Humidity is a strong predictor of Penicillium marneffei hospital admissions in Ho Chi Minh City, Vietnam, suggesting that humidity may contribute to exposure of susceptible individuals. We estimate the P. marneffei incubation period to be 1 week.
Keywords: Penicillium marneffei, penicilliosis, seasonality, humidity, HIV/AIDS
Abstract
Background. Penicillium marneffei is an emerging dimorphic mycosis endemic in Southeast Asia, and a leading cause of mortality among human immunodeficiency virus (HIV)–infected people in the region. Factors governing the seasonal incidence of P. marneffei infection are unknown, and may yield critical insights into possible reservoirs or modes of acquisition.
Methods. This study included HIV-infected patients presenting with P. marneffei (n = 719) and Cryptococcus neoformans (n = 1598) infection to the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam, from 2004 to 2010, and temperature, humidity, wind, precipitation, and HIV-related admissions data for the corresponding period. We used multivariate regression modeling to identify factors associated with P. marneffei and C. neoformans admissions. We estimated the P. marneffei incubation period by considering profile likelihoods for different exposure-to-admission delays.
Results. We found that P. marneffei admissions were strongly associated with humidity (P < .001), and that precipitation, temperature, and wind did not add explanatory power. Cryptococcus neoformans admissions were not seasonal, and P. marneffei admissions were more common relative to C. neoformans admissions during months of high (≥85%) humidity (odds ratio, 1.49; 95% confidence interval [CI], 1.10–2.01). Maximum likelihood estimation suggested a P. marneffei incubation period of 1 week (95% CI, 0–3 weeks).
Conclusions. Our findings suggest that humidity is the most important environmental predictor of P. marneffei admissions, and may drive exposure by facilitating fungal growth or spore release in the environment. In addition, it appears that a high proportion of penicilliosis patients present to the hospital with primary disseminated infection within 3 weeks of exposure.
Penicillium marneffei is an emerging dimorphic mycosis endemic in South and Southeast Asia, and a leading cause of mortality among human immunodeficiency virus (HIV)–infected persons in the region [1–4]. Penicillium marneffei ranks as the third most common opportunistic infection in the region, exceeded in prevalence only by tuberculosis and cryptococcal meningitis in Thailand and Vietnam and Pneumocystis jiroveci pneumonia (PCP) and tuberculosis in Hong Kong [1–4]. Critical aspects of the epidemiology of P. marneffei infection have yet to be elucidated, including its environmental reservoir, mode of acquisition, and incubation period. It has been observed that P. marneffei incidence is closely correlated with HIV type 1 prevalence interannually, and with rainy months intra-annually; however, specific seasonal drivers such as temperature, humidity, precipitation, and wind speed have not been studied [5]. In addition, seasonality has only been examined using data averaged or aggregated at the seasonal or annual level, negating the opportunity to discern among seasonal drivers that may vary within and across years.
Studies in Vietnam and Thailand comparing P. marneffei incidence with that of Cryptococcus neoformans found that P. marneffei infections varied seasonally with more infections during the rainy season, whereas C. neoformans infections were nonseasonal [4, 5]. One case-control study identified agricultural exposure to soil during the rainy season as an important risk factor for P. marneffei infection, but not exposure to the soil-burrowing bamboo rat (the only known nonhuman host of P. marneffei), suggesting that humans and rats may acquire the infection from a common soil reservoir [6]. However, as P. marneffei cases occur both in rural and urban settings, it is unclear whether infection ensues from exposure to an immediate soil reservoir, windblown spores, construction-related activities (especially in urban settings), or a combination of these factors. Analysis of specific seasonal drivers that influence such factors can provide clues to these questions. Our objective was therefore to test various hypotheses for the known seasonality of P. marneffei by examining the association between P. marneffei hospital admissions and a suite of environmental variables, including precipitation, humidity, wind speed, and temperature. We examined P. marneffei and C. neoformans hospital admissions to the Hospital for Tropical Disease (HTD) in Ho Chi Minh City, Vietnam, from 2004 to 2010 in relation to high-resolution weather and HIV admissions data from Ho Chi Minh City for the corresponding period. Using multivariate regression modeling, we sought to identify factors that could account for the observed seasonality of P. marneffei infection. We also generated a conditional estimate of the P. marneffei incubation period, which has been inaccessible to direct study owing to the paucity of serological data and lack of knowledge of the source of exposure, by incorporating different exposure-to-admission delays in our models and comparing the goodness-of-fit of these models.
PATIENTS AND METHODS
The present study included all patients admitted with P. marneffei infection, C. neoformans infection, and HIV/AIDS-related illness to the HTD in Ho Chi Minh City from January 2004 to June 2010. The HTD is the largest infectious disease referral hospital in Vietnam, caring for >5000 HIV-infected patients annually. Penicillium marneffei and C. neoformans cases were identified from hospital microbiology records and were defined as a compatible illness in which P. marneffei or C. neoformans was isolated from blood, skin scrapings, cerebrospinal fluid, bone marrow, lymph node, and/or other bodily fluids. Standard culture techniques were used and have been described elsewhere [7], as were data collection details [4]. Daily weather data from the Ho Chi Minh City weather station (latitude = 10.81, longitude = 106.66) for the 2004–2010 period were extracted from the website www.TuTiempo.net, which compiles global climactic data and has been used in other epidemiological studies [8, 9]. Weather variables included in this analysis were minimum, maximum, and mean temperature (°C), precipitation (mm), mean humidity (%), visibility (km), wind speed (km/hour), and maximum sustained wind speed (km/hour). All data were double-entered into Microsoft Excel 2008. The study was approved by the Scientific and Ethical Committee of the HTD.
Penicillium marneffei and C. neoformans admissions were aggregated by week and by month. Units of aggregation were selected to allow sufficient resolution to generate a conditional estimate of the incubation period (week), as well as sufficient sample sizes to detect an annual trend (month). Weather variables were averaged (temperature, humidity, visibility, wind speed) or summed (precipitation) over corresponding units of time. HIV admission numbers were aggregated by month. We performed univariate and multivariate negative binomial regressions (to account for overdispersion of count data), with weather variables as the independent variables, by week and by month to identify factors associated with P. marneffei and C. neoformans admissions. Factors found to be significantly associated with P. marneffei admissions in univariate analyses were included in multivariate regression models. We also stratified P. marneffei and C. neoformans cases by low (<70%), intermediate (70%–84%), and high (≥85%) monthly humidity (cutoffs were chosen to allow approximately equal case counts in the low and high categories) to evaluate the odds of P. marneffei relative to C. neoformans admissions at different levels of humidity.
We estimated the dates of penicilliosis disease onset by subtracting the patient-reported duration of symptomatic illness from the date of admission. We estimated the date of exposure by subtracting a range of hypothetical incubation periods (0–7 weeks) from the date of symptom onset, and from the date of hospital admission. Negative binomial regression models were fitted to examine hypothetical dates of P. marneffei exposure. By examining the likelihood scores corresponding to models fitted with different assumed incubation periods, we generated a conditional estimate of the P. marneffei incubation period (conditional on the assumption that pathogen exposure is directly linked to climate variables). A 95% confidence interval was calculated by standard methods of likelihood profiling, and included all values that yielded log-likelihood scores within 1.92 units of the maximum score [10]. All statistical analyses were conducted using the statistical software R, version 2.12.1 [11]. Regressions were performed using the glm.nb function in the MASS package, with a log link function [12]. We performed a likelihood-ratio test to determine that the negative binomial regression model was required instead of a standard Poisson model, due to overdispersion in the count data. We examined residuals and found that errors were not skewed across seasons.
RESULTS
This study included 719 HIV-infected patients admitted with P. marneffei, and 1598 HIV-infected patients admitted with C. neoformans, between 2004 and 2010. The clinical features of the P. marneffei cohort were consistent with disseminated infection (fever [82%], skin lesions [71%], anorexia [62%], hepatosplenomegaly [56%], and reticulonodular [50%] and interstitial [39%] findings on chest radiograph) and have been described in detail elsewhere [4]. The median CD4 cell count (n = 62) at admission was 7 cells/µL (interquartile range [IQR], 4–24 cells/µL) [4]. Penicillium marneffei admissions peaked annually during the rainy season (May–November) and decreased during the dry season (December–April). Cryptococcus neoformans admissions did not show a seasonal trend.
Associations between P. marneffei and C. neoformans admissions and environmental variables are reported in Table 1. Among all environmental variables examined, P. marneffei admissions were most closely associated with humidity (P = .0004) and precipitation (P = .001) in univariate regression analyses. The association between P. marneffei admissions and humidity was significant when examined at the weekly (P = .0004) and monthly levels (P = .002). The association between P. marneffei admissions and precipitation was also significant at the weekly (P = .001) and monthly levels (P = .004). Other weather variables, including minimum, maximum, and mean temperature, visibility, wind speed, and maximum sustained wind speed were not significantly associated with P. marneffei admissions. Cryptococcus neoformans admissions were significantly associated with low maximum wind speeds (P = .026), but not with other environmental variables.
Table 1.
Univariate and Multivariate Associations Between Monthly Penicillium marneffei Admissions, Cryptococcus neoformans Admissions, HIV/AIDS-Related Admissions, Total Admissions, and Environmental Variables, Ho Chi Minh City, Vietnam, 2004–2010
| Variable |
Penicillium marneffei |
Cryptococcus neoformans | |||
|---|---|---|---|---|---|
| Univariate P Value | β (SE) | Multivariate P Value | β (SE) | Univariate P Value | |
| Mean humidity, % | .0015 | .023 (0.0072) | .00082* | .023 (0.0069) | .87 |
| Precipitation, mm | .0040 | .0012 (0.00041) | .30** | .00055 (0.00053) | .46 |
| Min temperature, °C | .054 | .077 (0.040) | .081 | ||
| Max temperature, °C | .18 | .43 | |||
| Mean temperature, °C | .86 | .28 | |||
| Mean visibility, km | .25 | .92 | |||
| Mean wind speed, km/h | .73 | .19 | |||
| Max wind speed, km/h | .57 | .026 | |||
| HIV admissions | .0023 | .0015 (0.00040) | .00014** | .0017 (0.00052) | .071 |
| Total admissions | .011 | .00021 (0.000063) | .42** | .000084 (0.00010) | .27 |
Statistically significant associations are shown in boldface.
Abbreviations: HIV, human immunodeficiency virus; SE, standard error.
*P value generated by simultaneous examination of humidity and HIV admissions with regard to P. marneffei admission.
**P values generated by simultaneous examination of the variable in question and humidity with regard to P. marneffei admission.
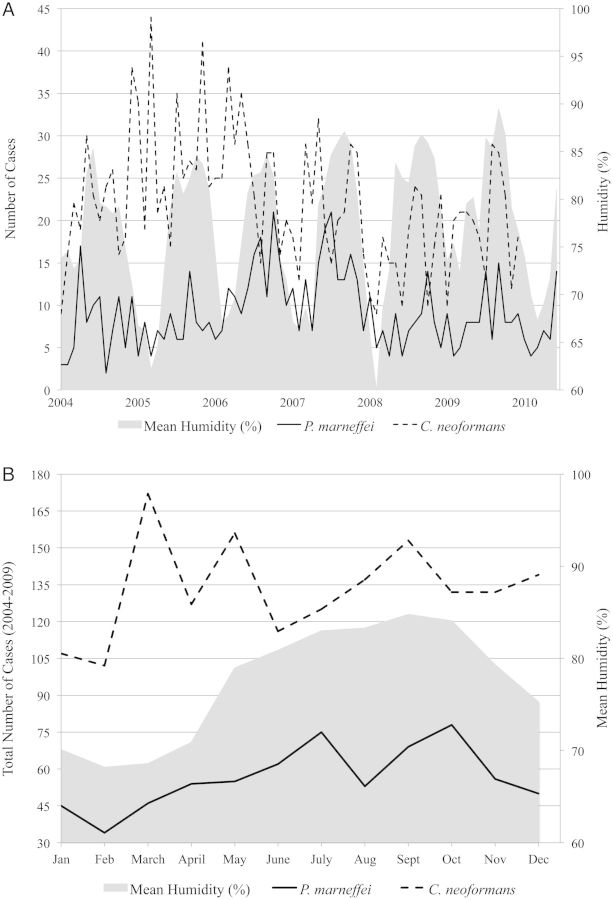
Upon simultaneous examination of humidity and precipitation in a multivariate regression model, only humidity remained significantly associated with P. marneffei admissions (P = .01). When humidity and HIV admissions were examined simultaneously, both remained significantly associated with P. marneffei admissions (P = .0008 and P = .0001, respectively; Table 1). When humidity and total admissions to HTD were examined simultaneously, only humidity was significantly associated with P. marneffei admissions (P = .036). The distribution of total P. marneffei and C. neoformans cases and mean humidity by month, during 2004–2010, is illustrated in Figure 1. When P. marneffei and C. neoformans admissions were stratified by monthly humidity (Table 2), we found that P. marneffei admissions were more common relative to C. neoformans admissions during months of intermediate (70%–84%) humidity (odds ratio [OR], 1.22; 95% confidence interval [CI], .96–1.55) and high (≥85%) humidity (OR, 1.49; 95% CI, 1.10–2.01).
Figure 1.
A, Monthly Penicillium marneffei and Cryptococcus neoformans hospital admissions and mean humidity (%), Ho Chi Minh City, Vietnam, 2004–2010. B, Distribution of total P. marneffei and C. neoformans cases and mean humidity by month, Ho Chi Minh City, Vietnam, 2004–2009.
Table 2.
Odds of Penicillium marneffei Admission Relative to Cryptococcus neoformans Admission by Low (<70%), Intermediate (70%–84%), and High (≥85%) Monthly Humidity
| Monthly Humidity | No. of Patients (2004–2009) |
Odds Ratio (95% CI) | |
|---|---|---|---|
| P. marneffei | C. neoformans | ||
| <70% | 115 | 330 | Reference (1.0) |
| 70–84% | 436 | 1025 | 1.22 (.96–1.55) |
| ≥85% | 126 | 243 | 1.49 (1.10–2.01) |
Abbreviation: CI, confidence interval.
The median duration of patient-reported illness in the penicilliosis cohort was 15 days (IQR, 7–30 days), and was not correlated with humidity (r2 = 0.09). Severity of disease, as measured by the risk of death upon hospital admission (mean = 20%), was also not correlated with humidity (r2 = 0.16). The CD4 cell counts at admission for patients presenting during the rainy season (May–November) and dry season (December–April) were similar (median, 6 and 7 cells/µL, respectively; P = .18, 2-tailed Student t test). We approached estimation of the date of penicilliosis disease onset by subtracting the patient-reported days of illness from the date of hospital admission for each patient. The association between the estimated dates of penicilliosis onset and monthly humidity was statistically significant (P = .01); however, the correlation between the calculated date of penicilliosis onset and humidity was lower (r = 0.28) than that between the date of admission and humidity (r = 0.39), leading us to hypothesize that the patient-reported duration of illness may have included illness time attributable to other HIV-related infections.
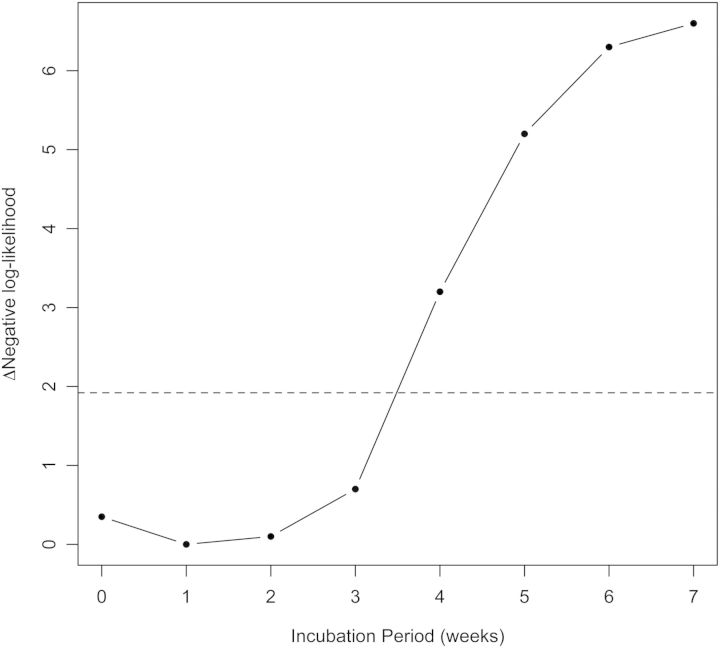
Subtraction of hypothetical incubation periods from the date of admission was a more successful approach to estimating the date of exposure. We incorporated hypothetical exposure-to-admission delays in our regression model linking P. marneffei cases to humidity, and compared the goodness-of-fit of these models for delays from 0 to 7 weeks. In this way we obtained an estimate of the P. marneffei incubation period, conditional on the validity of the association with humidity. The likelihood scores for the model with different values of the incubation period are shown in Figure 2, and reveal a maximum-likelihood estimate of the P. marneffei incubation period of 1 week (95% CI, 0–3 weeks). The association between P. marneffei admissions and precipitation was not significant at any incubation period.
Figure 2.
Maximum likelihood estimation of the Penicillium marneffei incubation period. The plot shows negative log-likelihood values obtained from negative binomial regression of weekly admission data and mean humidity, incorporating different exposure-to-admission delays (corresponding to incubation periods of 0–7 weeks). The maximum likelihood value (minimum negative log-likelihood) is 1-week incubation (95% confidence interval [CI], 0–3 weeks), suggesting an incubation period of ≤3 weeks. The dashed line corresponds to a difference of 1.92 log-likelihood units from the optimum value; points beneath this line fall within the 95% CI.
DISCUSSION
Several studies have reported the seasonality of penicilliosis infection, but it is unclear what factors are involved in maintaining this seasonal pattern [4–6]. In this study, we considered a suite of environmental variables, each aligned with hypothesized mechanisms of transmission, to see which could best account for our observed admissions data. We found that humidity, not rainfall, was the strongest predictor of P. marneffei hospital admissions. To our knowledge, our study is the first to perform a formal statistical analysis of P. marneffei seasonality with unaggregated data across seasons and years, and to report associations between infection and environmental factors at high resolution. Based on our findings, we suspect that humidity may drive P. marneffei incidence, perhaps by promoting expansion of the environmental reservoir or facilitating fungal growth or spore release in the environment. Our findings are consistent with proposed mechanisms of P. marneffei spillover from the environment into susceptible human populations (ie, inhalation of infectious spores or hyphal fragments from a soil reservoir) [13, 14], and with established risk factors for infection [5, 6]. As a dimorphic fungus, P. marneffei exists as a mold in its as-yet unidentified environmental reservoir. If humidity does in fact facilitate fungal growth, this may indicate that the mold is growing on air-exposed plant and soil surfaces, whereas rainfall would be expected to influence fungal growth in deeper soil and animal burrows. Therefore, our findings lead us to hypothesize that humidity-mediated fungal growth and/or spore release from air-exposed plant and soil surfaces may serve as a crucial step in the exposure and infection of immunocompromised human populations in endemic areas.
Prevailing opinion suggests that dimorphic yeasts, including those common in the United States (Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis), present clinically as either primary pulmonary infections or as disseminated infections arising from reactivation of latent infection upon significant impairment of cellular immunity [15–17]. The strong association of disseminated P. marneffei infections with seasonal factors in our study, primarily humidity, indicates that disseminated disease can result from primary infections among immunosuppressed patients shortly after exposure to the fungus in the environment. This hypothesis is predicated on the assumption that immunosuppression is not seasonal, as we did not observe a difference in CD4 count at admission during the rainy and dry seasons, and there is currently no evidence to support a link between immunosuppression and season. Unfortunately, longitudinal CD4 data for patients with P. marneffei were not available as most patients presented initially with P. marneffei. Such data could inform whether infection was driven primarily by environmental exposure or by a decline in host immune function and can guide prevention strategies. The strong seasonal signal we observed suggests that primary disseminated infection occurs soon after exposure; however, a background level of infection was consistently observed in nonhumid months as well, which indicates that exposure continues to occur during nonhumid months (albeit to a lesser degree), and/or that these cases are the result of reactivation of latent infection upon severe AIDS-related immunosuppression. Further research is necessary to determine the relative contribution of these various processes to the observed levels of disseminated infection at different times of the year. The high level of primary disseminated infection, as suggested by our strong seasonal signal, may suggest that P. marneffei pathophysiology differs somewhat from that of other dimorphic fungi, perhaps because of some fundamental difference in pathogen, transmission, or host response. For instance, P. marneffei may exhibit an enhanced ability to cause primary disseminated infection, relative to other dimorphic fungi, due to a greater inoculum or increased virulence upon initial infection. Alternatively, the high levels of primary disseminated infection we observed may simply reflect some unique characteristics of the epidemic in Vietnam (immune status of the population at risk, demographics, geography, setting, environmental factors). Certainly, the fact that C. neoformans, a pathogen with a largely overlapping suspected epidemiology and target population to P. marneffei, shows no seasonal pattern suggests that P. marneffei may exhibit a unique pathophysiology or route of acquisition.
To our knowledge, our study is the first to estimate the incubation period of P. marneffei infection. It has thus far been impossible to generate an estimate given the paucity of serological data, and so we used an unconventional approach of conditioning on the association with humidity to generate a first estimate, which we plan to test in subsequent work. Our maximum likelihood estimates suggest an incubation period of between 0 and 3 weeks. If we consider the possibility of delay in patient presentation to the hospital following symptom onset, as well as a possible lag for fungal growth or spore release in the reservoir, we can reasonably presume that the true incubation period is shorter, with an estimated upper bound of 3 weeks. Our finding that the estimated date of illness onset (based on patient-reported data) was less associated with humidity than the date of admission was surprising, as one would expect the onset of symptoms to more closely approximate the time of exposure than the date of admission. We suspect that this finding may have been biased by the inclusion of illness time not directly attributable to P. marneffei infection. Most symptoms of penicilliosis, with the exception of umbilicated skin lesions, which are present in approximately 70% of patients, are nonspecific. In addition, other opportunistic infections were documented in approximately 20% of the cohort (mainly tuberculosis), and may have accounted for some of the reported days of illness.
An alternative but perhaps less plausible explanation for the association between humidity and P. marneffei admissions is that, rather than contributing to exposure to P. marneffei, humidity may trigger the development of clinical manifestations (eg, skin lesions) in latently infected individuals. This seems less probable, however, as skin lesions in penicilliosis typically develop on relatively dry surfaces of the body (face, neck, chest, and back) and together with other manifestations of disseminated systemic infection that humidity would be unlikely to affect. Further investigation is planned to examine possible predictors of skin lesion development in infected patients. Assuming that humidity is indeed an indicator of exposure rather than onset, we can conclude that our approach of subtracting hypothetical incubation periods from the date of hospital admission was more accurate than subtracting the reported duration of illness in approximating the time of exposure, and that the estimated incubation period, given the limitations of this method, is ≤3 weeks. Our finding that C. neoformans admissions were not associated with most environmental variables was expected. We suspect that the association with low maximum wind speeds may be an artefactual result driven by multiple comparisons in the univariate analyses, as the P value is above the Bonferroni-corrected threshold for significance of .005 for 10 comparisons.
Our study is limited in that it did not include a metric of agricultural activity or construction work in Ho Chi Minh City, both of which could hypothetically contribute to exposure to P. marneffei and confound our results. Our study is strengthened by the inclusion of other potential confounders, such as HIV admissions, total admissions, and other weather variables. In addition, the large sample size and detailed admission and weather data over a 6-year period enabled precise quantification of associations between weather variables and P. marneffei admissions, as well as maximum likelihood estimation of the incubation period. Our findings should help guide efforts to understand the environmental reservoir and transmission of P. marneffei, which may inform the design and timing of much-needed prevention strategies for people living with HIV/AIDS in endemic regions of Asia. Earlier HIV diagnosis and placement on combination antiretroviral therapy, and seasonal antifungal drug prophylaxis (for patients with low CD4 counts in the early stages of combination antiretroviral therapy), may prevent penicilliosis in patients presenting very late [18–21]. Further investigation is planned to determine the causal and temporal relationships among humidity, exposure, immunosuppression, symptom onset, and ultimate presentation to the hospital, with the goal of broadening our understanding of the transmission and pathophysiology of this emerging dimorphic yeast.
Notes
Acknowledgments. We thank the Microbiology Department and the Scientific and Planning Office of the Hospital for Tropical Diseases for providing access to the hospital records. We thank members of the Lloyd-Smith lab, especially Ruian Ke and Claude Loverdo, for helpful discussion and comments.
Financial support. P. L. B. is supported by the UCLA-Caltech Medical Scientist Training Program and the Paul and Daisy Soros Fellowships for New Americans. T. L. is supported by the Fogarty International Clinical Research Fellowship, Hawaii Center for AIDS, and Wellcome Trust Major Overseas Program. J. O. L.-S. is supported by the De Logi Chair in Biological Sciences, and the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security and the Fogarty International Center, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated P. marneffei infection in southeast Asia. Lancet. 1994;344:110–3. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 2.Wong KH, Lee SS. Comparing the first and second hundred AIDS cases in Hong Kong. Singapore Med J. 1998;39:236–40. [PubMed] [Google Scholar]
- 3.Duong TA. Infection due to P. marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–30. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2011;52:945–52. doi: 10.1093/cid/cir028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson KE. Seasonal variation of disseminated penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis. 1996;173:1490–3. doi: 10.1093/infdis/173.6.1490. [DOI] [PubMed] [Google Scholar]
- 6.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case- control study of risk factors for P. marneffei infection in human immunodeficiency virus- infected patients in northern Thailand. Clin Infect Dis. 1997;24:1080–6. doi: 10.1086/513649. [DOI] [PubMed] [Google Scholar]
- 7.Viviani MA, Tortorano AM. In: P marneffei. 9th ed. Ajello L, Hay RJ, editors. Vol 4. London: Edward Arnold; 1998. pp. 409–19. Medical mycology Topley and Wilson's microbiology and microbial infections. [Google Scholar]
- 8.TuTiempo.net. Tutiempo Network. Available at: http://www.tutiempo.net/en/Climate/Ho_Chi_Minh/489000.htm . Accessed 1 January 2011. [Google Scholar]
- 9.Luque Fernandez MA, Bauernfeind A, Jimenez JD, Gil CL, El Omeiri N, Guibert DH. Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003- 2006: analysis of a time series. Trans R Soc Trop Med Hyg. 2009;103:137–43. doi: 10.1016/j.trstmh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Bolker B. Ecological models and data in R. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 11.R Development Core Team. R: a language and environment for statistical computing (version 2.12.1) 2010 http://www.R-project.org. Available at: Accessed 1 July 2011. [Google Scholar]
- 12.Venables WN, Ripley BD. Modern applied statistics with S. ed. New York, NY: Springer; 2002. 4th ed. ISBN 0-387-95457-0. [Google Scholar]
- 13.Nelson KE, Supparatpinyo K, Vanittanakom N. Penicilliosis. Essentials of Clinical Mycology. :399–414. In: Kauffman CA, Papas PG, Sobel J, Dismukes W, eds. New York, NY: Springer; 2011. [Google Scholar]
- 14.Vanittanakom N, Cooper CR, Fiser MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman CA. Histoplasmosis. Essentials of Clinical Mycology. :321–36. In: Kauffman CA, Papas PG, Sobel J, Dismukes W, eds. New York, NY: Springer; 2011. [Google Scholar]
- 16.Light BR, Kralt D, Embil JM, et al. Seasonal variations in the clinical presentation of pulmonary and extrapulmonary blastomycosis. Med Mycol. 2008;46:835–41. doi: 10.1080/13693780802132763. [DOI] [PubMed] [Google Scholar]
- 17.Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45:26–30. doi: 10.1128/JCM.02230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China national free antiretroviral treatment program. Ann Intern Med. 2009;151:241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 20.Chasombat S, McConnell MS, Siangphoe MS, et al. National expansion of antiretroviral treatment in Thailand, 2000–2007: program scale-up and patient outcomes. J Acquir Immune Defic Syndr. 2009;50:506–12. doi: 10.1097/QAI.0b013e3181967602. [DOI] [PubMed] [Google Scholar]
- 21.Vermund SH. Testing and linkage of patients to early care. AIDS. 2011;25:1547–8. doi: 10.1097/QAD.0b013e32834940b3. [DOI] [PubMed] [Google Scholar]