Abstract
Background
Given the nephrotoxicity of calcineurin inhibitors (CNIs), we asked whether their addition improved living related donor (LRD) human leukocyte antigen (HLA) identical kidney transplant recipient outcomes.
Methods
We performed a comprehensive literature review and a single-center study comparing patient survival (PS) and graft survival (GS) of LRD HLA-identical kidney transplants for three different immunosuppression eras: Era 1 (up to 1984): anti-lymphocyte globulin (ALG) induction and maintenance immunosuppression with prednisone and azathioprine (AZA) (n = 114); Era 2a (1984–99): CNI added; evolution from ALG to thymoglobulin; AZA to mycophenolate (n = 262). Era 2b (1999–2011): rapid discontinuation of prednisone (thymoglobulin induction, CNI and mycophenolate) in recipients having first or second transplant and not previously on prednisone (n = 77).
Results
Demographics differed by era: recipient (P < 0.0001) and donor age (P < 0.0001) increased and the proportion of Caucasian donors (P = 0.02) and recipients (P = 0.003) decreased with each advancing era. There was no significant difference in PS (P = 0.6); cause of death (P = 0.5); death-censored GS (P = 0.8) or graft loss from acute rejection by era. Graft loss from chronic allograft nephropathy (P = 0.02) and hypertension (P = 0.005) were greater in the CNI eras. There were no significant differences in the 1/creatinine slopes between eras for the first (P = 0.6), second (P = 0.9) or >2 years post-transplant (P = 0.4). Literature review revealed no clear benefits for CNI in these human leukocyte antigen (HLA) identical LRD graft recipients.
Conclusions
This study confirmed that there are no benefits of CNIs for HLA-identical LRD recipients. Moreover, we did find evidence of potential harm. Thus, monotherapy or early discontinuation of CNI should be given consideration in these patients.
Keywords: calcineurin inhibitors, cyclosporine, HLA identical, renal transplant, tacrolimus
INTRODUCTION
Since the early days of kidney transplantation, recipients of a living related donor (LRD) HLA-identical kidney grafts have had excellent short- and long-term outcomes [1–3]. From the 1950s when the first transplants were performed, there have been major advances in immunosuppression, among the most important, the discovery of calcineurin inhibitors (CNIs) including cyclosporine (CSA) and, later, tacrolimus (TAC). While providing substantial improvements in short- and long-term graft survival [4, 5], CNIs have also been implicated as the cause of irreversible histological changes that contribute toward late renal allograft loss [6, 7]. Likewise, CNI, at least in part, may be responsible for the substantial incidence of serious kidney disease, which develops several years after non-renal organ transplantation [8].
Since the outcome of LRD HLA-identical kidney transplantation was excellent prior to the availability of CNI, we asked whether the addition of CNI improved outcomes in these recipients. Graft rejection and a significant association between the presence of pre-transplant lymphocytotoxic antibodies and graft outcomes in HLA-identical transplant recipients [9] are proof that immunosuppression is required even in this cohort of patients. However, how much and what kind of immunosuppression is required, is an incompletely resolved issue. An in-depth review of the literature is presented here and, given that our experience with LRD HLA-identical kidney transplantation represents by far the largest such cohorts studied to date with the longest follow-up, we considered that analysis of outcomes in our recipients would help clarify this question.
STUDY SUBJECTS AND METHODS
A database of prospectively recorded demographics and outcomes data for all kidney transplants performed at the University of Minnesota (U of MN) since the program's inception was used to conduct a retrospective cohort study of all recipients of HLA-identical LRD grafts. Recipients were categorized as to whether or not they received CNI (with or without steroids) and were followed until graft loss/patient death or loss to follow-up. Patients with technical graft losses and those receiving Campath induction were excluded. The U of MN Institutional Review Board approved the study, including the waiver of informed consent (study number: 1006E84573).
Immunosuppressive protocols by era
Era 1 (up to 1984): anti-lymphocyte globulin induction and maintenance immunosuppression with steroids and azathioprine (AZA).
Era 2a (1984–99): CSA was added to the above protocol in 1984. The Era 2a protocol was further modified for most transplant recipients in 1996 to thymoglobulin induction with steroids, mycophenolate mofetil (MMF) and TAC maintenance.
Era 2b (1999–2011): steroid avoidance with thymoglobulin induction and CNI and MMF maintenance in patients with first or second transplants who were not on steroids at the time of transplant. Twenty-two patients on steroids as maintenance immunosuppression were excluded to avoid bias by indication including re-transplants (n = 10, three receiving their third graft), previous lung transplant recipient (n = 1), glomerular diseases or undocumented reasons (n = 11).
Literature review and data extraction
Full reports of clinical trials were searched via PubMed (http://www.ncbi.nlm.nih.gov) for the identification of eligible trials with the following MeSH terms: ‘cyclosporine’, ‘tacrolimus’, ‘kidney transplantation’ and ‘HLA identical’. No prospective or randomized or multicenter trials comparing CNI-inclusive versus CNI-free protocols were identified. Therefore, all available single-center studies comparing kidney transplant recipient and/or patient outcomes in CNI-inclusive versus CNI-free protocols were included and reviewed in detail.
Statistical analysis
The Chi-square test was used for nominal variables. Actuarial graft, acute rejection free and patient survival rates were computed by the Kaplan–Meier method. Graft, acute rejection free and patient survival were compared for patients in each era by log-rank analysis. Acute rejection was a categorical yes/no variable defined as yes if the patient was treated for rejection and no if not. In almost all cases, this diagnosis was based on allograft renal biopsy. Causes of graft loss were classified as acute rejection, non-compliance, recurrent disease, death with function and biopsy-proven chronic allograft nephropathy (CAN) more recently referred to as interstitial fibrosis and tubular atrophy (IF/TA) [10]. Hypertension was defined as patients requiring blood pressure medication since we did not have actual blood pressure measurements in the database and the definition of hypertension has changed over time. Slopes of 1/creatinine (Cr) versus time were plotted from serum Cr values at the time of discharge from transplant surgery to graft loss or patient death in each era. Slopes were compared for the first year, second year, and >2 years post-transplant for each era using the Kruskal–Wallis test. P-values <0.05 were considered statistically significant. All statistical analyses were performed using the SAS™ Software Version 9.2, Cary, NC, USA.
RESULTS
One hundred and fourteen HLA-identical LRD kidney transplant recipients in Era 1 (pre-CNI), 262 in Era 2a (CNI and steroids) and 77 in Era 2b (CNI with steroid-sparing regimens) met the study entry criteria; 35–40% patients in all eras were female (Table 1). Peak panel reactive antibody (PRA) was similar between eras. Transplant recipient and donor age increased with each advancing era (P < 0.0001 for both, Table 1). Two donors were <18 years of age and had a judge giving approval to these minors being donors on the grounds that emotional consequences to the HLA-identical donor for the potential loss of their sibling exceeded the risks of kidney donation. There were proportionally fewer Caucasian donors (P = 0.02) and recipients (P = 0.003) in the most recent era (Table 1). Although there were proportionally more patients with pre-transplant diabetes mellitus (DM) in Era 2a (47%) and 2b (30%) than Era 1 (21%) (P < 0.0001), DM was more commonly the cause of end-stage renal disease (ESRD) in Era 1 (46%) versus Era 2b (27%, P = 0.01).
Table 1.
Demographic characteristics by era
| Era | Era 1: pre-CNI (1963–83), n = 114 | Era 2a: CNI + steroids (1984–99), n = 262 | Era 2b: steroid-free CNI (1999–2011), n = 77 | P-value |
|---|---|---|---|---|
| Females (%) | 40 (35) | 93 (35) | 31 (40) | 0.7 |
| Recipient age (years): mean (range) | 31.7 (11.8–56.6) | 38.0 (15.0–74.8) | 42.1 (11.9–62.8) | <0.0001 |
| Donor age (years): mean (range) | 31.5 (15.5–54.8) | 37.0 (15.7–68.9) | 41.4 (18.8–59.6) | <0.0001 |
| Recipient race | 0.003 | |||
| Caucasian | 113 (99) | 256 (98) | 68 (88) | |
| Black | 0 | 1 (0.4) | 2 (2.5) | |
| Donor race (%) | 0.02 | |||
| Caucasian | 112 (98) | 255 (97) | 68 (88) | |
| Black | 0 | 2 (0.8) | 2 (2.5) | |
| Primary transplants | 108 (95) | 235 (90) | 69 (90) | 0.4 |
| ESRD etiology (%) | <0.0001 | |||
| Diabetes | 52 (46) | 108 (41) | 21 (27) | |
| FSGS | 1 (0.9) | 9 (3) | 4 (5) | |
| Glomerulonephritis | 32 (28) | 65 (25) | 20 (26) | |
| Anatomic/obstructive | 14 (12) | 38 (15) | 18 (23) | |
| Peak PRA (%) | 0.6 | |||
| 0% | 79 (69) | 199 (76) | 59 (77) | |
| 1–50% | 23 (20) | 42 (16) | 14 (18) | |
| 51–100% | 12 (11) | 21 (8) | 4 (5) | |
Patient survival
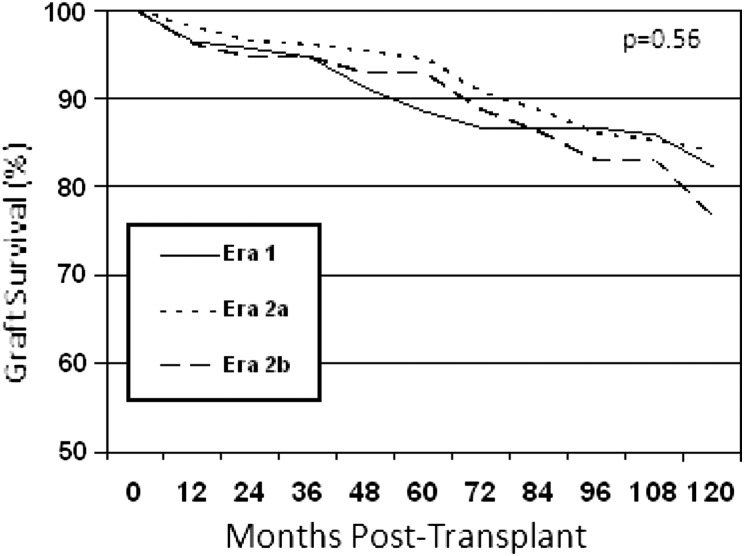
There were no statistically significant differences in patient survival rates in Eras 1, 2a and 2b (P = 0.6, Figure 1 and Table 2). Since donors and recipients were older in the later eras, transplant recipients aged 18–50 years at the time of transplant were compared. Patient survival rates at 1, 5 and 10 years were similar in Era 1 (98, 95 and 85%) and 2a (98, 91 and 88%). Data in Era 2b were insufficient for analysis.
FIGURE 1:
Patient survival by era.
Table 2.
Patient survival by era
| Era | Era 1: pre-CNI (1963–83), n = 114 | Era 2a: CNI + steroids (1984–99), n = 262 | Era 2b: steroid-free CNI (1999–2011), n = 77 |
|---|---|---|---|
| 1 year | 96% (n = 110) | 98% (n = 255) | 96% (n = 71) |
| 5 years | 89% (n = 100) | 95% (n = 243) | 93% (n = 46) |
| 10 years | 82% (n = 92) | 84% (n = 214) | Not sufficient for analysis |
| 20 years | 60% (n = 51) | 65% (n = 75) | N/A |
P = 0.56.
There were no significant differences in causes of death in the various eras (P = 0.5), but there was a numerical trend toward higher rates of death from infection with each advancing era: 3/64 (5%) in Era 1, 14/90(16%) in Era 2a and 2/10 (20%) in Era 2b. There were more cardio-vascular deaths in Era 1 (22/64 = 34%) versus Era 2a (16/90 = 18%, P = 0.01). There was no difference in rates of death with a functioning graft in Era 1 (47/64; 73% of the deaths) versus Era 2a (59/90; 66%; P = 0.3) and Era 2b (7/10; 70%; P = 0.8) (Table 4). Owing to the potential for lead-time bias and less follow-up time with each advancing era, and the smaller numbers in the most recent era, these results should be interpreted with caution.
Table 3.
Death-censored graft survival by era
| Era | Era 1: pre-CNI (1963–83), n = 114 | Era 2a: CNI + steroids (1984–99), n = 262 | Era 2b: steroid-free CNI (1999–2011), n = 77 |
|---|---|---|---|
| 1 year | 98% (n = 108) | 99% (n = 252) | 100% (n = 71) |
| 5 years | 96% (n = 98) | 93% (n = 228) | 94% (n = 43) |
| 10 years | 86% (n = 83) | 88% (n = 197) | Not sufficient for analysis |
| 20 years | 76% (n = 58) | 73% (n = 66) | N/A |
| 30 years | 74% (n = 21) | N/A | N/A |
P = 0.8.
Table 4.
Causes of graft loss by era
| Era | Era 1: pre-CNI (1963–83), n = 72 | Era 2a: CNI + steroids (1984–99), n = 117 | Era 2b: steroid-free CNI (1999–2011), n = 12 | P-value |
|---|---|---|---|---|
| Causes of graft loss | ||||
| Acute rejection | 0 (0%) | 3 (3%) | 0 (0%) | 0.4 |
| CAN | 8 (11%) | 29 (25%) | 4 (33%) | 0.02 |
| Recurrent disease | 10 (14%) | 8 (7%) | 1 (9%) | 0.2 |
| Non-compliance | 2 (3%) | 12 (10%) | 0 (0%) | 0.1 |
| Death with function | 47 (65%) | 59 (50%) | 7 (58%) | 0.1 |
| Other | 5 (7%) | 6 (5%) | 0 | 0.9 |
CAN, chronic allograft nephropathy including chronic rejection and CNI toxicity.
Graft function
There were no differences in the 1/Cr slopes between the various eras for the first (P = 0.6), second (P = 0.9) or >2 (P = 0.4) post-transplant years (Table 5).
Table 5.
1/creatinine slopes by era
| Era | Era 1: pre-CNI (1963–83), n = 114 | Era 2a: CNI + steroids (1984–99), n = 262 | Era 2b: steroid-free CNI (1999–2011), n = 77 | P-value |
|---|---|---|---|---|
| First year | 1.8 ± 7.5 | 0.02 ± 2.6 | 0.13 ± 0.8 | 0.6 |
| Second year | 0.1 ± 0.3 | 0.02 ± 0.3 | 0.02 ± 0.2 | 0.9 |
| After second year | 0.0004 ± 0.1 | −0.04 ± 0.3 | −0.03 ± 0.2 | 0.4 |
Graft survival
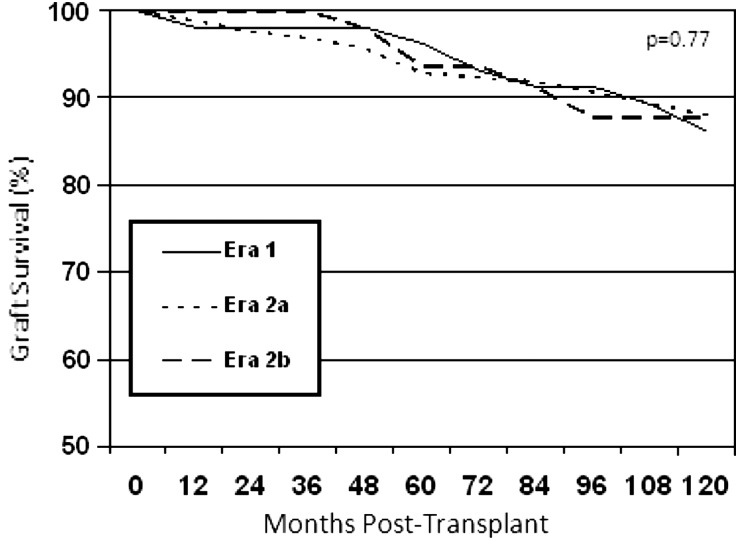
Graft loss including death with a functioning graft occurred in 72 patients (36%) in Era 1, 117 (58%) in Era 2a and 12 (6%) in Era 2b, but follow-up time was less with each advancing era. Death-censored graft survival was nearly identical in the three eras (Figure 2 and Table 3).
FIGURE 2:
Death-censored graft survival by era.
There were no group differences in graft loss from acute rejection. However, despite the shorter follow-up times, the incidence of graft loss from CAN was greater in the CNI eras (P = 0.02), (Table 4) as was biopsy-proven IF/TA (P = 0.002). Further analysis of recipients with at least 1 year of graft survival also demonstrated a higher incidence of graft loss from CAN of 12% (8), 27% (29) and 44% (4) in Era 1, 2a and 2b, respectively, although, as expected, numbers were small, resulting in statistical non-significance.
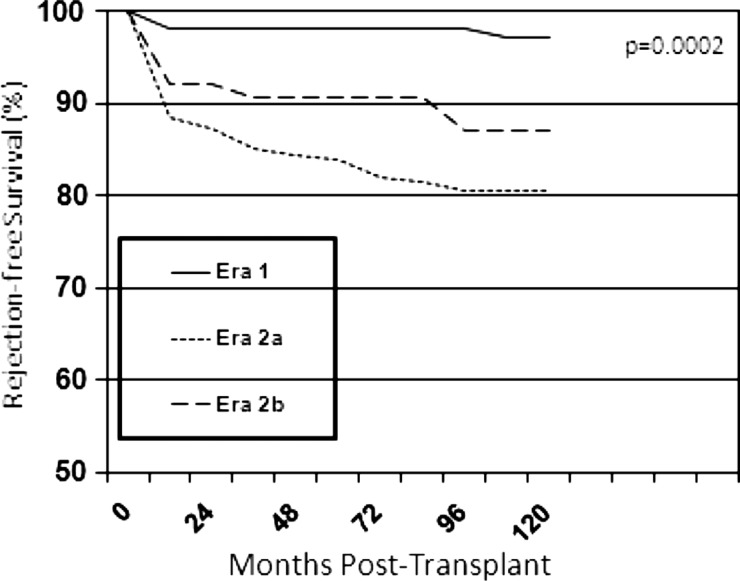
Graft rejection
There was a statistically significant decline in acute rejection-free survival with advancing eras (P = 0.0002) (Figure 3). Acute rejection-free survival was 98, 95 and 93% at 3 months; 98, 92 and 88% at 1 year and 98, 91 and 84% at 5 years in Era 1, 2a and 2b, respectively. However, this should be interpreted with caution since rejection definitions have been modified somewhat over time.
FIGURE 3:
Acute rejection-free survival by era.
CNI drug levels and comparison of side effects
In non-diabetic HLA-identical recipients, new-onset diabetes-free survival at 1, 5 and 10 years post-transplant was 99, 99 and 97% in Era 1; 97, 97 and 93% in Era 2a and 100, 100 and 90% in era 2b (P = 0.6). Hypertension was defined as patients requiring blood pressure medication. Fewer patients in Era 1 (48%) had post-transplant hypertension compared with 68 and 73% of Era 2a and 2b patients (P = 0.005). Although not statistically significant, there were numerically fewer patients in Era 1 (37%) with a post-transplant diagnosis of malignancy than Era 2a and 2b (45%) (P = 0.1). The same was true for post transplant lympho proliferative disorder rates which were 1% in pre-CNI era compared with 5% in the post-CNI eras (P = 0.07). However, skin cancer was diagnosed significantly less often in the pre-CNI (20%) than post-CNI eras (31%) (P = 0.02). CNI drug levels at the various post-transplant times are demonstrated in Table 6.
Table 6.
Mean CNI level in the post-CNI eras
| Time post-transplant | Mean CSA level* (ng/mL) |
Mean FK level* (ng/mL) |
||
|---|---|---|---|---|
| Era 2a | Era 2b | Era 2a | Era 2b | |
| 6 months | 152 | 146 | 11 | 7 |
| 9 months | 144 | 113 | 9 | 6.6 |
| 1 year | 120 | 105 | 8 | 6.6 |
| 2 years | 117 | 105 | 9 | 7.5 |
| 3 years | 105 | 92 | 8.8 | 6.7 |
| 4 years | 107 | 97 | 9 | 6.2 |
| 5 years | 95 | 93 | 9 | 6.3 |
*P < 0.0001
DISCUSSION
Transplant immunosuppression is a dynamic field and changes in immunosuppressive therapy have yielded much improved kidney graft survival rates since the first transplants >50 years ago. However, recipients of HLA-identical sibling living donor grafts represent <2% of renal transplants in the USA (UNOS), and are a particularly advantaged group that historically have always done well, even in the earlier days of kidney transplantation. We, therefore, asked whether the advances in immunosuppression have also benefited these recipients. An in-depth review of the literature (Table 7) of all available single-center trials (since randomized, prospective multicenter trials have, to our knowledge, not been done on this subject) provided no clear conclusions. The previously published studies were all smaller than the present study and, in fact, the present study virtually doubles the available information regarding outcomes of HLA-identical LRD kidney transplantation. In addition, our experience with LRD HLA-identical kidney transplantation emanating from among the oldest and most established LRD programs provided for a much longer patient follow-up than has previously been reported. This is especially important in the context of long-term graft outcomes in relation to possible CNI toxicity given that following non-renal organ transplantation, ESRD becomes markedly increased in frequency only >10 years [8].
Table 7.
Summary of key published results of CNI inclusive versus CNI-free immune-suppression regimens for HLA-identical transplant recipients
| Paper | Cohorts (year of transplant): number of patients | Patient and graft survival rates | Renal function | Other results | Follow-up (years) | Limitations |
|---|---|---|---|---|---|---|
| Sumrani et al. [19] | AZA/PDN (1972–83): 72 CSA/PDN (1983–89): 34 |
Patient survival at 1 and 5 years: AZA/PDN: 91 and 82% CSA/PDN: 100 and 96% Graft survival at 1 year: AZA/PDN: 85% CSA/PDN: 97% |
Mean serum Cr at 5 years: AZA/PDN :1.4 mg/dLa CSA/PDN:2.4 mg/dLb |
No differences in DM onset Increased HTN in CSA/PDN cohort |
AZA/PDN: 5 CSA/PDN: 2.9 |
Higher pre-tx DM in CSA/PDN (32%) than AZA/PDN cohort (8%) Information on CSA trough levels NA |
| Van Buren et al. [21] | Thymo induction and AZA/PDN (1980–92): 53 AZA/PDN/CSA (1986–92): 35 |
Patient survival at 1 and 5 years: AZA/PDN: 100%, 94% AZA/PDN/CSA: 100 and 91% Graft survival at 1 and 5 years: AZA/PDN: 96%, 77% AZA/PDN/CSA: 100%, 90% |
NA | Lower rejection rates in CSA cohorts | 5 | Higher pre-tx DM in CSA cohorts Information on CSA trough levels, HTN, malignancy, infection and renal function NA |
| Macdonald et al. [18] | AZA/PDN (1981–87): 15 CSA/PDN(1981–87): 21 CSA monotherapy (1981–87): 12 |
Patient survival at 1 and 3 years: AZA/PDN: 100%, 93% CSA/PDN: 100%, 100% CSA monotherapy: 100%, 100% Graft survival at 1 and 3 years: AZA/PDN: 100%, 86% CSA/PDN: 100%, 100% CSA monotherapy: 100%, 94% |
CSA cohorts had higher Cr at last follow-up | CSA cohorts required more anti-hypertensive therapy Rejection rates were lower in the combined CSA cohorts |
1–6.5 | Small patient numbers Short follow-up time |
| Flechner et al. [22] | AZA/PDN (since 1979): 20 CSA/PDN (since 1981): 28 |
Patient survival at 4.5 years: AZA/PDN: 95% CSA/PDN: 96% Graft survival at 3 and 4.5 years: AZA/PDN: 88%, 76% CSA/PDN: 96%, 96% |
No significant difference in serum Cr between AZA and CSA up to 4.5 years | Rejection rates lower in the combined CSA cohorts Lower hospitalization rates for infections in CSA cohorts |
4.5 | CSA inclusive cohort had significantly less pre-tx exposure to RBC transfusions |
| Keitel et al. [17] | AZA/PDN (before April 1995): 33 AZA/PDN/CSA (May 1995 to May 2000): 34 |
Graft survival was not significantly different between both cohorts | Estimated Cr clearance at 3 years: AZA/PDN: 76.6 ± 30.7 mL/min AZA/PDN/CSA: 71.6 ± 19.0 mL/min |
Rejection rate: AZA/PDN: 39.4% AZA/PDN/CSA: 14.7% |
0.5–5 | Information on CNI drug levels or side effects NA |
| Peddi et al. [16] | AZA/PDN (prior to January 1990): 15 AZA/PDN/CSA (January 1990–December 1996): 13 | Graft survival at 10 years: AZA/PDN: 69% AZA/PDN/CSA: 70% |
Rejection rate: AZA/PDN: 47% AZA/PDN/CSA: 0% CSA cohort had more HTN, DM and hyperlipidemia |
9 | Details of CSA levels and effects on renal function not provided Patient survival not compared Small numbers of patients |
|
| Vega et al. [15]. (article not available in English; these data are based on abstract alone) |
Double immunosuppressive therapy without CNI: 60 Triple immunosuppressive therapy with CNI: 25 |
No significant cohorts differences in graft survival | No significant difference in renal function at 60 months post-tx | 12 (53%) in the CNI-free cohort and 11 (47%) in the CNI-inclusive cohort required change in immunosuppressionc | CNI-free therapy: median 11.5 (2–25) CNI-inclusive therapy: median 4–5 (1–9) |
Full article not available in English so details of study not available |
AZA, azathioprine; PDN, prednisone; CSA, cyclosporine; DM, diabetes mellitus; HTN, hypertension; Thymo, Thymoglobulin induction; tx, transplant; CNI, calcineurin inhibitor; NA, not available.
aSerum Cr was relatively stable over time post-transplant
bSerum Cr steadily increased on a yearly basis post-transplant.
cThe major cause of change of therapy in the CNI-free cohort was leucopenia presumably due to AZA (five patients); and in the CNI-inclusive cohort was nephrotoxicity (six patients).
We evaluated the outcomes of transplants done in patients with HLA-identical sibling LRD in the era before the introduction of CNIs compared with those that received CNI with steroids or CNI without steroids in order to assess whether the nephrotoxic potential of CNI therapy might outweigh the immunological benefit.
Our center continues to do a large number of HLA-identical LRD transplantation, but the demographics here have changed over time. With each advancing era, donors and recipients are older and less homogenous, with proportionately fewer Caucasian donor–recipient pairs. Pre-transplant diabetes, especially type 2 DM was more common in the current era, this consistent with trends in the general population (http://www.cdc.gov/diabetes/statistics/incidence_national.html). Interestingly however, ESRD secondary to DM was higher in the previous era when the majority of the diabetic persons transplanted had type 1 DM (data not shown).
We demonstrated no difference in patient survival between HLA-identical transplant recipients in the various eras despite donors and recipients being older in advancing eras. A restricted analysis of transplants 18–50 years of age done to avoid confounding by older age recipients in later eras also showed no difference in patient survival. It was anticipated, however, that patient outcomes and survival would have improved with each advancing era for two reasons: (i) our program has evolved over time and our overall graft and patient survival outcomes have significantly improved as have those of other transplant programs [11] and (ii) improvements in the clinical management of DM have reduced post-transplant mortality and complications in more recent years. Thus, especially given the higher proportion of ESRD secondary to DM in the pre-CNI era (46%), we anticipated lower patient survival in the pre-CNI era. The unanticipated similar patient survival rates between eras could represent benefits of less immunosuppression in the pre-CNI era in the HLA-identical recipients. In support of this hypothesis, there was a numerical trend toward higher rates of death from infection with each advancing era. This is in keeping with the reports of increased hospitalization and death rates from infection observed with increasing intensity of immunosuppression [12, 13]. Unfortunately, data regarding CMV, BK virus and EBV from the pre-CNI era were insufficient for analysis.
There were no statistically significant differences between HLA-identical LRD recipients between the eras in death-censored graft survival (DCGS). DCGS between 10 and 20 years post-transplant decreased by 15% in Era 2a compared with 10% in the Era 1 recipients. Although not statistically significant by Chi-square analysis, this is a potentially worrisome numerical trend. The results were similar after eliminating patients with a renal failure diagnosis associated with substantial risk of graft loss from recurrent disease [focal segmental glomerulosclerosis (FSGS), primary hyperoxaluria, atypical hemolytic uremic syndrome and dense deposit disease] (data not shown). Over time our center has observed a statistically significant improvement in LRD kidney allograft survival comparing the era up to the early 1980s with the more recent eras (Matas et al. Unpublished data). This was not observed in this cohort of HLA-identical LRD kidney transplant recipients. This is a potentially worrisome observation, suggesting that not only does the addition of CNI to the immunosuppression regimen of HLA-identical LRD kidney transplant recipients not confer a measureable benefit, but may in fact be detrimental.
The use of depleting induction immunosuppression at our center, which is not universally given to HLA-identical transplant recipients at all centers, may limit the generalizability of our findings. Several single-center retrospective studies supported our finding [14–17] while others reported better graft survival in HLA-identical transplant recipients treated with CNI [18–22]. However, since most of these studies had shorter follow-up times and much smaller patient numbers with little or no CNI dose/level information, interpretation is difficult (Table 7). The two reports with long-term DCGS (>5 years) concurred with our findings of similar DCGS between eras despite decreased rejection with CNI therapy [15, 16].
Unfortunately, due to the retrospective nature of this study, we did not have detailed rejection data in the pre-CNI era but, contrary to the literature, our data suggest lower rejection rates among recipients in the pre-CNI era [16, 17, 20–22] (Table 7). This must be cautiously interpreted given changing definitions of graft rejection over time. We do not have donor-specific antibody data that are relevant since antibody-mediated injury is a major cause of kidney injury. We also lack information on killer-cell immunoglobulin-like receptor ligand mismatches which have been shown to be associated with reduced long-term graft survival in HLA-identical kidney transplant recipients [23]. Nonetheless, the absence of era differences in DCGS argues that CNI did not offer graft survival advantages to HLA-identical LRD transplant recipients.
Given the concern for the long-term nephrotoxicity of CNI, we also compared eras using 1/Cr slopes as a parameter of renal function change over time, and there were no significant differences between eras for the 1/Cr slopes during the first, second and >2 years post-transplant. This was different than previous retrospective studies which reported that HLA-identical recipients treated with CNI had higher median serum Cr concentrations than did AZA-treated patients [13, 18, 22]. However, in our study, there was statistically significantly increased graft loss from CAN/IFTA with each advancing era despite shorter follow-up times, and this could be a major reason why graft outcomes were similar in pre-CNI and more recent eras despite overall improvements in post-transplant care. This could also explain the decrease in DCGS by 15% between 10 and 20 years post-transplant in Era 2a compared with 10% in Era 1. Since HLA-identical sibling grafts, next to identical twin grafts, represent the closest approximation of a cure for ESRD, the possibility of additional graft losses due to long-term CNI should lead to rethinking of immunosuppressive strategies for these recipients.
In addition to increased risks of infections and nephrotoxicity, CNI therapy is associated with other side effects, including hypertension [24], gingival hypertrophy [25], new onset diabetes [26–28] and malignancy [29, 30]. In previous studies of HLA-identical living donor (LD) recipients, CNI was associated with increased incidence rates of hypertension [16, 19, 31], diabetes and hyperlipidemia [16]. Similarly, in our study population, pre-CNI HLA had less hypertension than post-CNI identical LRD transplant recipients.
Small non-randomized studies have assessed long-term monotherapy without CNI in HLA-identical transplant recipients. Walker et al. [32] discontinued TAC at 120 days and sirolimus at 1 year with subsequent mycophenolate mofetil (MMF) monotherapy in 17 HLA-identical graft recipients without prior acute rejection episodes. They noted no subsequent acute rejection or CAN and 100% DCGS with serum Cr at 2 years of 1.25 ± 0.29 mg/dL. Venot et al. [33] were successful in maintaining seven HLA-identical patients on MMF (n = 6) or sirolimus (n = 1) monotherapy with thymoglobulin induction alone, but follow-up time was limited. Van de Wetering et al. [34] tapered 27 of their HLA-identical LD recipients from triple or dual immunosuppression to 5 mg of prednisone daily at median 5.6 years post-transplant; 4 developed recurrence of their original disease but there was no acute rejection in any of the patients at 2 years of follow-up. Given the side effects of steroids, monotherapy with prednisone is unlikely to gain favor but a large prospective clinical trial could determine whether MMF/sirolimus monotherapy without CNI could offer a long-term advantage in HLA-identical sibling grafts. However, given the small number of HLA-identical LRD recipients, high success rates regardless of immunosuppressive strategies, as well as the long follow-up time needed to see the potential negative impact of CNI on graft outcomes, definitive randomized controlled clinical trials in this relatively privileged subset of recipients are unlikely to be done.
In summary, the addition of CNI to steroid inclusive or steroid avoidance immunosuppression regimens did not offer additional benefit to HLA-identical LRD transplant recipients for graft or patient survival. There were, however, increased graft loss secondary to CAN/IFTA, more hypertension and a trend toward more infection-related deaths in the CNI recipients. Since the recipients of HLA-identical LRD grafts may not need the immunosuppressive benefits of CNI therapy, but may be exposed to its risks, this may explain the failure to see the expected improved outcomes in more recent eras in this population. In the final analysis, next to identical twins, HLA-identical sibling grafts for non-recurrent diseases have the greatest potential for lifetime cure of ESRD and strategies should be developed with this long-term goal in mind. Given our findings, we suggest that monotherapy or early discontinuation of CNI should be given serious consideration in these patients.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Institutes of Health (DK013083). This study was partially presented as an abstract at the annual meeting of the 2013 American Transplant Congress in Seattle, WA, USA.
REFERENCES
- 1.Terasaki PI, Cho Y, Takemoto S, et al. Twenty-year follow-up on the effect of HLA matching on kidney transplant survival and prediction of future twenty-year survival. Transplant Proc. 1996;28:1144–1145. [PubMed] [Google Scholar]
- 2.Terasaki PI. The HLA-matching effect in different cohorts of kidney transplant recipients. Clin Transpl. 2000:497–514. [PubMed] [Google Scholar]
- 3.Shimmura H, Tanabe K, Ishida H, et al. Long-term results of living kidney transplantation from HLA-identical sibling donors under calcineurin inhibitor immunosuppression. Int J Urol. 2006;13:502–508. doi: 10.1111/j.1442-2042.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 4.Chavers BM, Matas AJ, Nevins TE, et al. Results of pediatric kidney transplantation at the University of Minnesota. Clin Transpl. 1989:253–266. [PubMed] [Google Scholar]
- 5.Hariharan S, Stablein DE. Improvements in long-term renal transplant graft survival. Am J Transplant. 2005;5:630–631. doi: 10.1111/j.1600-6143.2005.00746.x. Author reply 632–633. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL-S, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 7.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 9.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 10.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 11.Perez RV, Matas AJ, Gillingham KJ, et al. Lessons learned and future hopes: three thousand renal transplants at the University of Minnesota. Clin Transpl. 1990:217–231. [PubMed] [Google Scholar]
- 12.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. Am J Transplant. 2004;4:384–389. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 13.Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients—an analysis of USRDS data. Am J Transplant. 2007;7:653–661. doi: 10.1111/j.1600-6143.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 14.Gill IS, Hodge EE, Novick AC, et al. Azathioprine versus cyclosporine in recipients of HLA-identical renal allografts. Cleve Clin J Med. 1994;61:206–210. doi: 10.3949/ccjm.61.3.206. [DOI] [PubMed] [Google Scholar]
- 15.Vega O, Pérez-Gutiérrez A, Hernández-Ordóñez S, et al. Is a calcineurin inhibitor required as part of the immunosuppression scheme in kidney transplant recipients that share 2-haplotypes with their donors? Rev Invest Clin. 2010;62:200–205. [PubMed] [Google Scholar]
- 16.Peddi VR, Weiskittel P, Alexander JW, et al. HLA-identical renal transplant recipients: immunosuppression, long-term complications, and survival. Transplant Proc. 2001;33:3411–3413. doi: 10.1016/s0041-1345(01)02470-8. [DOI] [PubMed] [Google Scholar]
- 17.Keitel E, Santos AF, Alves MA, et al. Immunosuppression protocols for HLA identical renal transplant recipients. Transplant Proc. 2003;35:1074–1075. doi: 10.1016/s0041-1345(03)00313-0. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald AS, Belitsky P, Bitter-Suermann H, et al. Cyclosporine as primary therapy for A-matched living related donor kidney graft recipients. Transplant Proc. 1989;21(1 Pt 2):1667–1669. [PubMed] [Google Scholar]
- 19.Sumrani N, Delaney V, Ding ZK, et al. HLA-identical renal transplants: impact of cyclosporine on intermediate-term survival and renal function. Am J Kidney Dis. 1990;16:417–422. doi: 10.1016/s0272-6386(12)80053-9. [DOI] [PubMed] [Google Scholar]
- 20.Sumrani N, Delaney V, Ding Z, et al. Is cyclosporine indicated in HLA-identical renal transplant recipients? Transplant Proc. 1991;23(1 Pt 2):1239–1240. [PubMed] [Google Scholar]
- 21.Van Buren D, MacDonell R, Johnson HK, et al. Cyclosporine improves results in HLA-identical sibling renal transplants. Transpl Proc. 1994;26:2524–2515. [PubMed] [Google Scholar]
- 22.Flechner SM, Kerman RH, Van Buren CT, et al. Does cyclosporine improve the results of HLA-identical renal transplantation? Transplant Proc. 1987;19(1 Pt 2):1485–1488. [PubMed] [Google Scholar]
- 23.Van Bergen J, Thompson A, Haasnoot GW, et al. KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am J Transplant. 2011;11:1959–1964. doi: 10.1111/j.1600-6143.2011.03621.x. [DOI] [PubMed] [Google Scholar]
- 24.Luft FC. How calcineurin inhibitors cause hypertension. Nephrol Dial Transplant. 2012;27:473–475. doi: 10.1093/ndt/gfr679. [DOI] [PubMed] [Google Scholar]
- 25.Seymour RA, Ellis JS, Thomason JM. Drug-induced gingival overgrowth and its management. J R Coll Surg Edinb. 1993;38:328–332. [PubMed] [Google Scholar]
- 26.Joss N, Staatz CE, Thomson AH, et al. Predictors of new onset diabetes after renal transplantation. Clin Transplant. 2007;21:136–143. doi: 10.1111/j.1399-0012.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 27.Grewal HP, Thistlethwaite JR, Jr, Loss GE, et al. Corticosteroid cessation 1 week following renal transplantation using tacrolimus/mycophenolate mofetil based immunosuppression. Transplant Proc. 1998;30:1378–1379. doi: 10.1016/s0041-1345(98)00281-4. [DOI] [PubMed] [Google Scholar]
- 28.Marin M, Renoult E, Bondor CI, et al. Factors influencing the onset of diabetes mellitus after kidney transplantation: a single French center experience. Transplant Proc. 2005;37:1851–1856. doi: 10.1016/j.transproceed.2005.03.140. [DOI] [PubMed] [Google Scholar]
- 29.Shuttleworth D, Marks R, Griffin PJ, et al. Epidermal dysplasia and cyclosporine therapy in renal transplant patients: a comparison with azathioprine. Br J Dermatol. 1989;120:551–554. doi: 10.1111/j.1365-2133.1989.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 30.Morath C, Mueller M, Goldschmidt H, et al. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15:1582–1588. doi: 10.1097/01.asn.0000126194.77004.9b. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald AS, Belitsky P, Bitter-Suermann H, et al. Long-term follow-up (5 and 10 years) in recipients of HLA identical living related donor kidney grafts receiving continuous cyclosporine compared with azathioprine. Transplant Proc. 1997;29:190. doi: 10.1016/s0041-1345(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 32.Walker JK, Alloway RR, Roy-Chaudhury P, et al. A prospective trial of a steroid-free/calcineurin inhibitor minimization regimen in human leukocyte antigen (HLA)-identical live donor renal transplantation. Transplantation. 2009;87:408–414. doi: 10.1097/TP.0b013e318194515c. [DOI] [PubMed] [Google Scholar]
- 33.Venot M, Abboud I, Duboust A, et al. Calcineurin inhibitor-free monotherapy in human leukocyte antigen-identical live donor renal transplantation. Transplantation. 2011;91:330–333. doi: 10.1097/tp.0b013e3182033ef0. [DOI] [PubMed] [Google Scholar]
- 34.van de Wetering J, Gerrits JH, van Besouw NM, et al. Successful tapering of immunosuppression to low-dose monotherapy steroids after living-related human leukocyte antigen-identical renal transplantation. Transplantation. 2009;87:740–744. doi: 10.1097/TP.0b013e31819634eb. [DOI] [PubMed] [Google Scholar]