Abstract
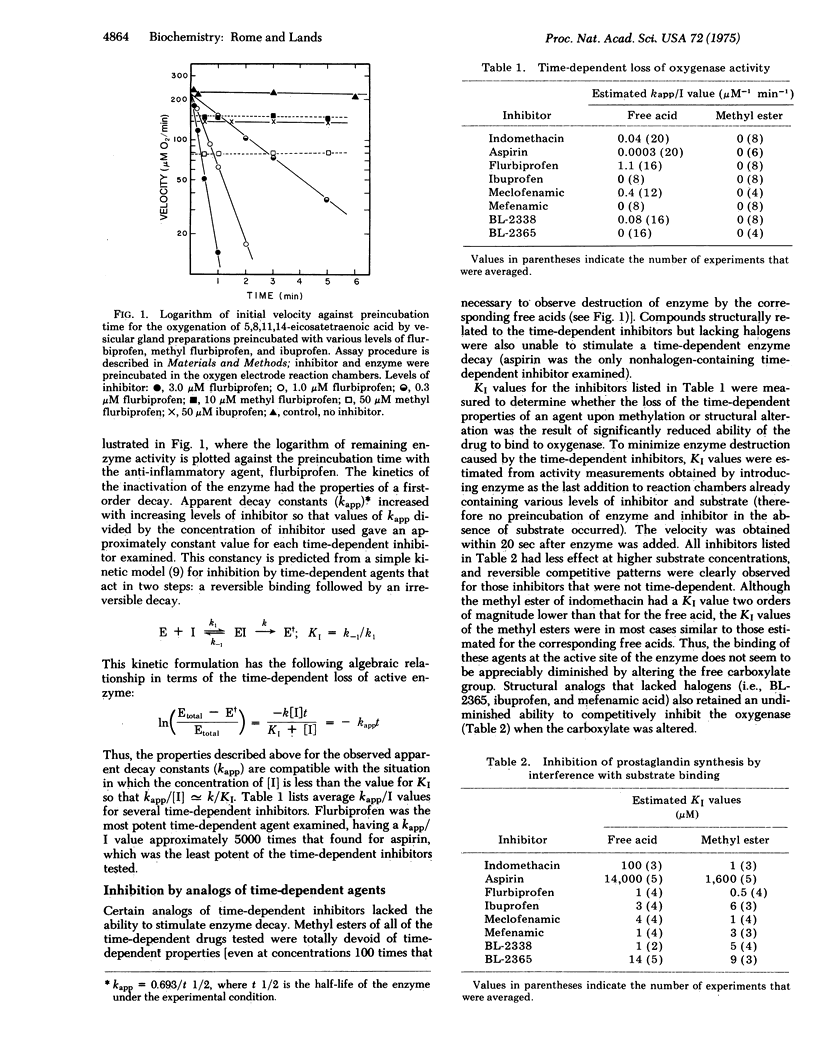
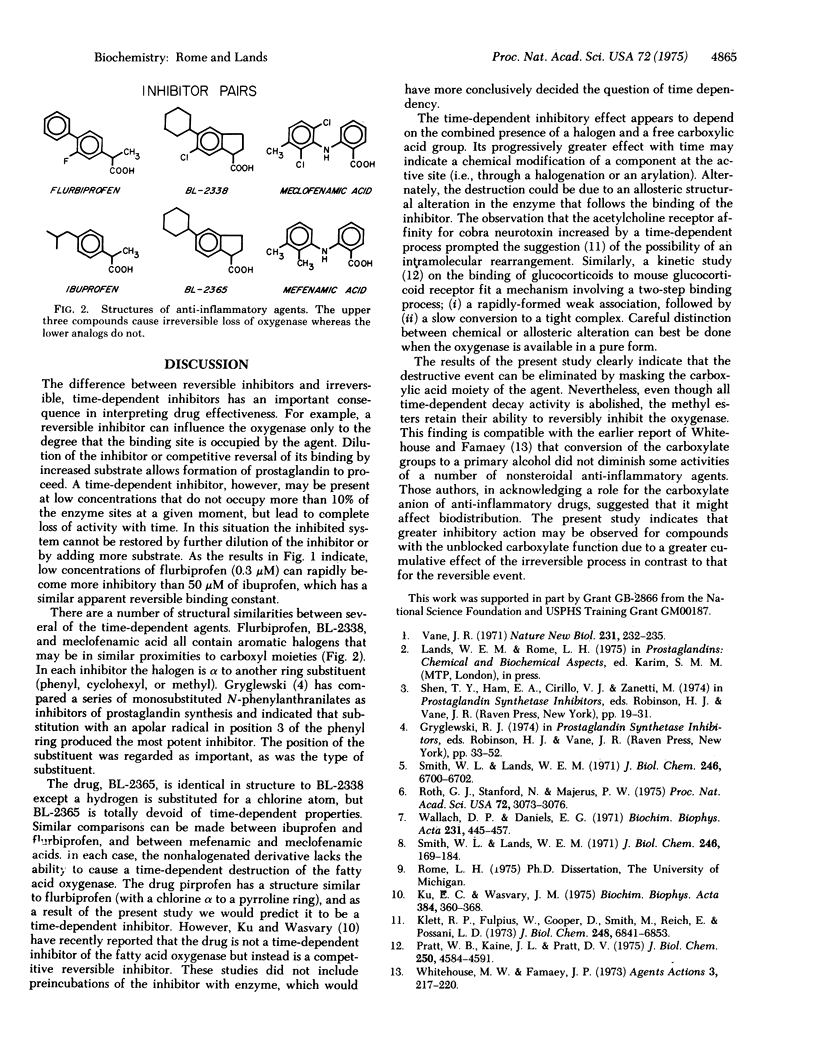
Several anti-inflammatory drugs have been examined for their ability to cause a time-dependent destruction of the fatty acid oxygenase that produces prostaglandins. All of the time-dependent inhibitors contained carboxylic acid moieties, and in addition all but one of the drugs contained a halogen atom. Structural analogs (of the time-dependent inhibitors) lacking halogen atoms were unable to cause a time-dependent destruction of the enzyme. The time-dependent property of an inhibitor was totally eliminated after methylation of the carboxylate group. Methylation did not, however, alter the ability of the inhibitors to competitively inhibit the oxygenase. Thus, the reversible binding of the agents at the active site was not appreciably dependent upon the free carboxyl group, whereas the subsequent irreversible process was.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Klett R. P., Fulpius B. W., Cooper D., Smith M., Reich E., Possani L. D. The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J Biol Chem. 1973 Oct 10;248(19):6841–6853. [PubMed] [Google Scholar]
- Ku E. C., Wasvary J. M. Inhibition of prostaglandin synthase by pirprofen. Studies with sheep seminal vesicle enzyme. Biochim Biophys Acta. 1975 Apr 19;384(2):360–368. doi: 10.1016/0005-2744(75)90037-6. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Kaine J. L., Pratt D. V. The kinetics of glucocorticoid binding to the soluble specific binding protein of mouse fibroblasts. J Biol Chem. 1975 Jun 25;250(12):4584–4591. [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971 Nov;246(21):6700–6702. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallach D. P., Daniels E. G. Properties of a novel preparation of prostaglandin synthetase from sheep seminal vesicles. Biochim Biophys Acta. 1971 May 4;231(3):445–457. doi: 10.1016/0005-2760(71)90112-3. [DOI] [PubMed] [Google Scholar]
- Whitehouse M. W., Famaey J. P. Concerning the pharmacological activity of non-steroid anti-inflammatory drugs: is the acidic function essential? Agents Actions. 1973 Nov;3(4):217–220. doi: 10.1007/BF01968545. [DOI] [PubMed] [Google Scholar]


