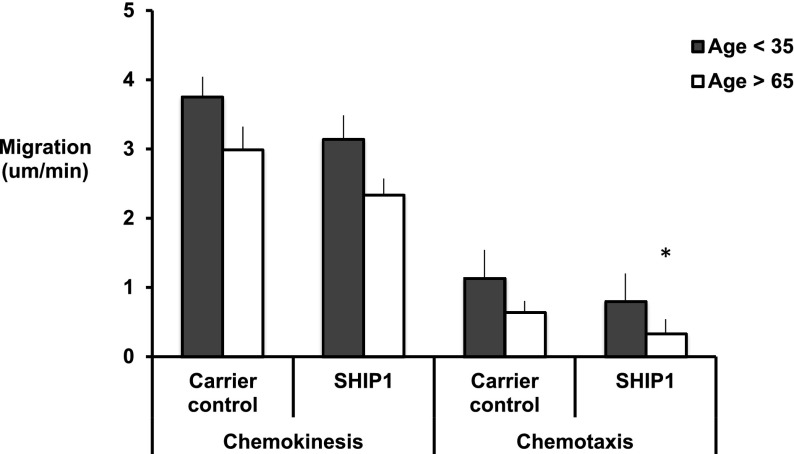
The cell-permeable steroidal compound 3AC, which selectively inhibits SHIP1 phosphatase activity toward PIP3, increasing PIP3 availability (50% inhibition concentration = 10 µM), further reduced chemotaxis in “old” neutrophils while inducing an “old migratory phenotype” in “young” cells (Figure 6). The p38 MAPK inhibitors did not significantly alter neutrophil migratory parameters in either old or young subjects, suggesting that this pathway did not have an impact on the migratory phenotype observed (data in supplemental Table 5).
Figure 6.
Chemokinesis and chemotaxis toward CXCL8 in the presence of a SHIP1 selective inhibitor. Neutrophils from healthy subjects (aged <35 or >65 years of age) (n = 6 each group) migrated toward CXCL8 (100 nM) following incubation with carrier control or the SHIP1 selective inhibitor for 45 minutes. Measurements were taken from 10 randomly selected cells from each individual. The average results for each subject were calculated, and an overall average was used for comparisons between groups. Bars represent the mean migratory parameter with SD shown as the error line. * indicates significant decrease in migratory parameter from carrier control data (P < .05).
To ensure that altered migration was not the result of changes in neutrophil viability, cell viability was assessed. There were no differences in cell viability in untreated cells compared with cells incubated with any inhibitor used, in either age group (data not shown).
Evidence of increased degranulation and neutrophil proteinase activity
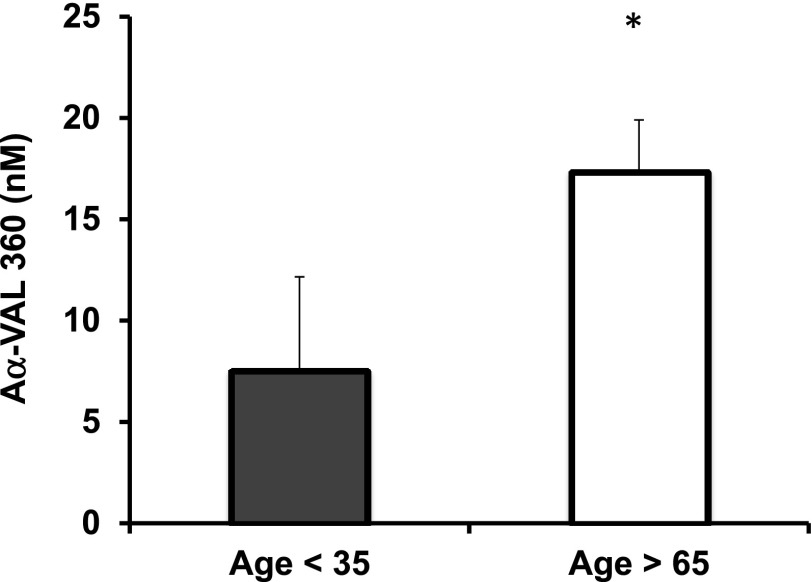
NE activity was assessed using the neutrophil proteinase-specific fibrinogen cleavage product Aα-VAL360, validated as a sensitive measure of systemic neutrophil proteinase activity.20 Aα-VAL360 plasma concentrations were higher in older healthy subjects (mean 17.3 nM + 2.6 standard error of the mean) compared with younger healthy subjects (mean 7.5 nM + 0.7 standard error of the mean), P = .006, consistent with increased NE activity in healthy aging (Figure 7).
Figure 7.
The mean systemic concentrations of Aα-VAL360 in young and older healthy adults. Aα-VAL360 is an NE-specific fibrinogen cleavage product. The cleavage product was measured by enzyme-linked immunosorbent assay in plasma in triplicate from all individuals, and healthy subjects aged over 65 and under 35 were compared (n = 20 each group). The mean concentration is shown for each group with the standard error given. * indicates significant difference from young adults (P < .05).
In keeping with these data, CD63 surface expression (a marker of primary granule degranulation) was higher in unstimulated neutrophils from older people compared with younger controls (median fluorescent index [and interquartile range]: old, 4364.8 [SD 258]; young, 3218 [SD 562]; P = .04, n = 20 each group).
Discussion
We describe differences in the migratory behavior of neutrophils from healthy older subjects compared with gender-matched healthy younger donors. Neutrophils from older subjects migrated with similar chemokinesis, but with reduced chemotaxis and less accuracy. This report is the first to assess neutrophil migration in this number of older subjects and with detailed migratory dynamics and analysis of chemokine receptor expression and signaling for a series of key chemoattractants believed to be of importance in neutrophil recruitment in inflammation and infection.22 This study is also the first to describe the migratory responses of neutrophils from healthy older humans toward infected, inflammatory biological secretions, which the cells would encounter during acute infections. Concentrations of all chemoattractants were physiological, as measured in this study and in previous work.23 The number of subjects tested and the range of stimuli used suggest that the age-associated migratory phenotype is generic, robust, and therefore likely to be a feature of the aging neutrophil response to inflammation and infection.
Novel cross-sectional studies showed a decline of migrational accuracy over time, with function being most compromised after the age of 60. This is consistent with current theories of the causal association between low-grade, systemic inflammatory exposure seen with advancing age and immunosenescence, organ dysregulation, and functional decline.24-26
Our data suggest that acute exposure to inflammation does not cause aberrant migration. This phenotype appears robust and not easily modified by acute environment. However, it is still possible that continued inflammatory exposure may alter cell function over time either during hematopoiesis or while in the circulation. This might be a chronic process, involving epigenetic influences,27 and thus an extrinsic defect. Although few studies report on bone marrow neutrophil functions in humans, some murine models support a diminution of function with advancing age.28 Prospective sampling over many years would be needed to confirm this (and to identify any relationship between neutrophil cell functions and infective episodes). Preserved chemokinesis with reduced accuracy is a crucial distinction because studies suggest that reduced neutrophil chemotaxis, rather than chemokinesis, is associated with higher mortality with age.7,29
Older people experience increased mortality and morbidity with bacterial infections.30 Neutrophil phagocytosis and superoxide production is reduced with age.4,5 If chemotaxis is also impaired, the cumulative effects are likely to impact bacterial clearance in disease. This is supported by murine models of aging, in which neutrophil chemotaxis to infection was impaired in aged mice, resulting in poor inflammation resolution and wound repair.31
Aging is associated with a decline in organ function including a reduction in lung function, which has been associated with systemic inflammatory burden in a dose-dependent manner.32 It has been hypothesized that the decline in organ function may be attributable in part to neutrophilic inflammation. During activation and migration, neutrophil proteinases are expressed on the neutrophil membrane,33,34 polarizing to the leading edge of the cell. A proportion of the proteinase is left behind as the cell moves on,9,35 leaving an area of obligate enzyme activity until proteinase concentrations have decreased by diffusion to match those of surrounding proteinase inhibitors.36 Inaccurate chemotaxis leads to “old” neutrophils spreading farther than those from younger controls (data not shown), and so the potential for collateral damage is increased, as membrane-bound proteinase is released over a wider area increasing the distribution of obligate enzyme activity and collateral tissue damage. Our data support this conclusion. Serum levels of a neutrophil proteinase-specific fibrinogen degradation product were significantly raised in older donors, and there was evidence of increased degranulation, as demonstrated by the augmented CD63 surface expression on quiescent neutrophils.
This study proposes dysregulated PI3K activity as a mechanism for age-related aberrant migration, demonstrating that PI3K inhibition (but not p38) can correct the defect, a potential therapeutic intervention. Increased PI3K activity was suggested by increased phosphorylated p85, and the relation to migration confirmed because PI3K-blocking strategies corrected flaws in neutrophil accuracy, whereas SHIP1 inhibition heightened them. Regulatory subunit p85 phosphorylation is necessary for the recruitment of PI3K to activated receptors, which then initiates the phosphorylation of PIP2 to PIP3.
PI3Kγ and PI3Kδ are enriched in leukocytes and are thought to be central to migration and phagocytosis.37,38 PIP3 generation, first via PI3Kγ,39 induces early polarization,40 whereas PI3Kδ is thought to facilitate later pseudopod localization,41 orienting the cell to chemoattractant cues.42 Reduced chemotactic responses and an inability to generate PIP3 have been reported in PI3Kγ−/− null mice or after PI3Kγ inhibition,43,44 although this has not been replicated in cell models.44 Classical pathways of PI3K signaling suggest that class 1A (δ) and class 1B (γ) are differentially regulated (the former by tyrosine kinases and the latter by G-protein–coupled receptors), and yet we describe improvements in the migration of “older” neutrophils in response to both δ and γ subclass selective inhibitors. The migration assay used an albumin-coated surface, a surrogate for intercellular adhesion molecule 1.45 Integrin binding has been shown to increase PI3Kδ activity via the nonreceptor tyrosine kinase Syk, while the chemoattractants used would increase PI3Kγ activity.41 However, both isoforms can be activated in the presence of fMLP44 with evidence of functional redundancy between class 1A and 1B isoforms in neutrophil apoptosis,46 suggesting that their activity is not as isolated as previously thought.47 Of note, mInsc-deficient neutrophils also migrate in an undirected manner.48 PI3K activity increases Cdc42, which binds to Par649 and increases mInsc-related activation, suggesting related mechanisms, but more studies would be required to determine this.
Increased PI3K activity could deregulate the PI3K/SHIP1/PTEN balance, leading to insensitivity to inflammatory signals or displacement of key signaling proteins within the cell, but this remains unclear. Currently, there are no published data of PIP3 accumulation in “older” neutrophils, although aging has been associated with altered PIP3 signaling in other human cell types (eg, neurones50). Increased PI3K activity might alter PIP3 localization, inhibiting focus to the leading edge of the cell. There is some evidence to support this; in vitro studies of fibroblasts suggest that increased PI3K activity leads to widespread PIP3 accumulation,51 and the absence of SHIP1 leads to diffuse translocation of Akt across migrating murine neutrophils.52 PTEN knockout models have preserved chemokinesis but reduced chemotaxis, which can be corrected by PI3K inhibition.52,53 These models suggest that the formation of the leading edge required for chemotaxis is governed by an exquisite balance of PI3K and PTEN/SHIP1 activity. Studies of relevant inhibitors have shown their abilities to reduce but not block PI3K downstream signaling,54,55 and it may be that inhibition “resets” PI3K/PTEN/SHIP balance and signaling during migration, allowing accurate signaling within the polarized cells, but this remains to be proved. The p38 MAPK inhibitors did not affect migration, suggesting that the p38 pathway is not central to the aging neutrophil migratory phenotype, and this supports the notion that altered function is PI3K-pathway specific.
Improving neutrophil responses by normalizing PI3Kγ or PI3Kδ signaling may improve outcomes during infection and reduce inflammation in the chronic diseases associated with age. Such strategies have been tested in animal models of chronic neutrophil-based inflammatory diseases (such as rheumatoid arthritis) with clinical benefits and altered neutrophil trafficking.56 Aberrant PI3K signaling with increasing age may be a function of systemic inflammation, but its modulation may reduce the resulting amplification of inflammation and restore neutrophil functions. Our data support further studies of the PI3K pathway as a potential therapeutic strategy to address aspects of immunosenescence.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of Mrs H. Chahal in assay preparation.
This work was supported by grants from the Medical Research Council, the University Hospital Birmingham Charities, the Academy of Medical Sciences, and the Biotechnology and Biological Sciences Research Council.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.S. conceived the project, recruited subjects, performed experimental assays, carried out the statistical analysis, and prepared the manuscript for publication; H.G. recruited patients, performed experimental assays, and carried out statistical analysis; E.M., G.W., A.L., and N.A. performed experimental assays; R.H.I. designed and oversaw analysis of all migratory assays; R.A.S. oversaw the Aα-VAL360 assay and contributed vital reagents; and J.M.L. conceived and oversaw the project and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth Sapey, Centre for Translational Inflammation Research, School of Clinical and Experimental Medicine, University of Birmingham, Birmingham, B15 2TT, United Kingdom; e-mail: elizabeth.sapey@uhb.nhs.uk.
References
- 1.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2(11):659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 3.Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochem Soc Trans. 2003;31(2):449–451. doi: 10.1042/bst0310449. [DOI] [PubMed] [Google Scholar]
- 4.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122(14):1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 5.Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67(1):40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor RR, Shalit M. Neutrophil function in healthy elderly subjects. J Gerontol. 1990;45(2):M55–M60. doi: 10.1093/geronj/45.2.m55. [DOI] [PubMed] [Google Scholar]
- 7.Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44(22):1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- 8.Fulop T, Larbi A, Douziech N, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3(4):217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 9.Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J Cell Sci. 1999;112(12):1937–1945. doi: 10.1242/jcs.112.12.1937. [DOI] [PubMed] [Google Scholar]
- 10.Kaynar AM, Houghton AM, Lum EH, Pitt BR, Shapiro SD. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2008;39(1):53–60. doi: 10.1165/rcmb.2007-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89(3):359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens LR, Ellson C, Hawkins PT. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14(2):203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 13.Hannigan MO, Huang CK, Wu DQ. Roles of PI3K in neutrophil function. Curr Top Microbiol Immunol. 2004;282:165–175. doi: 10.1007/978-3-642-18805-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 15.Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J Leukoc Biol. 2005;78(1):210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- 17.British Thoracic Society. Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994;88(3):165–194. [PubMed] [Google Scholar]
- 18.Mikami M, Llewellyn-Jones CG, Bayley D, Hill SL, Stockley RA. The chemotactic activity of sputum from patients with bronchiectasis. Am J Respir Crit Care Med. 1998;157(3):723–728. doi: 10.1164/ajrccm.157.3.9606120. [DOI] [PubMed] [Google Scholar]
- 19.Muinonen-Martin AJ, Veltman DM, Kalna G, Insall RH. An improved chamber for direct visualisation of chemotaxis. PLoS ONE. 2010;5(12):e15309. doi: 10.1371/journal.pone.0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter RI, Mumford RA, Treonze KM, et al. The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax. 2011;66(8):686–691. doi: 10.1136/thx.2010.154690. [DOI] [PubMed] [Google Scholar]
- 21.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 22.Woolhouse IS, Bayley DL, Stockley RA. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax. 2002;57(8):667–671. doi: 10.1136/thorax.57.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapey E, Bayley D, Ahmad A, Newbold P, Snell N, Stockley RA. Inter-relationships between inflammatory markers in stable COPD patients with bronchitis; the intra and inter patient variability. Thorax. 2008;63(6):493–499. doi: 10.1136/thx.2007.086751. [DOI] [PubMed] [Google Scholar]
- 24.Fulop T, Franceschi C, Hirokawa K, Pawelec G. Handbook on Immunosenescence: Basic Understanding and Clinical Application. Rotterdam, The Netherlands: Springer Publishing; 2009. [Google Scholar]
- 25.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 26.Cevenini E, Caruso C, Candore G, et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16(6):609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 27.Ward JR, Heath PR, Catto JW, Whyte MKB, Milo M, Renshaw SA. Regulation of neutrophil senescence by microRNAs. PLoS ONE. 2011;6(1):e15810. doi: 10.1371/journal.pone.0015810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udupa KB, Lipschitz DA. Effect of donor and culture age on the function of neutrophils harvested from long-term bone marrow culture. Exp Hematol. 1987;15(3):212–216. [PubMed] [Google Scholar]
- 29.Egger G, Aigner R, Glasner A, Hofer HP, Mitterhammer H, Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Med. 2004;30(2):331–334. doi: 10.1007/s00134-003-2111-6. [DOI] [PubMed] [Google Scholar]
- 30.Liang SY, Mackowiak PA. Infections in the elderly. Clin Geriatr Med. 2007;23(2):441–456, viii. doi: 10.1016/j.cger.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol. 2013;190(4):1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168(6):602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell EJ, Campbell MA, Owen CA. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol. 2000;165(6):3366–3374. doi: 10.4049/jimmunol.165.6.3366. [DOI] [PubMed] [Google Scholar]
- 34.Owen CA, Campbell MA, Boukedes SS, Campbell EJ. Cytokines regulate membrane-bound leucocytes elastase on neutrophils, a novel mechanism for effector activity. Am J Physiol Lung Cell Mol Physiol. 1997;272(3):L385–L393. doi: 10.1152/ajplung.1997.272.3.L385. [DOI] [PubMed] [Google Scholar]
- 35.Clayton A, Evans RA, Pettit E, Hallett M, Williams JD, Steadman R. Cellular activation through the ligation of intercellular adhesion molecule-1. J Cell Sci. 1998;111(4):443–453. doi: 10.1242/jcs.111.4.443. [DOI] [PubMed] [Google Scholar]
- 36.Liou TG, Campbell EJ. Quantum proteolysis resulting from release of single granules by human neutrophils: a novel, nonoxidative mechanism of extracellular proteolytic activity. J Immunol. 1996;157(6):2624–2631. [PubMed] [Google Scholar]
- 37.Ferguson GJ, Milne L, Kulkarni S, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9(1):86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 38.Martin EL, Souza DG, Fagundes CT, et al. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Respir Crit Care Med. 2010;182(6):762–773. doi: 10.1164/rccm.201001-0088OC. [DOI] [PubMed] [Google Scholar]
- 39.Ong E, Gao X-P, Predescu D, Broman M, Malik AB. Role of phosphatidylinositol 3-kinase-gamma in mediating lung neutrophil sequestration and vascular injury induced by E. coli sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L1094–L1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- 40.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121(2):205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 41.Schymeinsky J, Then C, Sindrilaru A, et al. Syk-mediated translocation of PI3Kdelta to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS ONE. 2007;2(11):e1132. doi: 10.1371/journal.pone.0001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18(2):226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suire S, Condliffe AM, Ferguson GJ, et al. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol. 2006;8(11):1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- 44.Boulven I, Levasseur S, Marois S, Paré G, Rollet-Labelle E, Naccache PH. Class IA phosphatidylinositide 3-kinases, rather than p110 gamma, regulate formyl-methionyl-leucyl-phenylalanine-stimulated chemotaxis and superoxide production in differentiated neutrophil-like PLB-985 cells. J Immunol. 2006;176(12):7621–7627. doi: 10.4049/jimmunol.176.12.7621. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Subbaraman R, Sano H, et al. A surrogate method for assessment of beta(2)-integrin-dependent adhesion of human eosinophils to ICAM-1. J Immunol Methods. 2000;240(1-2):157–164. doi: 10.1016/s0022-1759(00)00192-7. [DOI] [PubMed] [Google Scholar]
- 46.Juss JK, Hayhoe RP, Owen CE, et al. Functional redundancy of class I phosphoinositide 3-kinase (PI3K) isoforms in signaling growth factor-mediated human neutrophil survival. PLoS ONE. 2012;7(9):e45933. doi: 10.1371/journal.pone.0045933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Condliffe AM, Davidson K, Anderson KE, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106(4):1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 48.Kamakura S, Nomura M, Hayase J, et al. The cell polarity protein mInsc regulates neutrophil chemotaxis via a noncanonical G protein signaling pathway. Dev Cell. 2013;26(3):292–302. doi: 10.1016/j.devcel.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Hayase J, Kamakura S, Iwakiri Y, et al. The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells. J Cell Biol. 2013;200(5):635–650. doi: 10.1083/jcb.201208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costantini C, Scrable H, Puglielli L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J. 2006;25(9):1997–2006. doi: 10.1038/sj.emboj.7601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platek A, Vassilev VS, de Diesbach P, Tyteca D, Mettlen M, Courtoy PJ. Constitutive diffuse activation of phosphoinositide 3-kinase at the plasma membrane by v-Src suppresses the chemotactic response to PDGF by abrogating the polarity of PDGF receptor signalling. Exp Cell Res. 2007;313(6):1090–1105. doi: 10.1016/j.yexcr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Nishio M, Watanabe KI, Sasaki J, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9(1):36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 53.Tang M, Iijima M, Kamimura Y, Chen L, Long Y, Devreotes P. Disruption of PKB signaling restores polarity to cells lacking tumor suppressor PTEN. Mol Biol Cell. 2011;22(4):437–447. doi: 10.1091/mbc.E10-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herman SEM, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pomel V, Klicic J, Covini D, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49(13):3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 56.Camps M, Rückle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11(9):936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.