Abstract
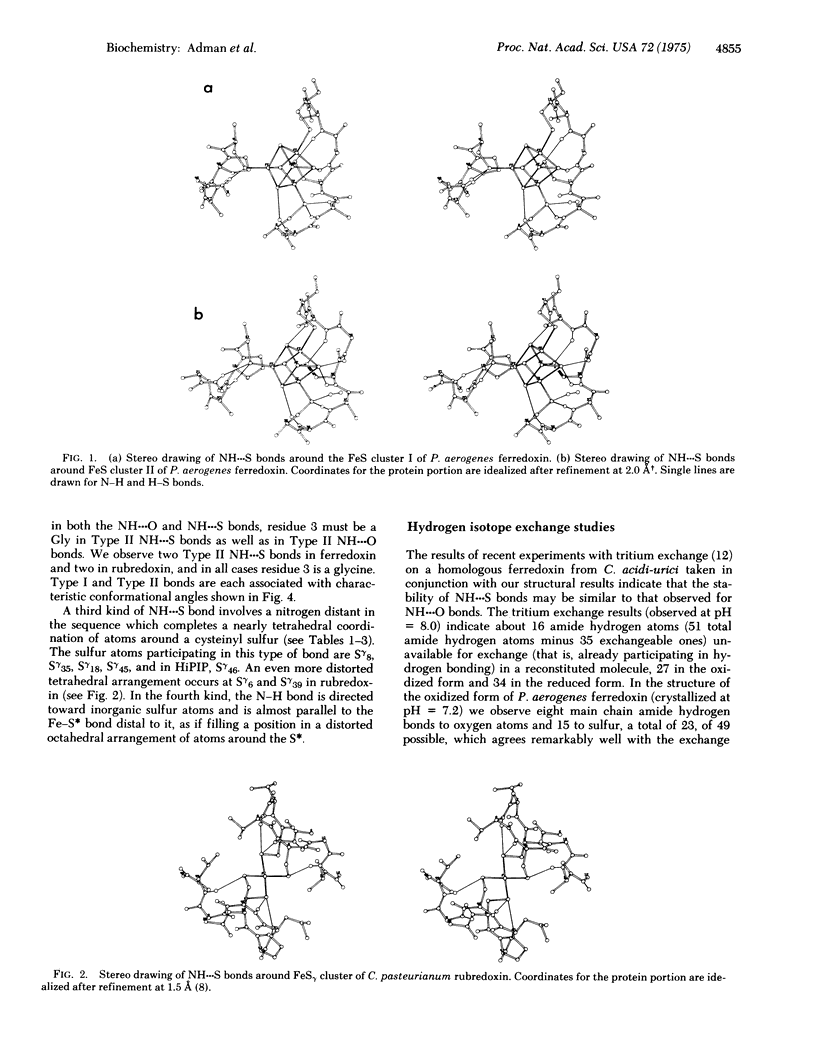
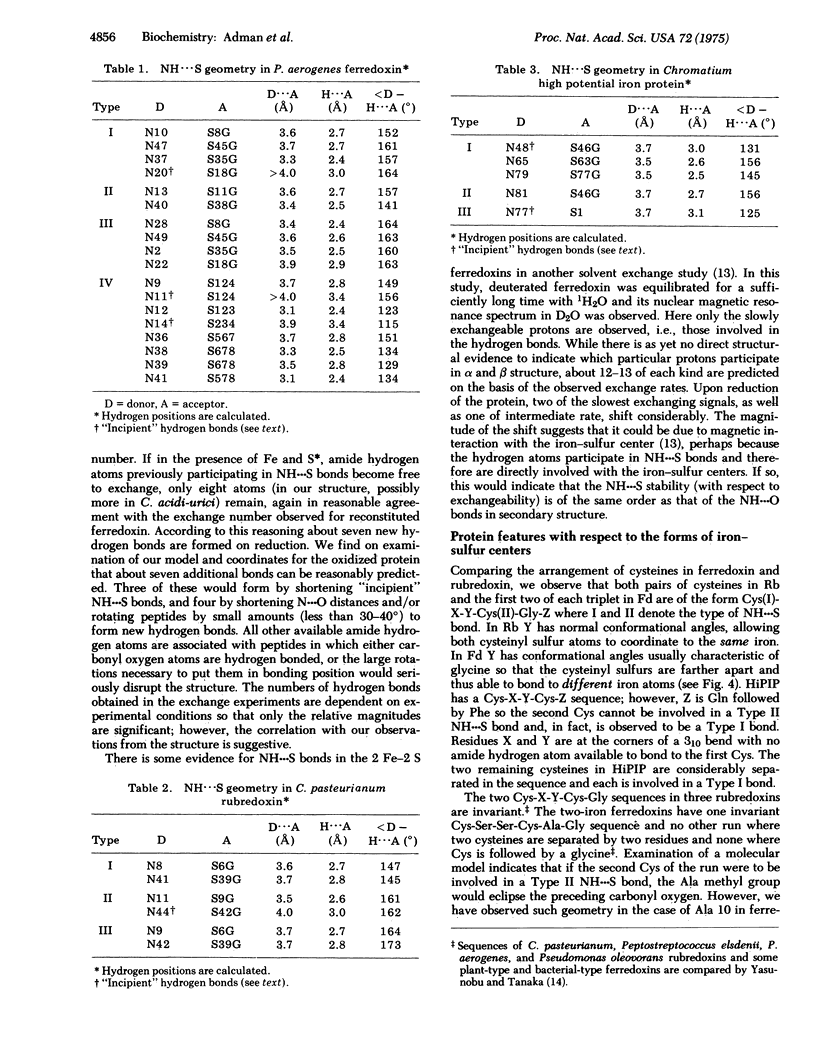
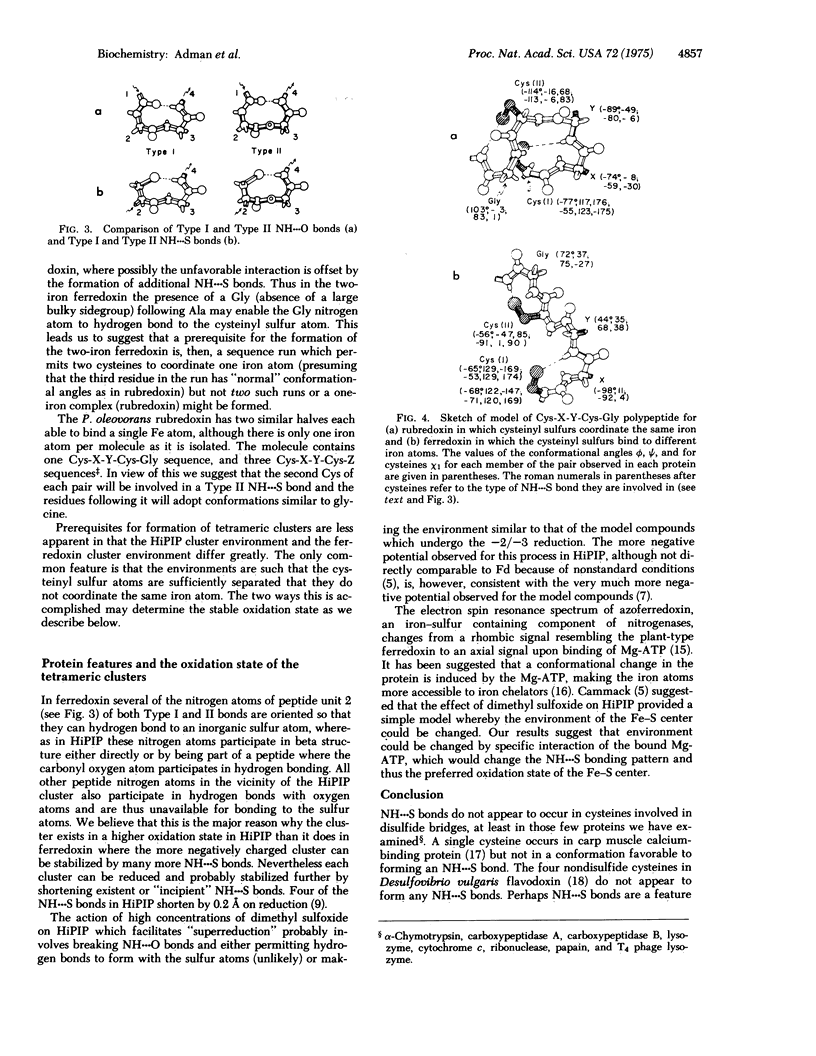
Results from refinement of the crystal structures of P. aerogenes ferredoxin and C. pasteurianum rubredoxin determined by x-ray diffraction show that there are 15-18 NH---S bonds in the former and six in the latter with lengths in the range 3.1-3.9 A. Earlier tritium exchange experiments are consistent with the presence of these hydrogen bonds in the ferredoxin structure and show that more peptide hydrogen atoms are available for exchange in apoferredoxin than in intact ferredoxin. Four types of NH---S bonds are observed and two of these are geometrically similar to the two types of 3(10) NH---O bonds. The existence of more NH---S bonds in ferredoxin than in high potential iron protein suggests why the -2 form of the Fe4S4 cluster is preferred in ferredoxin over the -1 form found in high potential iron protein. From comparison of Cys-X-Y-Cys sequences in rubredoxin, ferredoxin, and high potential iron protein we suggest that two Cys-X-Y-Cys-Z sequences, where Z may have conformation angles similar to glycine, are required to make a one-iron cluster, no more than one Cys-X-Y-Cys-Z-Gly sequence is required to form a Fe2S2 ferredoxin, and a Cys-X-Y-Cys-Gly sequence where Y has a conformation such that the cysteines bond to different iron atoms is necessary to form the tetrameric cluster.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Cammack R. "Super-reduction" of chromatium high-potential iron-sulphur protein in the presence of dimethyl sulphoxide. Biochem Biophys Res Commun. 1973 Sep 18;54(2):548–554. doi: 10.1016/0006-291x(73)91457-5. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A. Comparison of oxidation-reduction site geometries in oxidized and reduced Chromatium high potential iron protein and oxidized Peptococcus aerogenes ferredoxin. J Biol Chem. 1974 Oct 10;249(19):6339–6346. [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Nguyen-Huu-Xuong, Alden R. A., Bartsch R. G. Two-Angstrom crystal structure of oxidized Chromatium high potential iron protein. J Biol Chem. 1974 Jul 10;249(13):4212–4225. [PubMed] [Google Scholar]
- Crespi H. L., Kostka A. G., Smith U. H. Proton Magnetic resonance observations of hydrogen exchange rates and secondary structure in algal ferredoxin. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1407–1414. doi: 10.1016/s0006-291x(74)80440-7. [DOI] [PubMed] [Google Scholar]
- DePamphilis B. V., Averill B. A., Herskovitz T., Que L., Jr, Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. VI. Spectral and redox characteristics of the tetranuclear clusters (Fe4S4(SR)4).2-. J Am Chem Soc. 1974 Jun 26;96(13):4159–4167. doi: 10.1021/ja00820a017. [DOI] [PubMed] [Google Scholar]
- Donohue J. On N-H--S hydrogen bonds. J Mol Biol. 1969 Oct 28;45(2):231–235. doi: 10.1016/0022-2836(69)90102-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. Immunological properties and conformational differences detected by tritium-hydrogen exchange of clostridial ferredoxins and apoferredoxins. J Biol Chem. 1970 Oct 10;245(19):4995–5000. [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hamilton W. D., Jones T. L., Tso M. Y., Burris R. H., Shah V. K., Brill W. J. Electron paramagnetic resonance of nitrogenase and nitrogenase components from Clostridium pasteurianum W5 and Azotobacter vinelandii OP. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3142–3145. doi: 10.1073/pnas.69.11.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney W. V., Bearden A. J., Rabinowitz J. C. The electron paramagnetic resonance of oxidized clostridial ferredoxins. Biochem Biophys Res Commun. 1974 Jul 10;59(1):188–194. doi: 10.1016/s0006-291x(74)80192-0. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Walker G. A., Mortenson L. E. An effect of magnesium adenosine 5'-triphosphate on the structure of azoferredoxin from Clostridium pasteurianum. Biochem Biophys Res Commun. 1973 Aug 6;53(3):904–909. doi: 10.1016/0006-291x(73)90177-0. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Jensen L. H., Legall J., Dubourdieu M. Structure of the oxidized form of a flavodoxin at 2.5-Angstrom resolution: resolution of the phase ambiguity by anomalous scattering. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3185–3188. doi: 10.1073/pnas.69.11.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]