Abstract
Background
A new classification of diabetic nephropathy was reported by Tervaert et al., but the association between pathological findings and the clinical outcomes remains unclear.
Methods
Among 310 patients with diabetes mellitus who underwent renal biopsy from March 1985 to January 2010 and were confirmed to have diabetic nephropathy according to the Tervaert's classification, 205 patients were enrolled in this study. Cox proportional hazard regression analysis was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for death-censored renal death. Each regression analysis employed two levels of multivariate adjustment.
Results
After adjustment for age, gender, estimated glomerular filtration rate, type of diabetes, urinary protein excretion, systolic blood pressure, body mass index, HbA1c, diabetic retinopathy and red blood cells in urinary sediment at the time of renal biopsy, compared with glomerular class IIA, the HRs for death-censored renal death of glomerular classes I, IIB, III and IV were 0.21 (95% CI: 0.04–1.25), 2.12 (0.89–5.04), 4.23 (1.80–9.90), and 3.27 (1.32–8.10), respectively. Also, compared with an interstitial fibrosis and tubular atrophy score 1 group, HRs for score 0 group, score 2 group and score 3 group were 0.08 (0.01–0.57), 2.17 (0.96–4.91), 4.78 (1.96–11.68), respectively.
Conclusions
The progression of glomerular, tubulointerstitial and vascular lesions was associated with higher HRs for renal death. These results suggest the clinical utility of Tervaert's pathological classification.
Keywords: diabetic nephropathy, pathological classification, renal prognosis
INTRODUCTION
Type 2 diabetes was once considered to be a disease of Western countries, but today more than 60% of the world's diabetic patients are found in Asia because of rapid economic development in this region. Unlike Western countries, where older persons are most affected, diabetes in Asian countries is characterized by onset at a relatively young age and low body mass index. The increased prevalence of diabetes has led to an increase of patients with diabetic nephropathy (DN) and end-stage renal disease (ESRD) around the world. Asian patients with diabetes are more likely to develop ESRD than their Western counterparts [1, 2]. In general, 25% of patients with type 2 diabetes for 20 years develop DN and 20% of these patients progress to ESRD within 10 years [3]. Ritz et al. [4] reported that with respect to the prevalence of proteinuria after diagnosis or renal failure after the onset of proteinuria, there was no difference between type 1 diabetes and type 2 diabetes. Wada et al. [5] reported that diabetic patients who have albuminuria/overt proteinuria and a low glomerular filtration rate are at risk of developing cardiovascular events as well as renal events. De Boer et al. [6] suggested that intensive glycemic control, blood pressure reduction and diet are associated with an improved outcome. However, patients with diabetes and proteinuria were enrolled in these studies and were assumed to have DN without biopsy for histological confirmation. Renal biopsy is generally not done in patients with diabetes and DN because their renal prognosis is not influenced by pathological findings. In atypical cases, renal biopsy is often performed for differentiation from other renal diseases or to detect the coexistence of other diseases, but the long-term renal prognosis of a cohort of patients with histologically confirmed DN remains uncertain. For several renal diseases, including lupus nephritis, focal segmental glomerulosclerosis and IgA nephropathy, the pathological classifications have been revised extensively and studies based upon the new pathological classifications have been started for each disease. However, there is still no uniform classification for DN, even though it is the most frequent cause of ESRD. A working group that included Tervaert et al. [7] has proposed a new pathological classification of DN that is intended to improve communication between renal pathologists and clinicians, to provide a logical basis for prognostic interventional studies and to improve clinical management and efficiency. They expected that classifying the severity of disease in patients with pure DN could help to unravel various pathways leading to glomerulosclerosis, thus providing new possibilities for intervention to prevent the progression of DN.
In the present study, the renal prognosis of patients with DN classified according to Tervaert's criteria was followed for a long time.
METHODS
Patients and study design
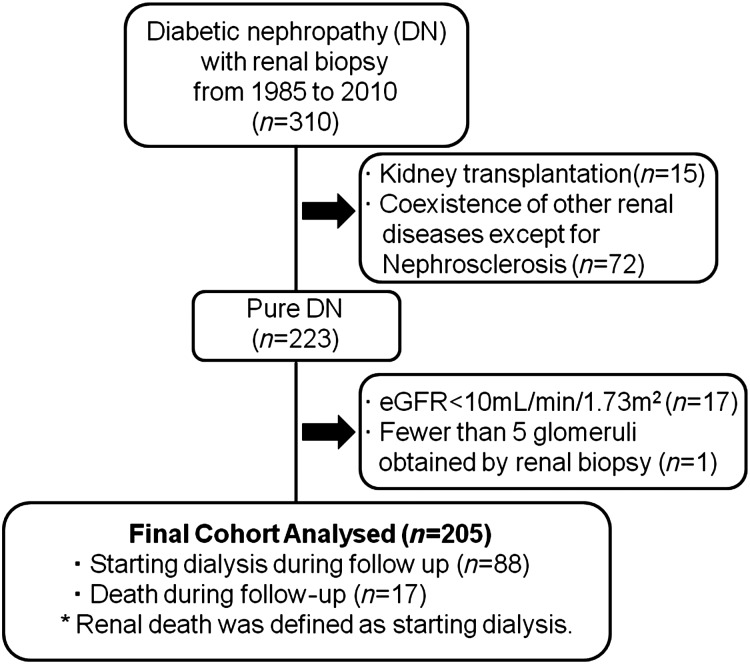
Among 310 patients with diabetes mellitus who underwent renal biopsy at our hospital from March 1985 to January 2010 and were confirmed to have DN, 205 patients were considered to be eligible and were enrolled in this study. DN was diagnosed by at least two renal pathologists and/or nephrologists, and the diagnosis was re-evaluated according to Tervaert's classification [7]. Exclusion criteria were kidney transplantation, coexistence of other renal diseases except for nephrosclerosis, estimated glomerular filtration rate (eGFR) <10 mL/min per 1.73 m2 at the time of renal biopsy and obtaining fewer than five glomeruli by renal biopsy (Figure 1).
FIGURE 1:
Flowchart of study participants. eGFR: estimated glomerular filtration rate.
Assessment of laboratory data and definitions
HbA1c was measured by high-performance liquid chromatography according to the standards of the Japanese Diabetes Society (normal range: 4.3–5.8%). Hematuria was defined as the detection of more than five erythrocytes per high-power field in at least two of three consecutive urine tests. All patients with hematuria had no more than one to five white blood cells per high-power field, no urinary tract malignancy and no stone disease. The average annual values were calculated for urinary protein excretion, systolic and diastolic blood pressure, HbA1c and hemoglobin during follow-up. When data for any of these variables were not available during follow-up, the average of the observations before and after the missing value was calculated and used. We employed U-Pro (g/gCr) if U-Pro (g/day) was not available.
Renal biopsy and pathological classification
All renal biopsies were performed based on the decisions by our department and/or primary doctor. Basically, indications of renal biopsy were proteinuria more than 0.5 g/day or atypical DN such as renal involvement without diabetic retinopathy and/or with hematuria. Renal tissue was obtained by needle biopsy. For light and electron microscopy, the biopsy specimens were processed according to standard procedures. Sections were stained with hematoxylin–eosin, periodic acid-Schiff, Weigert's elastica-van Gieson, Masson trichrome, or periodic acid methanamine silver stain. The mean number of glomeruli was 17.0 ± 11.1 (range, 5–63). Classification of DN and histological scoring were done according to the criteria of Tervaert et al. [7]. The glomerular classification was as follows and light microscopic changes in the glomerular basement membrane and epithelial foot process effacement by electron microscopy had no influence on the classification. Class I was defined as glomerular basement membrane thickening (>395 nm in females or >430 nm in males) without any of the criteria mentioned below for classes II, III or IV. The glomerular basement membrane of other cases from class II, III or IV was directly measured by electron microscopy. The mean thickness of the glomerular basement membrane was 529.6 ± 130.4 nm. Class IIA was mild mesangial expansion in >25% of the observed mesangial areas (mesangium < capillary lumen), and class IIB was severe mesangial expansion in >25% of the observed areas (mesangium > capillary lumen). Mesangial expansion was defined as an increase in the extracellular material in the mesangium such that the width of the interspace exceeded two mesangial cell nuclei in at least two glomerular lobules. Class III was nodular sclerosis, specifically defined as presence of at least one convincing Kimmelstiel-Wilson lesion and <50% global glomerulosclerosis. Class IV was advanced DN defined as more than 50% global glomerulosclerosis. The interstitial fibrosis and tubular atrophy (IFTA) scores were classified as follows: 0, absent; 1, <25%; 2, 25–50% and 3, >50% of the total area. Interstitial inflammation was scored as follows: 0, absent; 1, inflammation only related to IFTA and 2, inflammation in areas without IFTA. Arteriolar hyalinosis was scored as follows: 0, absent; 1, hyalinosis of at least one arteriole and 2, hyalinosis of more than one arteriole. Arteriosclerosis was scored in the most severely affected artery as follows: 0, no intimal thickening; 1, intimal thickening that was less than the medial thickness and 2, intimal thickening that was greater than the medial thickness. Exudative lesions (e.g. capsular drops) were also evaluated because such lesions were specific but not entirely pathognomonic of DN and were associated with disease progression [8]. Scoring was performed by the same pathologists.
End point
The primary end point was renal death, which was defined as commencement of dialysis due to ESRD. None of the patients received kidney transplantation during follow-up.
Statistical analysis
Data were summarized as percentages or the mean (±standard deviation [SD]) as appropriate. Categorical variables were analyzed with the χ2 test or Fisher's exact test as appropriate, and continuous variables were compared using t-test, Mann–Whitney U-test, Kruskal–Wallis H-test or ANOVA, as appropriate. Correlations among each histopathological finding were evaluated by Spearman's correlation. Cumulative survival was estimated with Kaplan–Meier survival curves, and was compared by using the log-rank test. The Cox proportional hazards model was used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for death-censored renal death. In the Cox proportional hazards model 1, each HR was adjusted for age, gender, eGFR, type of diabetes, urinary protein excretion, systolic blood pressure, body mass index and HbA1c at the time of renal biopsy. Also, in Model 2, each HR was adjusted for the above plus diabetic retinopathy and red blood cells in urinary sediment at the time of renal biopsy. A P-value of <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS (version 20.0, Chicago, IL, USA).
RESULTS
Of the 310 patients screened, 205 met the study entry criteria. A flowchart showing the disposition of the patients is displayed in Figure 1. The mean follow-up period was 62.9 ± 68.3 months.
Of the 205 patients, 150 were men (73.2%). The mean (±SD) age at the time of renal biopsy was 55.9 ± 13.0 years (range: 21–83 years). A total of 183 patients (89.3%) had type 2 diabetes, and 141 patients (68.8%) had diabetic retinopathy. Mean body mass index (BMI) was 24.0 ± 4.0 kg/m2. The mean systolic and diastolic blood pressures at admission were 145.7 ± 20.6 and 81.7 ± 12.8 mmHg, respectively. The mean baseline serum creatinine level was 1.65 ± 0.95 mg/dL (0.4–5.5), the mean creatinine clearance rate was 49.8 ± 28.3 mL/min (6.0–175.6) and the mean eGFR was 44.3 ± 22.6 mL/min per 1.73 m2 (10.0–123.0) [9]. Urinary protein excretion was 3.22 ± 3.27 g/day (0.03–20.5). Twenty-two patients (10.7%) had hematuria (i.e. more than five erythrocytes per high-power field). The mean hemoglobin was 12.1 ± 2.4 g/dL (6.6–17.9), mean HbA1c was 7.3 ± 1.9%, serum albumin was 3.2 ± 0.7 g/dL, total cholesterol was 217.4 ± 61.2 mg/dL, triglycerides were 169.4 ± 90.9 mg/dL and low-density lipoprotein (LDL) cholesterol was 141.0 ± 52.3 mg/dL (Table 1).
Table 1.
Baseline clinical findings and the number of glomeruli obtained by renal biopsy in groups stratified according to the glomerular classification of diabetic nephropathy
| All (n=205) | Class I (n=13) | Class IIA (n=44) | Class IIB (n=55) | Class III (n=57) | Class IV (n=36) | |
|---|---|---|---|---|---|---|
| Male (%) | 73.2 (n=150) | 61.5 (n=8) | 86.4 (n=38) | 70.9 (n=39) | 59.6 (n=34)c | 86.1 (n=31)g |
| Age (year) | 55.9 ± 13.0 | 40.2 ± 13.4 | 57.1 ± 13.5a | 53.9 ± 13.1a | 57.5 ± 10.6a | 60.6 ± 11.5a,f |
| BMI (kg/m2) | 24.0 ± 4.0 | 26.5 ± 4.4 | 24.7 ± 4.2 | 23.5 ± 3.7b | 23.6 ± 4.3b | 23.6 ± 3.3b |
| sBP (mmHg) | 145.7 ± 20.6 | 136.5 ± 14.0 | 139.8 ± 20.8 | 143.3 ± 15.8 | 151.2 ± 23.1b,d,f | 151.3 ± 21.7b,d |
| dBP (mmHg) | 81.7 ± 12.8 | 81.2 ± 15.5 | 80.8 ± 11.3 | 80.8 ± 11.0 | 82.4 ± 13.7 | 83.1 ± 14.9 |
| s-Cr (mg/dL) | 1.65 ± 0.95 (0.4–5.5) | 0.98 ± 0.30 (0.4–1.5) | 1.20 ± 0.51 (0.5–2.9) | 1.51 ± 0.79a,d (0.6–3.9) | 1.65 ± 0.85a,c (0.5–4.2) | 2.63 ± 1.18a,c,e,g (1.0–5.5) |
| CCr (mL/min) | 49.8 ± 28.3 | 78.3 ± 21.9 | 65.5 ± 30.6 | 51.9 ± 26.2a,d | 42.0 ± 22.5a,c | 28.9 ± 18.3a,c,e,g |
| eGFR (ml/min/1.73 m2) | 44.3 ± 22.6 | 68.8 ± 22.1 | 56.9 ± 22.4 | 45.6 ± 19.9a,d | 40.0 ± 19.3a,c | 24.7 ± 11.3a,c,e,g |
| U-Pro (g/day) | 3.22 ± 3.27 | 0.81 ± 0.50 | 1.35 ± 1.77 | 3.36 ± 3.36a,c | 4.27 ± 3.51a,c,e | 4.66 ± 3.29a,c,e |
| s-Alb (g/dL) | 3.2 ± 0.7 | 4.1 ± 0.5 | 3.6 ± 0.6a | 3.2 ± 0.6a,c | 2.8 ± 0.7a,c,e | 2.8 ± 0.5a,c,e |
| HbA1c (%) | 7.3 ± 1.9 | 7.9 ± 1.4 | 7.4 ± 1.6 | 7.6 ± 2.0 | 7.5 ± 2.1 | 6.4 ± 1.4a,c,f,g |
| Hb (g/dL) | 12.1 ± 2.4 | 14.8 ± 1.7 | 13.6 ± 1.8 | 12.1 ± 2.2a,c | 10.9 ± 1.8a,c,f | 10.9 ± 2.3a,c,f |
| Retinopathy (%) | 68.8 (n=141) | 15.4 (n=2) | 43.2 (n=19) | 76.4 (n=42)a,c | 86.0 (n=49)a,c | 80.6 (n=29)a,c |
| Type1 DM (%) | 10.7 (n=22) | 15.4 (n=2) | 9.1 (n=4) | 14.5 (n=8) | 12.3 (n=7) | 2.8 (n=1) |
| RBC in urinary sediment (%) | 10.7 (n=22) | 0 | 9.1 (n=4) | 10.9 (n=6) | 19.3 (n=11) | 2.8 (n=1)h |
| ACE-I or ARB (%) | 63.9 (n=131) | 15.4 (n=2) | 63.6 (n=28)a | 67.3 (n=37)a | 77.2 (n=44)a | 55.6 (n=20)b,h |
| Number of antihypertensive agent | 2.1 ± 1.4 | 0.5 ± 0.8 | 1.6 ± 1.3a | 2.1 ± 1.3a | 2.7 ± 1.5a,c,f | 2.4 ± 1.3a,c |
| ESA (%) | 7.8 (n=16) | 0 | 2.3 (n=1) | 1.8 (n=1) | 14.0 (n=8)f | 16.7 (n=6)d,f |
| OHA therapy (%) | 30.2 (n=62) | 7.7 (n=1) | 38.6 (n=17)b | 30.9 (n=17) | 29.8 (n=17) | 27.8 (n=10) |
| Insulin therapy (%) | 49.3 (n=101) | 15.4 (n=2) | 34.1 (n=15) | 54.5 (n=30)b,d | 63.2 (n=36)a,c | 50.0 (n=18)b,d |
| Number of glomeruli | 17.0 ± 11.1 | 11.9 ± 10.9 | 12.7 ± 6.7 | 14.8 ± 8.8 | 23.3 ± 13.2a,c,e | 17.6 ± 10.7b,d,h |
BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure; s-Cr, serum creatinine; CCr, creatinine clearance rate; eGFR, estimated glomerular filtration rate; U-Pro, urinary protein excretion; s-Alb, serum albumin; Hb, hemoglobin; retinopathy, diabetic retinopathy; RBC in urinary sediment, red blood cells >5/HPF in sediment; ACE-I or ARB, use of an angiotensin-converting enzyme inhibitor or angiotensin II type I receptor blocker, respectively; ESA, erythropoietin-stimulating agents; OHA, oral hypoglycemic agent; insulin therapy, treatment with insulin including basal supported oral therapy.
aP < 0.01: versus class I.
bP < 0.05: versus class I.
cP < 0.01: versus class IIA.
dP < 0.05: versus class IIA.
eP < 0.01: versus class IIB.
fP < 0.05: versus class IIB.
gP < 0.01: versus class III.
hP < 0.05: versus class III.
Clinical findings at the time of renal biopsy and the number of glomeruli obtained by biopsy are compared among the glomerular classes in Table 1. Patients in class I were significantly younger than patients in the other classes. The eGFR was significantly lower for patients in classes IIB or III than for those in classes I or IIA, and also significantly lower for patients in class IV than for those in all other classes. Urinary protein excretion was significantly higher in class III or IV patients than in class I, IIA or IIB patients, as well as in class IIB patients than in class I or IIA patients. HbA1c was significantly lower for patients in class IV than for those in all other classes. The hemoglobin was significantly lower in class IV patients than in those from classes I, IIA and IIB, and it was also significantly lower in class IIB or III patients than in those with lower classes. Coexistence of diabetic retinopathy was significantly more frequent in patients from classes IIB, III and IV than in those from classes I or IIA. Treatment with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II type I receptor blockers (ARB) was significantly less frequent and significantly fewer antihypertensive agents were used by class I patients than by patients from the other classes.
Clinical findings during the follow-up period and at final follow-up are shown in Table 2 for each glomerular class. Average urinary protein excretion during follow-up was greater in patients from the higher classes than in those from the lower classes. The average systolic blood pressure was significantly higher in patients from classes III and IV than in those from classes I, IIA or IIB. Average HbA1c was significantly lower in class IV patients than in those from the other classes. There were no significant differences in the use of ACE-I or ARB during follow-up among the patients from different glomerular classes, except between those from classes I and IIA. At final follow-up, class I patients were using significantly fewer antihypertensive agents than patients from the other classes.
Table 2.
Clinical findings during follow-up and at final follow-up in groups stratified according to the glomerular classification of diabetic nephropathy
| All (n=205) | Class I (n=13) | Class IIA (n=44) | Class IIB (n=55) | Class III (n=57) | Class IV (n=36) | |
|---|---|---|---|---|---|---|
| U-Pro (g/day or g/gCr) | 3.66 ± 3.08 | 0.85 ± 0.90 | 1.99 ± 2.05b | 3.61 ± 2.65a,c | 4.62 ± 3.37a,c | 5.27 ± 3.19a,c,e |
| sBP (mmHg) | 141.0 ± 15.9 | 135.0 ± 11.5 | 137.2 ± 15.7 | 136.6 ± 12.3 | 146.4 ± 17.6b,c,e | 146.2 ± 15.9b,c,e |
| dBP (mmHg) | 78.1 ± 9.8 | 80.1 ± 10.7 | 76.6 ± 8.2b | 76.2 ± 8.4b | 79.5 ± 9.7 | 79.9 ± 12.4 |
| HbA1c (%) | 7.0 ± 1.4 | 7.6 ± 1.3 | 7.2 ± 1.2 | 7.1 ± 1.6 | 7.0 ± 1.4 | 6.4 ± 1.3b,c,f,g |
| Hb (g/dL) | 11.5 ± 2.1 | 14.3 ± 1.4 | 12.9 ± 1.8b | 11.8 ± 2.0a,c | 10.5 ± 1.5a,c,e | 10.1 ± 1.7a,c,e |
| ACE-I or ARB (%) | 82.4 (n=169) | 61.5 (n=8) | 90.9 (n=40)b | 85.5 (n=47) | 82.5 (n=47) | 75.0 (n=27) |
| Final number of antihypertensive agent | 2.9 ± 1.7 | 1.2 ± 1.2 | 2.7 ± 1.7a | 3.1 ± 1.6a | 3.4 ± 1.6a,d | 2.9 ± 1.7a |
| Final ESA (%) | 52.2 (n=107) | 15.4 (n=2) | 38.6 (n=17) | 56.4 (n=31)a | 52.6 (n=30)a | 75.0 (n=27)a,c,g |
| Final OHA therapy (%) | 22.9 (n=47) | 38.5 (n=5) | 27.3 (n=12) | 18.2 (n=10) | 26.3 (n=15) | 13.9 (n=5) |
| Final insulin therapy (%) | 60.0 (n=123) | 23.1 (n=3) | 56.8 (n=25)b | 67.3 (n=37)a | 66.7 (n=38)a | 55.6 (n=20)b |
| Number of renal death | 88 | 2 | 10 | 25 | 27 | 24 |
U-Pro, average annual urinary protein excretion; sBP, average annual systolic blood pressure; dBP, average annual diastolic blood pressure; HbA1c, average annual HbA1c; Hb, average annual hemoglobin level; ACE-I or ARB, use of an angiotensin-converting enzyme inhibitor or angiotensin II type I receptor blocker, respectively, for more than 3 months or half of the follow-up period (n = 154, respectively), final number of antihypertensive agent, final ESA, final OHA therapy; final insulin therapy, respectively, the number of antihypertensive agents, use of erythropoietin-stimulating agents, use of oral hypoglycemic agents and insulin therapy (including basal supported oral therapy) at the last follow-up or immediately before commencement of dialysis.
aP < 0.01: versus class I.
bP < 0.05: versus class I.
cP < 0.01: versus class IIA.
dP < 0.05: versus class IIA.
eP < 0.01: versus class IIB.
fP < 0.05: versus class IIB.
gP < 0.05: versus class III.
Renal death occurred in 88 patients during follow-up, and the number of renal deaths in each glomerular class is shown in Table 2. A total of 17 patients died during follow-up, with the cause of death being heart disease in 2, infection in 3 (pneumonia in 2 and lower limb gangrene in 1), stroke in 2 (cerebral hemorrhage and cerebral infarction in 1 each), hepatic disease in 2 (hepatic encephalopathy in 1 and bleeding varices in 1), malignancy (cholangiocarcinoma) in 1, gastrointestinal hemorrhage in 1, acute exacerbation of interstitial pneumonia in 1 and undefined in 5.
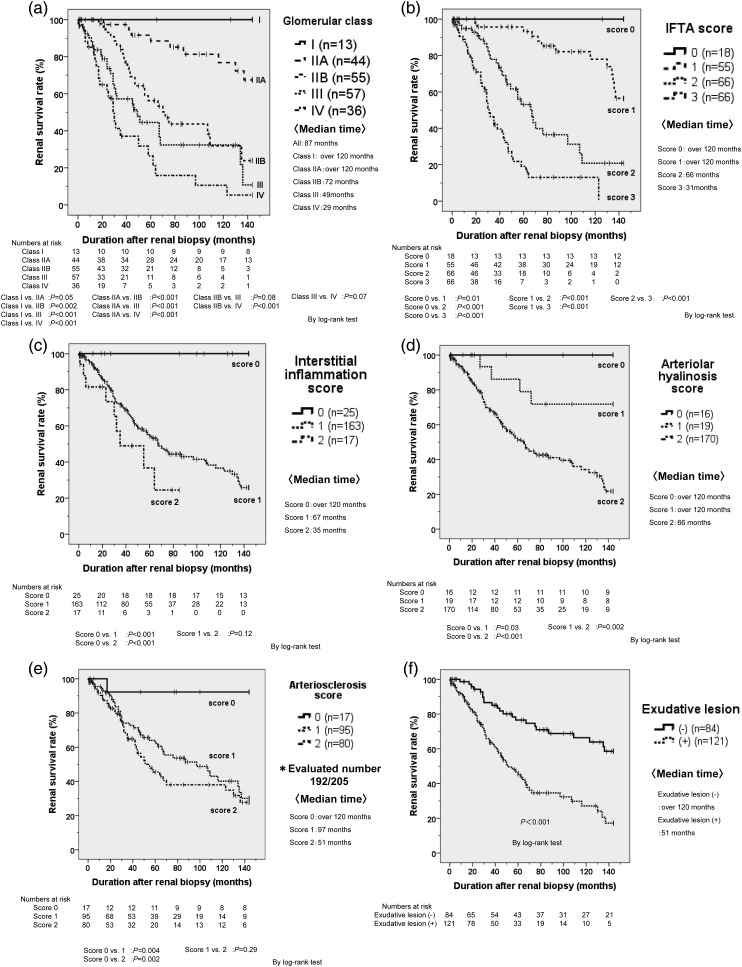
Kaplan–Meier survival curves stratified according to the glomerular class, the scores for IFTA, interstitial inflammation, arteriolar hyalinosis or arteriosclerosis, and the presence of exudative lesions are shown in Figure 2. There was a significant difference in the renal survival rate among most of the glomerular classes, except between class I versus IIA, class IIB versus III and class III versus IV (Figure 2a). There was also a significant difference in renal survival among each IFTA score or interstitial inflammation score, except between interstitial inflammation scores of 1 and 2 (Figure 2b and c). Furthermore, there was a significant difference in renal survival among each arteriolar hyalinosis score (Figure 2d) and among each arteriosclerosis score (except between scores of 1 and 2, Figure 2e). Finally, patients without exudative lesions had a significantly better renal survival rate than those with such lesions (Figure 2f).
FIGURE 2:
(a) Renal survival rates in glomerular classes. The 5-year renal survival rate in our study was estimated as 100% in glomerular class I, 88.5% in class IIa, 53.3% in class IIb, 36.4% in class III and 21.2% in class IV. (b) Renal survival rates in interstitial fibrosis and tubular atrophy (IFTA) scores. (c) Renal survival rates in interstitial inflammation scores. (d) Renal survival rates in arteriolar hyalinosis scores. (e) Renal survival rates in arteriosclerosis scores. Large vessel was not obtained in 13 patients. (f) Renal survival rates in the presence or nothing of exudative lesion. Abbreviation: Median time, median time for introduction of dialysis after renal biopsy
Correlations among the histopathological findings are displayed in Table 3. The glomerular class showed a strong correlation with IFTA (correlation coefficient (r) = 0.66), as well as correlations with interstitial inflammation (r = 0.43), arteriolar hyalinosis (r = 0.40) and exudative lesions (r = 0.46). IFTA was also correlated with each of these findings.
Table 3.
Correlation coefficients among histopathological findings
| Glomerular class | IFTA | Interstitial inflammation | Arteriolar hyalinosis | Arteriosclerosis | Exudative lesion | |
|---|---|---|---|---|---|---|
| Glomerular class | – | 0.66 | 0.43 | 0.40 | 0.33 | 0.46 |
| IFTA | – | – | 0.47 | 0.48 | 0.46 | 0.52 |
| Interstitial inflammation | – | – | – | 0.55 | 0.27 | 0.33 |
| Arteriolar hyalinosis | – | – | – | – | 0.31 | 0.36 |
| Arteriosclerosis | – | – | – | – | – | 0.20 |
| Exudative lesion | – | – | – | – | – | – |
IFTA, interstitial fibrosis and tubular atrophy scores.
The results of Cox proportional hazards analysis of clinical variables at the time of renal biopsy are shown in Table 4. In Models 1 and 2, urinary protein excretion demonstrated a significant independent association with renal survival, a finding that is consistent with previous reports [5, 10]. In both models, type 1 diabetes was also an independent predictor of the renal outcome.
Table 4.
Factors affecting renal outcome at baseline; adjusted for patient sex, age and body mass index (BMI)
| HR | 95% CI | P-value | |
|---|---|---|---|
| Model 1 | |||
| U-Pro | 1.13 | 1.05–1.21 | 0.001 |
| eGFR | 0.95 | 0.93–0.96 | <0.001 |
| sBP | 1.01 | 1.00–1.02 | 0.09 |
| HbA1c | 0.89 | 0.80–1.01 | 0.07 |
| DM type | 0.47 | 0.23–0.95 | 0.04 |
| Model 2 | |||
| U-Pro | 1.12 | 1.04–1.20 | 0.002 |
| eGFR | 0.95 | 0.93–0.96 | <0.001 |
| sBP | 1.01 | 1.00–1.02 | 0.06 |
| HbA1c | 0.89 | 0.79–1.02 | 0.08 |
| DM type | 0.44 | 0.22–0.88 | 0.02 |
| RBC in urinary sediment | 1.73 | 0.90–3.34 | 0.10 |
| Retinopathy | 0.95 | 0.56–1.62 | 0.85 |
Model 1 includes duration from renal biopsy to event, patient sex, age, BMI, urinary protein excretion (U-Pro), estimated glomerular filtration rate (eGFR), systolic blood pressure (sBP), HbA1c and type of diabetes (DM type). Model 2 includes duration from renal biopsy to event, patient sex, age, BMI, U-Pro, eGFR, sBP, HbA1c, DM type, red blood cells (RBC) in urinary sediment and presence/absence of diabetic retinopathy (Retinopathy). Reference and abbreviation: U-Pro, per 1 g/day; eGFR, per 1 mL/min per 1.73 m2; sBP, per 1 mmHg; HbA1c, per 1%; DM type, type 2 diabetes is referent. RBC in urinary sediment: red blood cells ≦5/HPF in urinary sediment is referent. Red blood cells in urinary sediment were classified as ≦5/HPF and >5/HPF. Retinopathy: Absence of diabetic retinopathy is referent. HR, hazard ratio; CI, confidence interval.
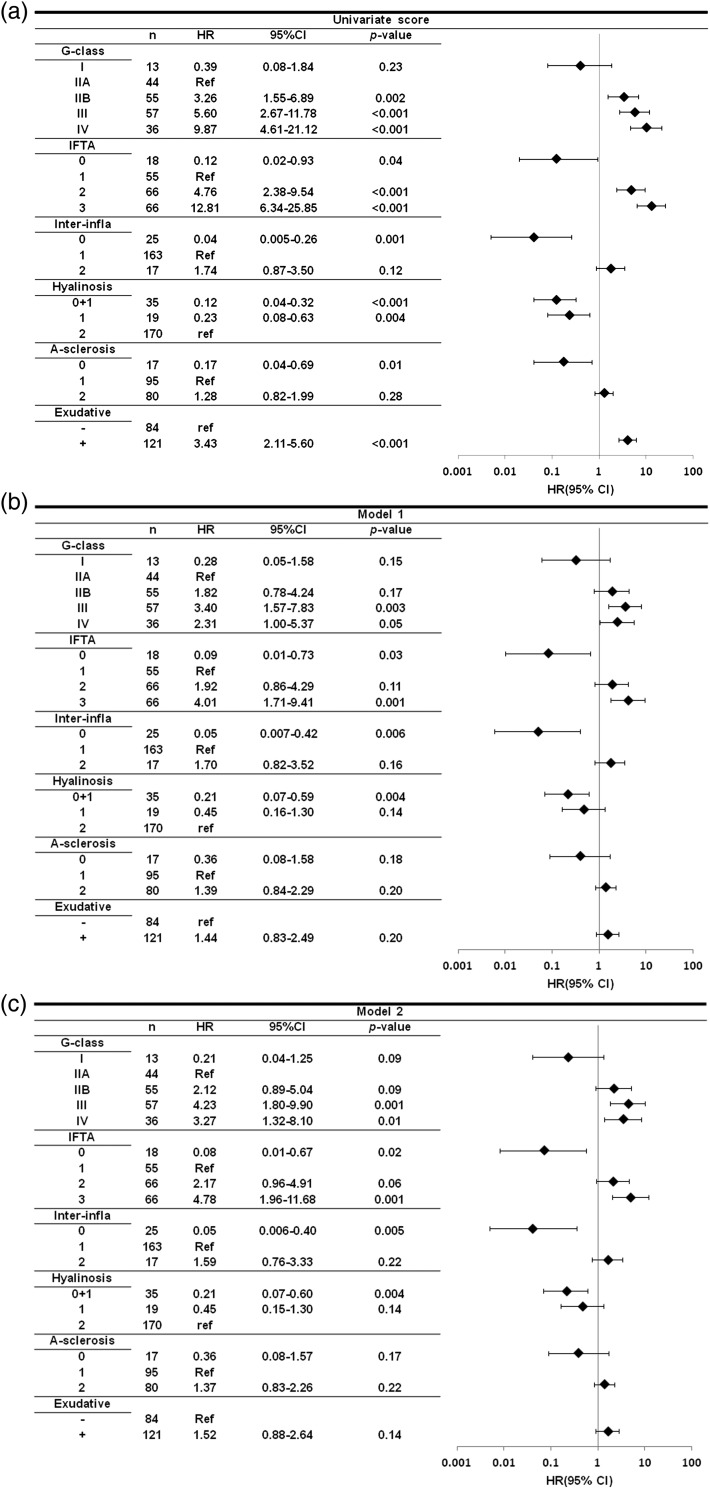
The adjusted HRs of various pathological factors for renal survival are shown in Figure 3. Compared with glomerular class IIA, the HRs for glomerular classes IIB, III and IV were more than 1.8 (corresponding to the HR for glomerular class IIB in Model 1) in both models. There were also significant differences of renal survival between glomerular class IIA and classes III or IV in both models. Compared with class IIB, the overall HR for class III was significantly higher, being 1.87 (95% CI: 1.03–3.42) in Model 1 and 1.99 (95% CI: 1.08–3.67) in Model 2. With respect to the IFTA classification, the HR for a score of 3 was significantly higher compared with that for a score of 1 in both models and the groups with higher IFTA scores had much higher HRs. In both models, higher interstitial inflammation scores, arteriolar hyalinosis scores and arteriosclerosis scores were associated with higher HRs, although there were no significant differences among some of the pairs. Only one patient with an arteriolar hyalinosis score of 0 progressed to hemodialysis at 319 months after renal biopsy. Finally, exudative lesions were associated with a higher HR in both models.
FIGURE 3:
Univariate and multivariate Cox proportional hazard models by pathological variables at renal end point. Model 1: adjusted for age, gender, estimated glomerular filtration rate, type of diabetes, urinary protein excretion, systolic blood pressure, body mass index and HbA1c at the time of renal biopsy. Model 2: adjusted for the above plus diabetic retinopathy and red blood cells in urinary sediment at the time of renal biopsy. HR: hazard ratio, 95% CI: 95% confidence interval. G-class, glomerular class; IFTA, interstitial fibrosis and tubular atrophy scores; interinfra, interstitial inflammation scores; hyalinosis, arteriolar hyalinosis scores; A-sclerosis, arteriosclerosis scores; exudative, presence of exudative lesions.
DISCUSSION
Before Tervaert's classification was proposed, some authors reported a relation between mesangial matrix volume and renal function, as well as a difference of the renal prognosis between DN with diffuse lesions and DN with nodular lesions [11–13]. With regard to tubulointerstial lesions, Ueno et al. [14] found that interstitial expansion combined with thickening of the tubular basement membrane existed before the onset of proteinuria and these changes progressed in parallel with glomerular and arteriolar lesions. White et al. [15] reported a significant correlation between an increase of the interstitial volume and decreased creatinine clearance, while proteinuria was only correlated with glomerular pathology.
After Tervaert's pathological classification of DN was proposed, Okada et al. evaluated renal biopsy specimens from 69 patients with type 2 diabetes and frank proteinuria. They found that the glomerular class was not a significant independent variable, but IFTA and interstitial inflammation were independently associated with the renal end point (HR: 3.36, 95% CI: 1.21–9.32 and HR: 4.74, 95% CI: 1.26–17.91, respectively). They also found no significant difference in renal survival between glomerular classes IIa and IIb combined (diffuse lesions) and glomerular class III (nodular lesions) [10]. In addition, Oh et al. performed renal biopsy in 126 patients with type 2 DM and frank proteinuria from January 2000 to December 2007, revealing that 50 patients had pure DN, 65 had nondiabetic renal disease (NDRN) and 11 had coexisting DN and NDRN. ESRD occurred in 44.0% of the DN group, 18.2% of the mixed group and 12.3% of the NDRD group (P < 0.001 by the χ2 test). Among patients with pure DN, the 5-year renal survival rate was estimated to be 100.0% in class I (n = 2) and class IIa (n = 6), 75.0% in class IIb (n = 12), 66.7% in class III (n = 9) and 38.1% in class IV (n = 21) (P = 0.002) [16].
In our retrospective study, IFTA and interstitial inflammation had a strong impact on the renal prognosis, as well as in Okada's study [10], and this result was also in agreement with the findings reported for other renal diseases such as IgA nephropathy and lupus nephritis [17, 18]. However, with respect to glomerular lesions, there was a significant difference in the renal survival rate between glomerular class IIA and classes IIB or III in our study, unlike the findings of Okada [10]. The reasons for this discrepancy may be that our cohort was larger and that we studied patients with worse renal function. Actually, among our 222 patients with pure DN (including those with an eGFR < 10 mL/min per 1.73 m2), there was a significant difference in the renal survival rate among each glomerular class (data not shown). Moreover, our analysis clearly showed that the HR for class III (DN with nodular lesions) was significantly higher than that for class IIB (DN with diffuse lesions), even after adjusting for other variables. On the other hand, the estimated 5-year renal survival rate in our subjects was lower for each glomerular class compared with the pure DN subjects studied by Oh et al. [16], possibly because of differences in the background factors of the patients.
This study had several limitations. First, it had a relatively small sample size and all of the subjects were Japanese. However, this study was the first investigation of the relationship between the renal prognosis and all pathological findings, including early DN (glomerular class I), unlike previous studies. Second, this was a retrospective cohort study and the indications for renal biopsy were not standardized, making it undeniable that there was selection bias in our study. Third, factors related to treatment during follow-up that could have a strong influence on the renal prognosis, such as use of renin-angiotensin inhibitors (ACE-I and ARB), glycemic control and blood pressure control, were not adequately examined and adjusted. However, there were no significant differences of ACE-I or ARB use during follow-up among the glomerular classes, except between classes I and IIA. The reason for the low rate of ACE-I or ARB use in class I was that fewer patients had hypertension in this class than in other classes, as indicated by the average systolic blood pressure during follow-up and the final number of antihypertensive agents. With respect to glycemic control during follow-up, there were no marked differences among the glomerular classes, and this was supported by the average HbA1c and hemoglobin levels during follow-up as well as the final use of erythropoietin-stimulating agents. In our study, there was no significant difference in the average HbA1c during follow-up among the glomerular classes, except for classes III and IV. The lowest hemoglobin and the highest rate of final use of erythropoietin-stimulating agents may have been associated with the significantly lower HbA1c in class IV than in the other classes. With regard to blood pressure control, average systolic and diastolic blood pressures during follow-up were lower than at baseline for each glomerular class, while the number of antihypertensive agents in use showed an increase at final follow-up. However, the average systolic blood pressure during follow-up exceeded 140 mmHg in classes III and IV, being significantly higher than in classes I, IIA and IIB. Fourth, the minimum number of glomeruli required for the classification of glomerular lesions was five in this study. Therefore, inclusion of patients with only a few glomeruli in our study may have influenced the classification of renal pathology, especially in patients who had late-stage DN such as glomerular classes III and IV because of the large number of glomeruli with global sclerosis in those classes. However, more glomeruli were obtained by biopsy in classes III or IV than in classes I, IIA or IIB (Table 1), and fewer than 10 glomeruli were rarely obtained (8 (14%) in class III and 7 (19%) in class IV). Finally, the formula used to estimate GFR in our study was derived from the Modification of Diet in Renal Disease (MDRD) Study equation [9]. According to Horio, the precision of the Japanese GFR equations used in our study was significantly better at a GFR of 0–29 mL/min per 1.73 m2, was statistically equivalent at a GFR of 30–59 mL/min per 1.73 m2 and was significantly worse at GFRs of 60–89 or 90–119 mL/min per 1.73 m2 compared with the coefficient-modified Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation based on serum creatinine (0.813 × CKD-EPIcr) [19]. This study included many patients with a GFR of <60 mL/min per 1.73 m2 (77% estimated by the Japanese GFR equations), and our estimated GFR values were valid for those patients. However, it is undeniable that the estimated GFR values for patients with less advanced CKD (GFR >60 mL/min per 1.73 m2) were of lower validity than if we had employed the coefficient-modified CKD-EPI equation.
In conclusion, we examined whether Tervaert's pathological classification could predict the renal prognosis of patients with DN. Cox proportional hazards analysis showed that HRs were much higher for higher glomerular classes and higher tubulointerstitial lesion scores. The progression of glomerular, tubulointerstitial and vascular lesions was associated with renal survival, suggesting that Tervaert's pathological classification of DN is useful for predicting the renal prognosis.
AUTHORS’ CONTRIBUTIONS
The contributions of the authors are detailed as follows: K.M. contributed to analysis and interpretation of data including pathological findings, and to writing the manuscript and collecting clinical data including follow-up. J.H. contributed to analysis and interpretation of data and writing the manuscript. Y.U. contributed to analysis and interpretation of data and writing the manuscript. K.S. contributed to managing patients and assessing data. R.H. contributed to managing patients and assessing data. E.H. contributed to managing patients and assessing data. M.Y. contributed to managing patients and assessing data. N.H. contributed to managing patients and assessing data. T.S. contributed to managing patients and assessing data. N.S. contributed to managing patients and assessing data. T.F. contributed to classification of pathological findings. K.O. contributed to classification of pathological findings. S.H. contributed to managing patients and assessing data. K.T. contributed to analysis and interpretation of data.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Okinaka Memorial Institute for Medical Research. We are grateful to Hirofumi Makino, Masakazu Haneda and Takashi Wada for their helpful comments on this study. We are also grateful to Shoji Kawatsu (The Institute for Adult Diseases, Asahi Life Foundation) and to Ayako Hakura, Masafumi Yokota, Yukio Maruyama, Tomio Onuma and Ai Terai for providing patient data and medications after renal biopsy.
REFERENCES
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 3.Cowie CC, Port FK, Wolfe RA, et al. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 4.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–1133. doi: 10.1056/NEJM199910073411506. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Shimizu M, Toyama T, et al. Clinical impact of albuminuria in diabetic nephropathy. Clin Exp Nephrol. 2012;16:96–101. doi: 10.1007/s10157-011-0508-z. [DOI] [PubMed] [Google Scholar]
- 6.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the diabetes control and complications trial/epidemiology of diabetes interventions and complications cohort. Arch Intern Med. 2011;171:412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 8.Takazakura E, Nakamoto Y, Hayakawa H, et al. Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes. 1975;24:1–9. doi: 10.2337/diab.24.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Okada T, Nagao T, Matsumoto H, et al. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 2012;17:68–75. doi: 10.1111/j.1440-1797.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 11.Perrin NE, Torbjornsdotter TB, Jaremko GA, et al. The course of diabetic glomerulopathy in patients with type I diabetes: a 6-year follow-up with serial biopsies. Kidney Int. 2006;69:699–705. doi: 10.1038/sj.ki.5000146. [DOI] [PubMed] [Google Scholar]
- 12.Hong D, Zheng T, Jia-qing S, et al. Nodular glomerular lesion: a later stage of diabetic nephropathy? Diabetes Res Clin Pract. 2007;78:189–195. doi: 10.1016/j.diabres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Heaf JG, Lokkegaard H, Larsen S. The relative prognosis of nodular and diffuse diabetic nephropathy. Scand J Urol Nephrol. 2001;35:233–238. doi: 10.1080/003655901750292024. [DOI] [PubMed] [Google Scholar]
- 14.Ueno M, Kawashima S, Nishi S, et al. Tubulointerstitial lesions in non-insulin dependent diabetes mellitus. Kidney Int Suppl. 1997;63:S191–S194. [PubMed] [Google Scholar]
- 15.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11:1667–1673. doi: 10.1681/ASN.V1191667. [DOI] [PubMed] [Google Scholar]
- 16.Oh SW, Kim S, Na KY, et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract. 2012;97:418–424. doi: 10.1016/j.diabres.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Alamartine E, Sauron C, Laurent B, et al. The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol. 2011;6:2384–2388. doi: 10.2215/CJN.01170211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh C, Chang A, Brandt D, et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011;63:865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horio M, Imai E, Yasuda Y, et al. Performance of GFR equations in Japanese subjects. Clin Exp Nephrol. 2013;17:352–358. doi: 10.1007/s10157-012-0704-5. [DOI] [PubMed] [Google Scholar]