Abstract
Backgrounds
Predicting the development of acute kidney injury (AKI) in the critical care setting is challenging. Although several biomarkers showed somewhat satisfactory performance for detecting established AKI even in a heterogeneous disease-oriented population, identification of new biomarkers that predict the development of AKI accurately is urgently required.
Methods
A single-center prospective observational cohort study was undertaken to evaluate for the first time the reliability of the newly identified biomarker semaphorin 3A for AKI diagnosis in heterogeneous intensive care unit populations. In addition to five urinary biomarkers of L-type fatty acid-binding protein (L-FABP), neutrophil gelatinase-associated lipocalin (NGAL), IL-18, albumin and N-acetyl-β-d-glucosaminidase (NAG), urinary semaphorin 3A was measured at intensive care unit (ICU) admission.
Results and conclusion
Three hundred thirty-nine critically ill adult patients were recruited for this study. Among them, 131 patients (39%) were diagnosed with AKI by the RIFLE criteria and 66 patients were diagnosed as AKI at post-ICU admission (later-onset AKI). Eighty-four AKI patients showed worsening severity during 1 week observation (AKI progression). Although L-FABP, NGAL and IL-18 showed significantly higher area under the curve (AUC)-receiver operating characteristic (ROC) values than semaphorin 3A in detecting established AKI, semaphorin 3A was able to detect later-onset AKI and AKI progression with similar AUC-ROC values compared with the other five biomarkers [AUC-ROC (95% CI) for established AKI 0.64 (0.56–0.71), later-onset AKI 0.71 (0.64–0.78), AKI progression 0.71 (0.64–0.77)]. Urinary semaphorin 3A was not increased in non-progressive established AKI, while the other biomarkers were elevated regardless of further progression. Finally, sepsis did not have any impact on semaphorin 3A while the other urinary biomarkers were increased with sepsis. Semaphorin 3A is a new biomarker of AKI which may have a distinct predictive use for AKI progression when compared with other AKI biomarkers.
Keywords: acute kidney injury, AKI progression, biomarker, mixed ICU, semaphorin 3A
INTRODUCTION
Acute kidney injury (AKI) is a frequent and serious complication in critically ill patients in intensive care unit (ICU) settings. Currently, AKI is diagnosed using serum creatinine, which is not sufficiently sensitive and specific for detecting tubular injury in AKI. Moreover, serum creatinine will only diagnose the existing renal dysfunction but cannot predict the development of AKI. Lack of early sensitive diagnostic tests for AKI is a major hindering factor for developing an effective therapy for prevention and treatment of AKI and the associated mortality. A decade of research led to the identification of several candidate biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) [1–4], interleukin-18 (IL-18) [5], L-type fatty acid-binding protein (L-FABP) [6, 7] and kidney injury molecule-1 [8] for early diagnosis of AKI in a different patient population. Although these biomarkers can detect AKI in the early phase even before the elevation of serum creatinine, their performance of predicting AKI progression has not been sufficiently evaluated and their translation into the clinic is not available so far.
In search for a new biomarker, we have recently identified and characterized a protein called semaphorin 3A which can predict the development of AKI in an experimental animal model and in a pediatric human population who underwent cardiopulmonary bypass surgery [9]. Since this cohort is relatively homogeneous in terms of fewer comorbidity and more readily apparent onset of renal insult, the necessity of evaluating the usefulness of new biomarkers with more heterogeneous populations should be addressed. Therefore, the current study was carried out to validate the newly identified biomarker semaphorin 3A as an early diagnostic test using urine samples from critically ill adult patients treated in mixed ICU.
Semaphorins, formerly known as collapsins, belong to a large family of repulsive guidance cues consisting of eight classes of secreted and membrane-bound polypeptides that have been identified in both invertebrates and vertebrates [10, 11]. Class 3 semaphorin (Sema3) are a large subgroup of these polypeptides, containing members A-G. Semaphorin 3A is expressed in the developing kidney and in distal tubular epithelial cells of the adult kidney [12]. Our animals and human studies have determined that semaphorin 3A is secreted into urine in response to hypoxia and drug treatment [9]. Since ICU patients experience multiple renal insults, we proposed that semaphorin 3A could be a promising AKI biomarker for complex clinical conditions.
MATERIALS AND METHODS
Study design and data collection
The original study with these samples was published previously [6]. This study was performed as a prospective observational study. Adult patients >20 years who had been admitted to the mixed ICU in the University of Tokyo Hospital during December 2008 to May 2009 were eligible for enrollment. Patients with end-stage renal disease or renal transplant were excluded. The study protocol was approved by the University of Tokyo institutional review board and Georgia Health Sciences University. Informed consent was obtained from each participant or the participant's family. The presence of AKI was assessed daily by calculating the change in serum creatinine from the baseline to the maximum serum creatinine on each day according to the RIFLE (Risk, Injury, Failure) criteria [13]; AKI was defined as a 50% increase from the baseline. Baseline serum creatinine was defined as the last outpatient value within 3 months before admission. For a patient with no creatinine measurement within the prior 3 months or any previously known creatinine value, the baseline was estimated using the MDRD equation for the lower end of the normal range (i.e. 75 mL/min per 1.73 m2), as the RIFLE criteria suggests [13]. The GFR was estimated using the MDRD equation [14]. The AKI severity was also categorized according to the RIFLE criteria with one exception: patients who needed renal replacement therapy (RRT) were categorized as failure because patients receiving RRT are included in stage 3 of the Acute Kidney Injury Network criteria [15]. The AKI severity was assessed for 1 week after ICU admission. Later-onset AKI was defined as follows: no AKI diagnosis was made at ICU admission, but rise in serum creatinine was seen during post-ICU admission that meets RIFLE criteria or if RRT was started within 1 week. Progression of AKI was defined as worsening of RIFLE stage (from non-AKI to AKI of any stage, from risk to either injury or failure, from injury to failure).
Diagnosis of sepsis was made according to the American College of Chest Physicians and the Society of Critical Care Medicine Consensus Conference Committee guidelines [16]. Whether the presence of sepsis would have an impact on the performances of AKI biomarkers was also evaluated.
Urinary biomarker measurement
Urine samples were collected within 12 h of ICU admission and were then frozen at −80°C within 1 h of collection. Urinary semaphorin 3A was measured using commercially available enzyme-linked immunosorbent assay kits (Cat # MBS732622, My Biosource, CA, USA). Urinary L-FABP, NGAL and IL-18 were measured using commercially available ELISA kits [Human L-FABP Assay Kit; CMIC Co. Ltd., Tokyo, Japan; NGAL ELISA Kit (KIT 036); BioPorto, Gentofte, Denmark and Human IL-18 ELISA Kit (No. 7620); MBL Co. Ltd., Nagoya, Japan]. Urinary NAG and albumin were measured at the University of Tokyo Hospital Clinical Laboratory using the 4-HP-NAG substrate method (L-Type NAG; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and immunonephelometry (Autokit Micro Albumin; Wako Pure Chemical Industries, Ltd.), respectively.
Statistical analysis
Data were expressed as median (interquartile range) and continuous variables were compared using Wilcoxon rank-sum test or Steel–Dwass test for multiple comparison. Categorical variables were compared using the Pearson chi-square or Fisher's exact test. The performance of urinary biomarkers was determined using receiver operating characteristic (ROC) curve analysis. The differences in areas of under the ROC curves were tested with a non-parametric method as described in the study by Delong et al. [17]. Bonferroni adjustments were used to account for multiple comparisons. To identify the best predictors of AKI progression, we examined the independent relationship using stepwise logistic regression analysis. These calculations were performed using JMP version 9.0 (SAS Institute, Inc., Cary, NC). A conventional criterion of level 0.05 was used to determine statistical significance.
RESULTS
Patient characteristics
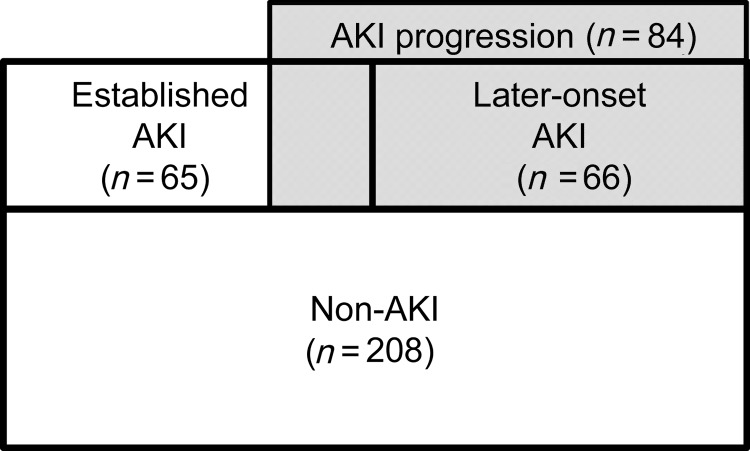
For the analysis, 339 adult ICU patients were enrolled. Table 1 presents baseline clinical data and outcomes of the enrolled patients. Of the 339 ICU patients, AKI was diagnosed in 131 (38.6%) within 1 week after ICU admission. A significantly higher serum creatinine at the time of ICU admission, lower mean blood pressure, higher APACHE II score, longer length of ICU stay and higher 14-day in-hospital mortality were observed in patients with AKI. In patients with AKI, contrast exposure within 48 h before ICU admission was less frequent than the non-AKI. This might indicate avoidance of contrast injection to high-risk patients. Complication with sepsis occurred more frequently in patients with AKI than in non-AKI patients. Of 131 patients with AKI, 66 patients were diagnosed as later-onset AKI, whereas the other 65 patients already had AKI at ICU admission (established AKI). Eighty-four patients were determined as progression of AKI, whereas the other 47 AKI patients did not show any progression (Figure 1). Of these 84 patients with AKI progression, 18 patients who showed progression from established AKI had a high mortality of 22.2%.
Table 1.
Baseline clinical data and outcomes
| Non-AKI (n = 208, 61.4%) | AKI (n = 131, 38.6%) | P-value (vs. non-AKI) | |
|---|---|---|---|
| Age | 66 0.0 [53.3–74.0] | 66.0 [55.0–73.0] | 0.650 |
| Male, n (%) | 132 (63.5) | 91 (69.5) | 0.255 |
| Admission type, n (%) | |||
| Medical | 98 (47.1) | 66 (50.4) | 0.746 |
| Elective surgery | 80 (38.5) | 45 (34.4) | |
| Emergent surgery | 30 (14.4) | 20 (15.3) | |
| Reason of ICU admission, n (%) | |||
| Cardiac surgery | 66 (31.7) | 52 (39.7) | 0.160 |
| Neurosurgery | 16 (7.7) | 0 (0.0) | 0.0003 |
| Other surgery | 27 (13.0) | 16 (12.2) | 0.869 |
| Cardiology | 45 (21.6) | 25 (19.1) | 0.680 |
| Other medicine | 30 (14.4) | 29 (22.1) | 0.078 |
| Others | 24 (11.5) | 9 (6.9) | 0.190 |
| Location before ICU admission, n (%) | |||
| General ward | 126 (60.6) | 76 (58.0) | 0.896 |
| Emergency room | 71 (34.1) | 48 (36.6) | |
| Others | 11 (5.3) | 7 (5.3) | |
| Baseline Cre (mg/dL) | 0.78 [0.64–0.99] | 0.88 [0.65–1.05] | 0.105 |
| Baseline eGFR (mL/min/1.73 m2) | 68.1 [53.2–81.6] | 65.1 [48.9–80.5] | 0.3652 |
| eGFR < 30, n (%) | 6 (3.9) | 11 (12.2) | 0.014 |
| Unknown, n (%) | 54 (26.4) | 41 (31.3) | 0.334 |
| Cre at admission (mg/dL) | 0.78 [0.64–1.01] | 1.28 [0.94–1.98] | <0.0001 |
| Diabetes, n (%) | 57 (27.4) | 37 (28.2) | 0.866 |
| Contrast exposure preceding 48 h, n (%) | 76 (36.5) | 27 (20.6) | 0.002 |
| Mean blood pressure (mmHg) | 70.0 [66.7–77.3] | 68.0 [58.3–73.3] | 0.001 |
| APACHE II | 10.0 [7.0–13.0] | 15.0 [10.0–22.0] | <0.0001 |
| Sepsis, n (%) | 30 (14.4) | 36 (27.5) | 0.0031 |
| 14-day in-hospital mortality, n (%) | 2 (1.0) | 12 (9.2) | 0.0003 |
| ICU stay (day) | 3.0 [3.0–5.3] | 5.0 [3.0–9.0] | <0.0001 |
Cre, serum creatinine concentration; eGFR, estimated glomerular filtration rate. Values are presented as proportions or median [interquartile range].
FIGURE 1:
Later-onset AKI and AKI progression. Eighteen established AKI and all the later-onset AKI (n = 66) were determined as AKI progression (n = 84). Fourteen-day in-hospital mortality rate for each group is as follows: non-AKI 1.0%, established AKI without progression 8.5%, established AKI with further progression 22.2% and later-onset AKI 6.1%.
Semaphorin 3A and AKI
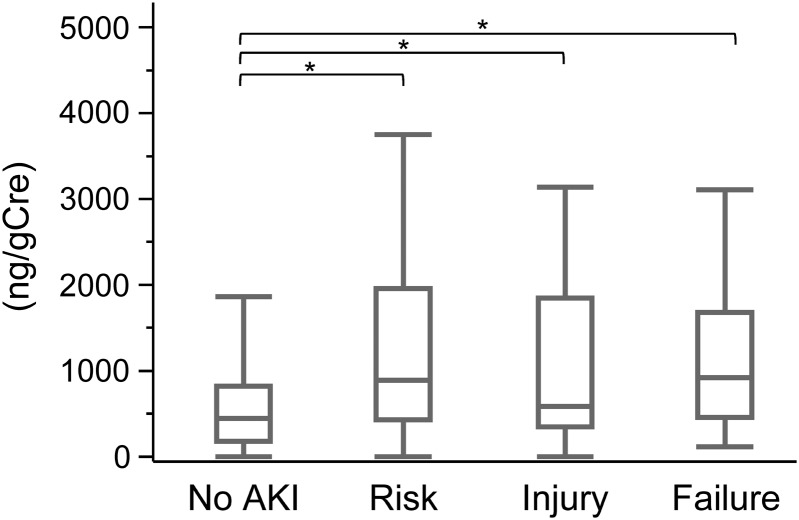
Urinary semaphorin 3A measured at ICU admission was significantly higher in patients with AKI than in non-AKI patients as other urinary markers of L-FABP, NGAL, IL-18, NAG and albumin (Table 2). The ROC curve analysis for detecting AKI revealed that the area under the curve of ROC curve (AUC-ROC) for semaphorin 3A was 0.68 (95% confidence interval 0.62–0.74) which was similar to the other five biomarkers. There was no significant difference of AUC-ROC values among the measured urinary markers. Semaphorin 3A values were compared with the severity of AKI determined using the RIFLE criteria (Figure 2). Although urinary semaphorin 3A levels in each RIFLE group were significantly higher than the non-AKI group, they were not significantly different among the AKI groups indicating that urinary semaphorin 3A was not able to reflect the AKI severity.
Table 2.
Urinary biomarkers for total AKI detection
| Non-AKI (n = 208, 61.4%) | AKI (n = 131, 38.6%) | P value (vs. non-AKI) | AUC-ROC [95% CI] | |
|---|---|---|---|---|
| Semaphorin 3A (ng/gCre) | 441.5 [166.0–844.0] | 873.5 [404.8–1818.2] | <0.0001 | 0.68 [0.62–0.74] |
| L-FABP (μg/gCre) | 14.7 [3.4–72.4] | 131.3 [26.7–628.1] | <0.0001 | 0.75 [0.69–0.80] |
| NGAL (μg/gCre) | 39.5 [15.1–143.0] | 171.2 [51.3–684.6] | <0.0001 | 0.72 [0.66–0.77] |
| IL-18 (ng/gCre) | 134.4 [63.8–352.2] | 445.1 [180.8–1107.4] | <0.0001 | 0.74 [0.68–0.79] |
| Alb (mg/gCre) | 87.6 [42.1–189.2] | 277.3 [116.8–658.3] | <0.0001 | 0.74 [0.68–0.79] |
| NAG (IU/gCre) | 19.9 [9.5–41.9] | 38.1 [21.6–71.4] | <0.0001 | 0.67 [0.61–0.73] |
Values are presented as median [interquartile range].
AUC; area under the curve.
FIGURE 2:
Urinary biomarker semaphorin 3A values grouped by acute kidney injury (AKI) severity. Values of semaphorin 3A measured at ICU admission are shown in each AKI severity category [no AKI (n = 208), risk (R) (n = 54), injury (I) (n = 33) and failure (F) (n = 44)]. Boxes enclose the range of lower to upper quartile values; lines inside the boxes represent the median values. Error bars represent the lowest datum still within 1.5 interquartile range of the lower quartile and the highest datum still within 1.5 interquartile range of the upper quartile, *P < 0.05.
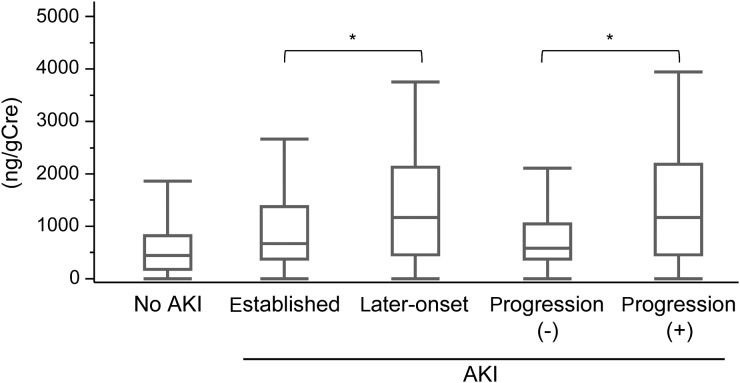
Comparisons of AUC-ROC for detecting established AKI revealed that semaphorin 3A showed significantly lower AUC-ROC values when compared with L-FABP, NGAL and IL-18 (Table 3). However, urinary semaphorin 3A showed similar performance for later-onset AKI and AKI progression compared with other five biomarkers. It is noteworthy that urinary semaphorin 3A in the later-onset AKI and the AKI progression groups was significantly higher than the established AKI and the non-progression groups, respectively (Figure 3). Moreover, urinary semaphorin 3A in 47 established AKI patients who did not show any progression of AKI severity was as low as non-AKI, whereas the other five markers were significantly increased in these 47 patients (Table 4). These observations may suggest that urinary semaphorin 3A has a distinct characteristic from the other five markers. It should be noted that 95% confidence interval ranges of AUC-ROC values for the three categories of AKI (established AKI, later-onset AKI and AKI progression) overlapped in all the six urine biomarkers (Table 3).
Table 3.
AUC-ROC values for established AKI, later-onset AKI and AKI progression
| Established AKI (n = 65) | Later-onset AKI (n = 66) | AKI progression (n = 84) | |
|---|---|---|---|
| Semaphorin 3A (ng/gCre) | 0.64 [0.56–0.71] | 0.71 [0.64–0.78] | 0.71 [0.64–0.77] |
| L-FABP (μg/gCre) | 0.76 [0.69–0.82]# | 0.73 [0.66–0.79] | 0.71 [0.64–0.77] |
| NGAL (μg/gCre) | 0.78 [0.70–0.84]# | 0.66 [0.59–0.73] | 0.66 [0.59–0.73] |
| IL-18 (ng/gCre) | 0.78 [0.71–0.84]# | 0.70 [0.62–0.77] | 0.68 [0.62–0.75] |
| Alb (mg/gCre) | 0.78 [0.70–0.84] | 0.70 [0.62–0.77] | 0.68 [0.59–0.72] |
| NAG (IU/gCre) | 0.67 [0.60–0.74] | 0.68 [0.60–0.74] | 0.66 [0.59–0.72] |
| Serum creatinine at ICU admission (mg/dl) | 0.80 [0.75–0.84] | 0.68 [0.61–0.75] | 0.64 [0.57–0.70] |
Values are presented as AUC-ROC [95% confidence interval]. #P<0.05 vs. Semaphorin 3A.
AUC; area under the curve.
FIGURE 3:
The urinary biomarker semaphorin 3A in later-onset AKI and AKI progression. Values of semaphorin 3A in the later-onset AKI and the AKI progressors were significantly higher than the established AKI and the non-AKI progressors, respectively. Boxes enclose the range of lower to upper quartile values; lines inside the boxes represent the median values. Error bars represent the lowest datum still within 1.5 interquartile range of the lower quartile and the highest datum still within 1.5 interquartile range of the upper quartile, *P < 0.05.
Table 4.
Urinary biomarkers in established AKI without further progression
| Non-AKI (n = 208, 61.4%) | Established AKI without progression (n = 47, 13.8%) | AKI progression (n = 84, 24.8%) | |
|---|---|---|---|
| Semaphorin 3A (ng/gCre) | 441.5 [166.0–844.0] | 586.7 [354.6–1063.6] | 1157.7 [453.4–2185.9]*# |
| L-FABP (μg/gCre) | 14.7 [3.4–72.4] | 79.4 [30.6–723.6]# | 154.6 [23.2–623.7]# |
| NGAL (μg/gCre) | 39.5 [15.1–143.0] | 278.9 [56.4–934.0]# | 151.1 [51.1–665.4]# |
| IL-18 (ng/gCre) | 134.4 [63.8–352.2] | 472.5 [177.1–1088.3]# | 427.2 [181.4–1132.5]# |
| Alb (mg/gCre) | 87.6 [42.1–189.2] | 269.7 [162.5–759.1]# | 290.4 [111.4–629.8]# |
| NAG (IU/gCre) | 19.9 [9.5–41.9] | 35.4 [21.2–49.9]# | 41.9 [21.7–86.5]# |
Values are presented as median [interquartile range].
*P < 0.05 versus established AKI without progression; #P < 0.05 versus non-AKI.
To clarify the contribution to AKI progression for each parameter, stepwise logistic regression analysis incorporating mean arterial pressure and serum creatinine at ICU admission, complication of sepsis and the six urinary biomarkers were conducted. Results revealed that among those parameters mean arterial pressure, urinary L-FABP and semaphorin 3A were significantly associated with AKI progression (Table 5).
Table 5.
Multiple logistic analysis for AKI progression
| Variable | β (SE) | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Mean arterial pressure (mmHg) | −0.03 (0.01) | 0.97 (0.95–0.99) | 0.0171 |
| Log10 L-FABP (μg/gCre) | 0.25 (0.06) | 1.29 (1.14–1.47) | <0.0001 |
| Semaphorin 3A (μg/gCre) | 0.23 (0.11) | 1.26 (1.04–1.59) | 0.0168 |
SE, standard error; CI, confidence interval.
Semaphorin 3A and sepsis
Sepsis-induced complications were frequently found in the AKI group when compared with the non-AKI group (Table 1). Whereas five urinary biomarkers (L-FABP, NGAL, IL-18, NAG and albumin) in the sepsis group were significantly increased when compared with the non-sepsis group, urinary semaphorin 3A did not differ between septic and non-septic patients (Table 6). We further divided patients into the four groups with or without AKI. All biomarkers except semaphorin 3A in the AKI patients complicated with sepsis (AKI/sepsis) showed the highest values. AKI did not increase urinary semaphorin 3A in septic patients, although the sample size of these subgroups was not sufficiently large (Supplementary data, Table S1).
Table 6.
Urinary biomarkers and sepsis
| Non-sepsis (n = 273, 80.5%) | Sepsis (n = 66, 19.5%) | P value (vs. non-sepsis) | |
|---|---|---|---|
| Semaphorin 3A (ng/gCre) | 573.5 [206.2–1251.7] | 513.1 [318.9–1180.1] | 0.8600 |
| L-FABP (μg/gCre) | 26.4 [4.7–166.6] | 62.6 [21.6–376.3] | 0.0016 |
| NGAL (μg/gCre) | 48.1 [17.8–167.1] | 395.9 [102.6–2040.3] | <0.0001 |
| IL-18 (ng/gCre) | 160.2 [70.6–423.8] | 703.3 [278.8–1734.3] | <0.0001 |
| Alb (mg/gCre) | 109.4 [47.1–291.7] | 285.4 [105.0–476.0] | <0.0001 |
| NAG (IU/gCre) | 22.4 [11.0–46.5] | 41.4 [26.5–74.0] | <0.0001 |
Values are presented as median [interquartile range].
DISCUSSION
This is the first study to demonstrate that urinary excretion of semaphorin 3A is a predictive biomarker of human AKI, especially for later-onset AKI and AKI progression. While other urinary biomarkers of L-FABP, NGAL and IL-18 showed significantly higher AUC-ROC values than semaphorin 3A in detecting established AKI at ICU admission, semaphorin 3A showed similar AUC-ROC values for predicting acute decline of renal function after ICU admission. Moreover, in contrast to other urinary biomarkers, semaphorin 3A was not influenced by sepsis. These observations suggest the distinct characteristics of urinary semaphorin 3A as an AKI biomarker.
Although semaphorins are originally reported as being responsible solely for the axonal guidance in the developing nervous system [10], recent evidence suggests that they are pleiotropic in nature, fulfilling many additional roles including the regulation of angiogenesis, organogenesis, tumorogenesis, cell migration, cytokine release and immune modulation [12, 18–20]. However, despite the increasing evidence regarding the different functions of the semaphorins within the human body, the complete in vivo biological role of these molecules still remains unclear. The role of semaphorin 3A in kidney pathophysiology is unknown. Animal study using mice revealed that semaphorin 3A is not expressed in proximal tubular epithelial cells and glomerular mesangial cells but highly expressed in the distal tubular and collecting duct epithelial cells as well as podocytes in normal physiological condition. Semaphorin 3A is undetectable in normal urine. In a model of ischemia reperfusion, urinary semaphorin 3A can be detected within a few hours after reperfusion [9]. This suggests that urinary semaphorin 3A can detect renal hypoxic injury. Administration of recombinant semaphorin 3A induced proteinuria through disruption of podocyte foot process [21]. Semaphorin 3A is known to have anti-angiogenic molecules but whether it regulates angiogenesis is unknown. In addition, semaphorin 3A is known to regulate cell migration and adhesion. Since epithelial cell proliferation and migration are known to occur immediately after acute injury, it is possible that semaphorin may be involved in these processes.
AKI progression is frequently observed in the ICU in the context of development of multiple organ failure [22]. Although the usefulness of biomarkers has been widely validated for early detection of AKI, only a few studies were done for predicting AKI progression in the clinical settings. Considering the development for new AKI therapeutics, biomarkers for AKI progression will be useful to identify the high risk and most beneficial patients. Koyner et al. [23] evaluated whether AKI biomarkers measured at the time of AKI diagnosis after cardiac surgery can predict AKI worsening. Urinary IL-18, albumin and plasma NGAL were associated with AKI progression. A recent multicenter study has evaluated two additional biomarkers [tissue inhibitor of metalloprotease-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGFBP-7)] in a populations of critically ill patients including sepsis, shock, major surgery and trauma. These two biomarkers have been shown to be superior to other established biomarkers [24]. Again, AKI progression was not examined in that study. Our study evaluated AKI progression in a more heterogeneous population of an adult mixed ICU cohort and demonstrated for the first time that urinary semaphorin 3A could predict later-onset AKI and AKI progression after ICU admission.
Urinary semaphorin 3A failed to detect the severity of AKI defined by the RIFLE criteria, whereas the other biomarkers, especially L-FABP, could discriminate the severity of AKI in this cohort [6]. It should be noticed that the severity of AKI in this study depended on the maximum serum creatinine levels within 1 week after ICU admission. Although the peak serum creatinine values might be correlated with the degree of tubular injury in AKI in general, it could not directly reflect tubular epithelial cell injury. It is possible that semaphorin 3A in the urine detected medullary hypoxic injury that is not closely related to a decrease in glomerular filtration. Reportedly, urinary L-FABP can also detect hypoxia in proximal tubular cells [25, 26]. Urinary L-FABP could detect established AKI better than semaphorin 3A possibly because L-FABP is highly induced in response to injuries which occurred at the renal cortico-medullary junction, a hypoxia prone region of the kidney. Further investigation is necessary to clarify the role of these two biomarkers in detecting renal hypoxia in humans. Another possibility is that urinary semaphorin 3A was saturated even in the mild AKI of risk. Although the regulatory mechanism of semaphorin 3A expression induced by renal insult is unclear, there might be an upper limit of up-regulation of semaphorin 3A by acute stress signals. Consistent with this observation, we see a similar result even in pediatric patients where higher levels of semaphorin 3A is seen in a risk group (RIFLE criteria) when compared with injury or failure group [9].
Another distinct feature of urinary semaphorin 3A is that sepsis did not affect its levels. Reportedly, urinary and blood NGAL can be influenced by sepsis [27, 28]. Urinary L-FABP in septic shock patients was extraordinarily high [29]. Although septic AKI is more severe than other AKI such as contrast-induced or post-cardiac surgery AKI and biomarkers that respond both to sepsis and AKI will be helpful for predicting mortality, biomarkers that are not affected by sepsis may be used for evaluation of kidney injury in severe multiple organ failure. Our data indicate urinary semaphorin 3A might be able to monitor renal injury independent of severity of the systemic illness.
Our study has strengths. First, we have identified a new biomarker for AKI and validated it in human samples. Secondly, we prospectively recruited a relatively heterogeneous cohort of adult subjects with various different etiologies for AKI and no apparent onset. Even with this heterogeneous cohort, urinary semaphorin 3A was useful for AKI diagnosis. Thirdly, we demonstrated that urinary semaphorin 3A can show a superior performance not only in detecting established AKI but also in predicting AKI progression as other AKI biomarkers such as NGAL [1–3], IL-18 [5], L-FABP [6, 7] and kidney injury molecule-1 [8]. These biomarker-based tests are currently not available for standardized clinical application, and all biomarkers have individual strengths and weaknesses. Given the multifactorial etiologies of AKI [30], emerging AKI biomarker panels will enable the timely initiation of interventions such as atrial natriuretic peptide [31] and insulin-like growth factor [32] that have been successful in smaller, phase II-level efficacy studies but not in larger phase. III trials. Possibly, these interventions will be successful in the early stages of AKI that are predicted to progress (e.g. based on semaphorin 3A measurement) in combination with other early diagnostic biomarkers (e.g. NGAL and L-FABP) rather than serum creatinine based late intervention.
The current study has limitations. Diagnosis of AKI and AKI progression depend on the baseline serum creatinine. Measured baseline serum creatinine was not available for 95 patients (28.0%) in this study. Although the RIFLE criteria recommends estimating the baseline value using the MDRD equation for the lower end of the normal range (i.e. 75 mL/min per 1.73 m2), this method might cause misclassification of AKI diagnosis [33]. Secondly, causes of AKI were not clearly determined in this study because most of the AKI patients had complications due to several different potential causes such as sepsis, hypovolemia and exposure to nephrotoxins. Thirdly, it is a single-center study. Thus, these results will need to be validated in a larger population. Fourthly, we have not measured serum semaphorin 3A levels in this cohort of patients to determine whether semaphorin 3A level in serum also has a predictive value for AKI. Due to its size (95 KDa) and distal tubular expression pattern, urinary semaphorin 3A is unlikely to be derived from glomerular filtration. Finally, we measured urinary biomarkers at one single time point. Sequential measurement should be performed in the future study because it will remarkably improve the performance of biomarkers.
CONCLUSION
We evaluated a new biomarker of urinary semaphorin 3A in comparison with other AKI biomarkers of urinary NGAL, L-FABP, IL-18, NAG and albumin. Urinary L-FABP showed good performances in detecting established AKI and predicting later-onset AKI and AKI progression. While these five biomarkers showed similar performance in detecting established AKI, later-onset AKI and AKI progression, urinary semaphorin 3A showed equal AUC-ROC values to the five biomarkers only in predicting later-onset AKI and AKI progression. Urinary semaphorin 3A in non-progressive established AKI patients was not increased, while other five markers in established AKI were elevated regardless of future progression. Finally, sepsis did not have any impact on urinary semaphorin 3A. These observations differentiate semaphorin 3A from other biomarkers and suggest the possible contribution of semaphorin 3A to the development of emerging AKI biomarker panels.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST
Dr Ramesh and Georgia Regents University have submitted a provisional patent application for the use of urine semaphorin 3A for the early diagnosis of AKI. All other authors declared no competing interest.
Supplementary Material
ACKNOWLEDGEMENTS
G.R. is supported by an R01 grant from NIH-NIDDK (1R01DK083379-01A3) and a startup grant from Georgia Regents University.
REFERENCES
- 1.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 3.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 5.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Negishi K, Ishizu T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- 7.NOIRI E, Doi K, Negishi K, et al. Urinary fatty acid binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296:F669–679. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Jayakumar C, Ranganathan P, Devarajan P, et al. Semaphorin 3A is a new early diagnostic biomarker of experimental and pediatric acute kidney injury. PLoS ONE. 2013;8:e58446. doi: 10.1371/journal.pone.0058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 11.Roth L, Koncina E, Satkauskas S, et al. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reidy K, Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci. 2003;116:3463–3470. doi: 10.1242/jcs.00674. [DOI] [PubMed] [Google Scholar]
- 20.Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. doi: 10.1023/a:1026579711033. [DOI] [PubMed] [Google Scholar]
- 21.Tapia R, Guan F, Gershin I, et al. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2007;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 22.Russell JA, Singer J, Bernard GR, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28 doi: 10.1097/00003246-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashani K, Al Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, NOIRI E, Ono Y, et al. Renal L-type fatty acid-binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 26.Doi K, Katagiri D, Negishi K, et al. Mild elevation of urinary biomarkers in prerenal acute kidney injury. Kidney Int. 2012;82:1114–1120. doi: 10.1038/ki.2012.266. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw S, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intens Care Med. 2010;36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1927–1030. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi K, NOIRI E, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38:2037–2042. doi: 10.1097/CCM.0b013e3181eedac0. [DOI] [PubMed] [Google Scholar]
- 30.Shusterman N, Strom BL, Murray TG, et al. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83:65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- 31.Allgren RL, Marbury TC, Rahman SN, et al. Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med. 1997;336:828–834. doi: 10.1056/NEJM199703203361203. [DOI] [PubMed] [Google Scholar]
- 32.Hirschberg R, Kopple J, Lipsett P, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 33.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.