Abstract
Aims
Various pathological changes lead to the development of heart failure (HF). HDL is dysfunctional in both acute coronary syndrome, as measured by the HDL inflammatory index (HII) assay, and stable coronary disease, as measured by cholesterol efflux capacity. We therefore hypothesized that these functions of HDL are also impaired in subjects with ischaemic cardiomyopathy.
Methods and results
A case–control study was performed on subjects in the University of Pennsylvania Catheterization Study (PennCath) cohort of patients with angina. Cases had EF <50% and angiographic CAD (≥70% stenosis of any vessel; n = 23); controls included those with EF ≥55% and no CAD (n = 46). Serum from subjects was apolipoprotein-B depleted to isolate an HDL fraction. To measure HDL anti-oxidative capacity, the HDL fraction was incubated with LDL and a reporter lipid that fluoresces when oxidized. To measure cholesterol efflux capacity, the HDL fraction was also incubated with macrophages and tritium-labelled cholesterol. Mean HII was higher and efflux capacity lower in subjects with ischaemic cardiomyopathy (HII 0.26 vs. –0.028; efflux 0.80 vs. 0.92; P < 0.05). In a multivariable logistic regression model, both high HII and low efflux capacity were significant risk factors for HF [HII odds ratio (OR) 2.8, 95% confidence interval (CI) 2.0–3.9, P = 0.002; efflux OR 2.1, 95% CI 1.5–3.0, P = 0.03]. These effects persisted after adjustment for covariates and traditional risk factors for HF.
Conclusion
Subjects with reduced EF from ischaemia have lower HDL concentration and also impaired HDL function. HDL is a versatile lipoprotein particle with various anti-inflammatory and vasoprotective functions, whose impairment may contribute to ischaemic heart failure.
Keywords: Heart failure, Coronary artery disease, HDL cholesterol
Introduction
HDL is a versatile lipoprotein particle that has been studied in various populations based on its many putative functions, including anti-oxidative capacity in countering inflammation and reverse cholesterol transport in countering atherogenesis. The HDL level correlates with incident congestive heart failure (HF) in patients with ischaemia,1,2 and predicts HF exacerbations and adverse cardiovascular events in patients with and without ischaemia.1–6 However, few studies have confirmed that HDL function influences this correlation.
HDL anti-oxidative capacity may be important in this population, as HF patients exhibit reduced capacity to counter inflammation and oxidative stress, with higher basal levels of tumour necrosis factor-α (TNF-α)7−9 and lipopolysaccharide (LPS).10 However, it remains unclear how HDL might attenuate such inflammatory mediators. In one cohort, the HDL-associated protein paraoxonase-1 (PON-1) demonstrated reduced serum arylesterase activity in HF patients,11 and a genetic polymorphism study previously showed that serum arylesterase activity may correlate with cardiac risk.12 Additionally, HDL putatively prevents oxidation of LDL in lipid core plaque, which can be measured in vitro using the HDL inflammatory index (HII) assay.13 We have demonstrated that the HII is impaired in patients with acute coronary syndrome (ACS).14
However, HDL also putatively participates in the reverse cholesterol transport pathway, transferring cholesterol from very low-density lipoprotein (VLDL) and LDL to the liver for eventual excretion. This HDL cholesterol efflux capacity is reduced in patients with stable CAD.15 These two assays of HDL function provide independent and orthogonal information, as cholesterol efflux capacity is not reduced in patients with acute ischaemia, nor is the HII increased in stable CAD patients. Given these roles of HDL as both an anti-inflammatory particle and a promoter of cholesterol efflux, we hypothesized that patients with reduced EF and chronic ischaemia have impaired HDL anti-oxidative capacity and reduced cholesterol efflux capacity.
Methods
Patients and study design
The University of Pennsylvania Catheterization Study is a population-based cohort study examining associations between biochemical and genetic markers in predominantly white male patients undergoing cardiac catheterization for evaluation of angina. The study is institutional review board approved (Hospital of the University of Pennsylvania) and complies with the Declaration of Helsinki, and all subjects provided written consent. We selected a new nested case–control sample from this population in a manner previously described.14 Briefly, 69 patients were chosen consecutively from the median enrolment period in a 2:1 controls:cases fasion. Control subjects (n = 46) had no angiographic CAD and EF ≥ 55%; and cases (n = 23) had EF <50% and either angiographic CAD ( ≥ 1 vessel with ≥ 70% stenosis), history of prior coronary artery bypass grafting, or history of prior percutaneous coronary intervention. Clinical signs and symptoms of heart failure were not required for inclusion. Patients with active ischaemia (elevation of cardiac biomarkers or dynamic electrocardiographic changes) were excluded. Prior, non-revascularized myocardial infarction was not an exclusion criterion for control subjects, consistent with previous studies.16 The EF fraction was determined by echocardiography, radionucleography, or ventriculography. Conductors of the biochemical assays were blinded to group assignment.
Measurement of the high-density lipoprotein inflammatory index
The HII measures the ability of apolipoprotein (apo)-B depleted serum, which includes HDL, apo-A1, apo-A2, and HDL-associated particles, to inhibit or enhance the oxidation of LDL in the presence of a fluorescent organic substrate. The assay was performed as previously described.14 After polyethylene glycol precipitation of apo-B from patient sera, HDL-containing supernatant was used in the assay. Samples were stored at –80°C until use for the assays. LDL (Lipid Core Lab, Children's Hospital of Philadelphia, PA, USA) was oxidised at 37°C in CuSO4 for 48 h, purified by serial dialyses in phosphate-buffered saline (PBS) at 4°C, then diluted in PBS to a final cholesterol concentration of 100 μg/mL prior to use.17 All subsequent assays used this same batch of purified, oxidized LDL. The organic phospholipid 2',7'-dichlorodihydrofluorescein diacetate (DCF) from Molecular Probes (Eugene, OR, USA) was prepared as previously described.14 Oxidized LDL (final concentration 1.4 μg/mL), DCF (final concentration 2.9 μg/mL), and a fixed volume of apo- B-depleted serum from study subjects (5 μL) were incubated with PBS to a final volume of 175 μL. Samples were incubated at 37 degrees Celsius in a microplate reader (Spectra Max, Gemini XS, Molecular Devices, Sunnyvale, California, USA). Serial excitations at 485 nm were performed every 90 s, and fluorescence at emission wavelength 530 nm and cutoff of 515 nm was measured after 1 h. Samples were plated in duplicate, and mean fluorescence recorded. Fluorescence value was divided by the fluorescence of a standardized control. The mean intra-assay coefficient of variation for all samples was 4.1%.
Measurement of high-density lipoprotein cholesterol efflux capacity
Preparation of samples and measurement of efflux capacity were performed as previously described.15 Briefly, murine macrophage J774 cells were plated and radiolabelled with 2 μCi/mL of [3H]cholesterol. After 6 h incubation with 0.3 mM 8-(4-chlorophenylthio)-cAMP to up-regulate ATP-binding cassette transporter A1 (ABCA1) at 37°C, efflux medium with 2.8% apo-B-depleted serum was added for 4 h. Liquid scintillography was used to quantify efflux of radioactive cholesterol from the cells. Cholesterol efflux capacity is reported as a ratio of the percentage efflux for each subject divided by the percentage efflux for a control sample. Assays were performed in duplicate. and the mean intra-assay coefficient of variation was 4.3%.
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables as mean with standard deviation if normally distributed and median with interquartile range if skewed. The HII was logarithmically transformed prior to use in all analyses to achieve normal distribution. Association of the HII and efflux capacity with clinical variables was assessed using Pearson correlation coefficients when normally distributed and Spearman correlation when skewed. Study groups were compared using the Student's t-test for continuous variables and χ2 test for dichotomous variables. Skewed data were compared using the Wilcoxon rank sums test (bivariate associations) or Mann–Whitney test (group differences). A multivariable logistic regression model was used to estimate the association between the II, efflux, and HF. Variables that had some association (P < 0.1) with HF on bivariate analysis and common risk factors for cardiomyopathy were included as covariates in different models. Odds ratios (ORs) are per standard deviation change in the HII or efflux capacity, consistent with previous publications. All P-values are two-tailed, with P < 0.05 having statistical significance. Analysis was performed using JMP software, version 8.0 (SAS Institute, Cary, NC, USA).
Results
Bivariate associations with ischaemic heart failure
Baseline characteristics of cases and controls are presented in Table 1. Cases were patients with ischaemic cardiomyopathy and thus had significantly lower EF and more reported history of myocardial infarction. Patients with HF also had significantly lower HDL concentration and higher creatinine. They had insignificantly elevated triglyceride and blood urea nitrogen levels, and more history of diabetes and hypertension.
Table 1.
Baseline characteristics of cases and controls
| Variable | Controls (n = 46) | Cases (n = 23) | P-value |
|---|---|---|---|
| Age (years) | 57.8 ± 8 | 58.2 ± 10 | NS |
| Female | 23 (35%) | 6 (26%) | NS |
| Smoking | 32 (52%) | 11 (50%) | NS |
| Diabetes | 6 (9%) | 5 (22%) | 0.06 |
| Prior MI | 8 (13%) | 7 (30%) | <0.01 |
| HTN | 30 (48%) | 14 (63%) | 0.08 |
| BMI | 27 (24–27) | 30 (25–32) | NS |
| TG (mmol/L)a | 1.0 (0.66–1.7) | 1.7 (1.0–2.2) | 0.05 |
| TC (mmol/L)a | 4.3 (3.9–5.4) | 4.4 (3.8–5.3) | NS |
| HDL (mmol/L)a | 1.1 (0.9–1.8) | 0.9 (0.8–1.0) | <0.01 |
| LDL (mmol/L)a | 2.6 (2.2–3.3) | 2.5 (2.1–3.4) | NS |
| BUN (mmol/L)a | 4.3 (3.2–5.7) | 6.4 (5.0–7.9) | 0.05 |
| Cr (μmol/L)a | 82 (70–88) | 88 (82–106) | 0.05 |
| SBP | 133 (120–150) | 130 (115–150) | NS |
| DBP | 73 (70–80) | 70 (60–80) | NS |
| EF (%) | 65 (62–66) | 40 (35–45) | N/A |
Continuous variables are presented as mean ± standard deviation if normally distributed and median with interquartile range if skewed; categorical variables are presented as frequency and percentage.
BMI, body mass index; BUN, blood urea nitrogen; Cr, creatine; DBP, diastolic blood pressure; HTN, hypertension; MI, myocardial infarction; N/A, not applicable; NS, non-significant; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
aSI measurements. To convert to mg/dL, multiply TG by 89, TC, HDL, or LDL by 39, BUN by 2.14, and Cr by 0.011.
The high-density lipoprotein inflammatory index is increased and efflux capacity is decreased in ischaemic heart failure
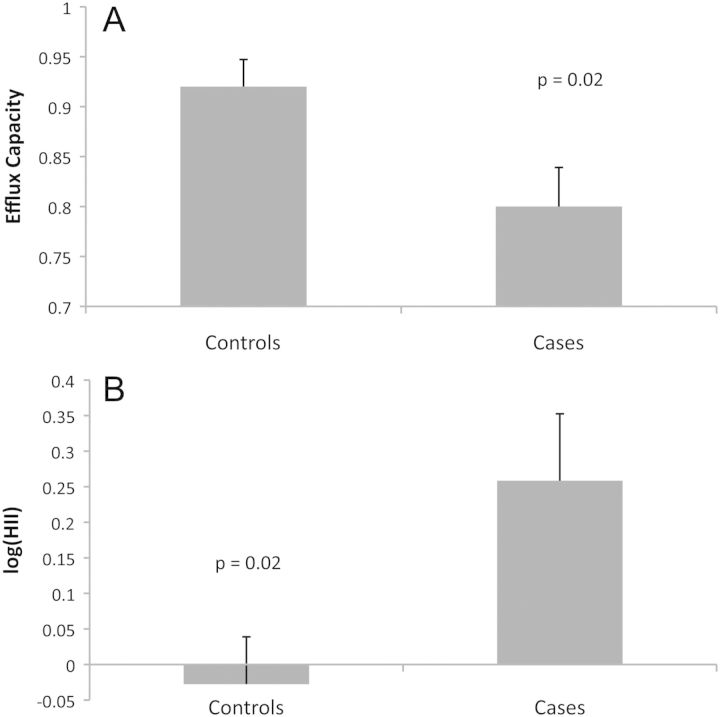
Impaired HDL anti-oxidation and cholesterol efflux capacity were present in those with ischaemic cardiomyopathy (Figure 1). Those with HF had a higher average HII and lower cholesterol efflux compared with controls (0.26 vs. –0.028, P = 0.02; 0.80 vs. 0.92, P = 0.02, respectively). Furthermore, previous studies showed a dramatic dependence on HDL concentration,14,15 and correction for HDL concentration strengthened these associations (HII P = 0.004; efflux P = 0.02).
Figure 1.
Associations of the high-density lipoprotein inflammatory index (HII) and efflux capacity with heart failure (HF). HII values were skewed and were therefore log-transformed. Comparison of the mean HII in cases (ischaemic HF) and controls (no ischaemia, no HF) by the Student's t-test revealed a significant difference (0.26 vs. –0.028, P = 0.02). Efflux values were normally distributed, and comparison of means by the Student's t-test also showed a significant difference (0.80 vs. 0.92, P = 0.02). HF correlates with a high HII (impaired anti-oxidant capacity) and low efflux capacity ratio (reduced cholesterol efflux capacity). Error bars represent the standard error of the mean.
We further assessed which clinical variables other than HF associated with the HII and efflux in our population (Table 2). The HII was associated with body mass index (BMI), triglyceride level, and HDL concentration. Cholesterol efflux capacity was associated with BMI, total cholesterol, LDL, and HDL. As expected, a higher HDL concentration was associated with a reduced HII (better anti-oxidative capacity) but increased efflux capacity (r2 = 0.09, P = 0.01; and r2 = 0.23, P = 0.0005, respectively). The HII and efflux capacity did not, however, correlate with each other (P = 0.7).
Table 2.
Associations of clinical variables with the high-density lipoprotein inflammatory index and efflux capacity
| HII |
Efflux |
|||||
|---|---|---|---|---|---|---|
| Correlation | R-value | P-value | Correlation | R-value | P-value | |
| BMI | + | 0.28 | 0.02 | – | 0.27 | 0.05 |
| TG | + | 0.33 | 0.01 | |||
| HDL | – | 0.30 | 0.01 | + | 0.48 | 0.01 |
| TC | + | 0.52 | 0.01 | |||
| LDL | + | 0.32 | 0.03 | |||
BMI, body mass inedex; HII, HDL inflammatory index; TG, triglycerides.
Associations between the high-density lipoprotein inflammatory index, efflux, and heart failure persist after adjustment for other clinical variables
Finally, we created multivariable logistic regression models of variables that associate with HF (Table 3). A high HII and low cholesterol efflux capacity are risk factors for HF [HII OR 2.8, 95% confidence interval (CI) 2.0–3.9, P = 0.002; efflux OR 2.1, 95% CI 1.5–3.0, P = 0.03]. When adjusted for clinical variables that showed some association (P < 0.1) with HF, both the HII and efflux continue to be risk factors for HF (HII OR 3.7, 95% CI 2.3–5.9, P = 0.006; efflux OR 2.6, 95% CI 1.6–4.1, P = 0.04). When adjusted for traditional risk factors for HF, the HII and systolic blood pressure, and efflux capacity emerged as risk factors for HF (HII OR 3.1, 95% CI 1.7–5.9, P = 0.04; systolic blood pressure OR 1.1, 95% CI 1.0–1.2, P = 0.01; efflux OR 10, 95% CI 3.2–31, P = 0.04). However, if the HII and efflux are included in the same model, neither variable has a significant influence on HF (data not shown; all P-values >0.05).
Table 3.
Multivariable logistic regression analysis of high-density lipoprotein function and ischaemic heart failure
| Variable | Unadjusted | Adjusted for correlatesa | Adjusted for risk factorsb |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| HII | 2.8 (2.0–3.9) | 3.7 (2.3–5.9) | 3.1 (1.7–5.9) |
| Efflux | 2.1 (1.5–3.0) | 2.6 (1.6–4.1) | 10 (3.2–31) |
CI, confidence interval; HII, HDL inflammatory index; OR, odds ratio.
All P-values <0.05. Odds ratios and 95% confidence intervals are for high HII (impaired anti-oxidative capacity) and low efflux capacity (impaired reverse cholesterol transport).
aBivariate correlates of heart failure include: diabetes, hypertension, blood urea nitrogen, creatinine, and HDL mass.
bRisk factors for heart failure include: age, blood pressure, creatinine, total cholesterol, LDL, and HDL mass.
Discussion
We demonstrate here that two separate assays of HDL function, HDL anti-oxidative capacity as measured by the HII and reverse cholesterol transport as measured by efflux capacity, are both impaired in HF patients with chronic ischaemia. We deliberately excluded patients with ACS, given potential confounders of an active inflammatory milieu and stunned or transiently depressed myocardium without true, stable HF.
Others have proposed mechanisms for each of these findings. There is some evidence that stable ischaemic HF patients have active inflammation. Blood collected from HF patients had lower HDL and was more susceptible to LPS-induced TNF-α production than than that of control subjects.18 Additionally, HF patients have reduced HDL and higher triglycerides at baseline, and demonstrated a significant association between total cholesterol level and inflammatory markers such as TNF-α and one of its receptors, sTNF-R2.6 Here we demonstrate not only an inverse relationship between HDL concentration and prevalent HF, but also a correlation between impaired HDL anti-oxidative capacity and HF.
Furthermore, the cholesterol level does not have a clear correlation with HF incidence and prognosis. Treatment of an elderly population of stable HF patients with a statin did significantly lower LDL cholesterol but did not affect cardiovascular outcomes.19 In acute HF patients, a lower total cholesterol level predicted worse outcome.20 Yet in patients with severe myocardial infarction causing systolic dysfunction, early treatment with a statin reduced LDL and cardiovascular mortality.21 These findings do not apply to HDL cholesterol, though, and some studies show favourable prognosis in HF patients with high HDL.4,22 A more nuanced understanding of these cholesterol effects involves examining the functionality of cholesterol particles; the present study shows an inverse correlation between HF prevalence and the ability of HDL to promote reverse cholesterol transport or to prevent lipid oxidation. This effect persisted even after adjustment for HDL concentration; however, the CIs are large and may not be clinically relevant. It remains unclear, moreover, whether these functions of HDL play a role in HF pathogenesis, and further translational research is important to answer this question.
Some suggestive evidence comes from a large, long-term cohort study of the Framingham Heart Study population; a high HDL concentration remained predictive of incident HF even after adjustment for interim ischaemic events.23 This is especially intriguing because it posits a role for HDL in the pathogenesis of HF independent of its anti-atherogenic properties. For example, in a select population of patients with ischaemic cardiomyopathy and high C-reactive protein levels, treatment with a statin reduced HF hospitalizations more than coronary endpoints.8 We have previously shown that statin therapy improves the HII in subjects without ACS.14 We posit here that HDL may influence development of HF in a cohort of patients with angina by both improved cholesterol efflux and enhanced anti-oxidative capacity.
However, similarly to previous studies,14 we noted in this population that the HII and efflux capacity did not correlate with one another, despite each having an association with HF. Furthermore, in the multiple regression model, the predictive power of each variable dissipated when the other variable was included. Unlike a previous population of those with ACS, where the HII and efflux provided information orthogonal to the endpoint, this population of HF subjects may possess a synergistic influence from the HII and efflux.
A strength of our study is the uniform collection of samples and subsequent use of the same patient serum for two different assays simultaneously. This minimized potential bias introduced by sample preparation and assay administration. However, our population was small and cross-sectional, including a majority of white males. The correlations are merely suggestive and hypothesis-generating. Furthermore, ischaemic aetiology for HF in this population depended on angiographic but not functional evaluation. Nevertheless, data from previous studies indicate reasonable correlation between our inclusion criteria (any vessel with > 70% stenosis and EF <50%) and clinical progression of ischaemic HF.16 Finally, the inherent difference between cases and controls, the presence of revascularized CAD, confounds interpretation of HDL dysfunction as unique to the HF population. Nevertheless, the presence of prior, non-revascularized CAD, myocardial infarction, and other cardiovascular risk factors, when included in multivariable analysis, did not significantly reduce the influence of HDL function on HF.
In conclusion, we have demonstrated here that patients with stable, chronic ischaemic HF have impaired HDL anti-oxidative capacity as well as reduced cholesterol efflux capacity. Our research supports further evaluation of the proposed mechanisms for HDL to exert its effects on HF pathogenesis.
Funding
The National Heart, Lung, and Blood Institute (NHBLI; grants HL 22633 and P50 HL70128); Doris Duke Charitable Foundation (a Distinguished Clinical Scientist Award).
Conflicts of interest: none declared.
References
- 1.Holme I, Strandberg TE, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Larsen ML, Lindahl C, Pedersen TR Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. Congestive heart failure is associated with lipoprotein components in statin-treated patients with coronary heart disease Insights from the Incremental Decrease in End points Through Aggressive Lipid Lowering Trial (IDEAL) Atherosclerosis. 2009;205:522–527. doi: 10.1016/j.atherosclerosis.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS, Ahn C. Frequency of congestive heart failure in older persons with prior myocardial infarction and serum low-density lipoprotein cholesterol>or=125 mg/dl treated with statins versus no lipid-lowering drug. Am J Cardiol. 2002;90:147–149. doi: 10.1016/s0002-9149(02)02438-4. [DOI] [PubMed] [Google Scholar]
- 3.Freitas HF, Barbosa EA, Rosa FH, Lima AC, Mansur AJ. Association of HDL cholesterol and triglycerides with mortality in patients with heart failure. Braz J Med Biol Res. 2009;42:420–425. doi: 10.1590/s0100-879x2009000500004. [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant. 2009;28:876–880. doi: 10.1016/j.healun.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Christ M, Klima T, Grimm W, Mueller HH, Maisch B. Prognostic significance of serum cholesterol levels in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2006;27:691–699. doi: 10.1093/eurheartj/ehi195. [DOI] [PubMed] [Google Scholar]
- 6.Rauchhaus M, Koloczek V, Volk H, Kemp M, Niebauer J, Francis DP, Coats AJ, Anker SD. Inflammatory cytokines and the possible immunological role for lipoproteins in chronic heart failure. Int J Cardiol. 2000;76:125–133. doi: 10.1016/s0167-5273(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 8.McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentrations of tumour necrosis factor in ‘cachectic’ patients with severe chronic heart failure. Br Heart J. 1991;66:356–358. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79:1426–1430. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 10.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Wu Y, Mann S, Pepoy M, Shrestha K, Borowski AG, Hazen SL. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circ Heart Fail. 2011;4:59–64. doi: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, Mackness M, Holvoet P. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–1646. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- 14.Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58:2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 17.Guy RA, Maguire GF, Crandall I, Connelly PW, Kain KC. Characterization of peroxynitrite-oxidized low density lipoprotein binding to human CD36. Atherosclerosis. 2001;155:19–28. doi: 10.1016/s0021-9150(00)00524-4. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R, von Haehling S, Rauchhaus M, Bolger AP, Genth-Zotz S, Doehner W, Oliver B, Poole-Wilson PA, Volk HD, Coats AJ, Adcock IM, Anker SD. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur J Heart Fail. 2005;7:479–84. doi: 10.1016/j.ejheart.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, Givertz MM, Mansoor GA, Ponikowski P, Teerlink JR, Voors AA, Fiuzat M, Wojdyla D, Chiswell K, Massie BM. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 21.Dobre D, Rossignol P, Murin J, Parkhomenko A, Lamiral Z, Krum H, van Veldhuisen DJ, Pitt B, Zannad F. Statin therapy and clinical outcomes in myocardial infarction patients complicated by acute heart failure: insights from the EPHESUS trial. Eur J Heart Fail. 2013;15:221–227. doi: 10.1093/eurjhf/hfs128. [DOI] [PubMed] [Google Scholar]
- 22.Sakatani T, Shirayama T, Suzaki Y, Yamamoto T, Mani H, Kawasaki T, Sugihara H, Matsubara H. The association between cholesterol and mortality in heart failure. Comparison between patients with and without coronary artery disease. Int Heart J. 2005;46:619–629. doi: 10.1536/ihj.46.619. [DOI] [PubMed] [Google Scholar]
- 23.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]