Abstract
Subependymal heterotopia (SEH) is a neuronal migration disorder characterized by nodules of gray matter along the lateral ventricular walls and often associated with other brain malformations. We present two cases of SEH associated with ventriculomegaly and cerebellar abnormalities diagnosed by fetal magnetic resonance imaging (MRI) at 20 and 23 weeks’ gestation respectively. Fetal MRI findings of this association of abnormalities have never been reported in literature. This report emphasizes the role of fetal MRI in recognition of subependymal heterotopia and other associated brain anomalies at early age of gestation along with its importance for a more targeted counseling and management strategies.
Keywords: Subependymal heterotopia, Fetal MRI, Ventriculomegaly, Cerebellar abnormalities
CASE REPORT
CASE 1
A 37-year-old primigravid woman had an apparently uneventful pregnancy until 22 weeks of gestation, when she was referred to our hospital due to fetal ventriculomegaly detected during ultrasound (US) examination performed at another institution (Figure 1). Maternal serum TORCH screening was negative and she had no relevant medical history. At 23 weeks of gestation we performed a fetal MRI exam on a 1.5-Tesla unit (Somatom Avanto®, Siemens, Erlangen, Germany) using a multi-channel phased array coil to allow increased coverage of the fetal head and increased signal-to-noise ratio. T2-weighted HASTE, FLAIR, T1 FLASH 2D and diffusion-weighted images (DWI) were obtained in planes that were approximately sagittal, coronal and axial with respect to the head of the fetus. We chose to use a slice thickness of 3 mm to better study the fetal brain. The supine position, feet first, has been used throughout the examination in order to minimize fetal movements. No fetal or maternal sedation was administered.
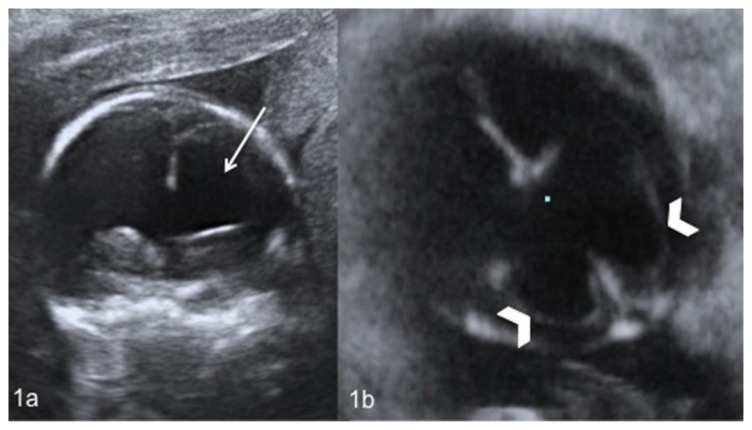
Figure 1.
Fetal brain at 22 weeks of gestation (normal male; 46, XY karyotype) with bilateral subependymal heterotopia, ventriculomegaly and cerebellar asymmetry. Coronal (1a) and axial (1b) US images show enlargement of the frontal (white arrow) (1a) and occipital (white arrowheads) (1b) horns of the lateral ventricles. (GE Voluson 730 Expert, 3.5 MHz convex transducer).
The presence of bilaterally increased lateral ventricular atrial diameter measuring at the occipital horns 12 mm at right and 13 mm at left (normal value: below 10 mm), confirmed the ultrasound diagnosis of ventriculomegaly (Figure 2a, 2c). The MRI also disclosed multiple, round, subcentimetric nodular depositions located just beneath and abutted the ependymal lining of the occipital horns of the lateral ventricles resulting in an irregular ventricular outline. The nodules presented low signal intensity, similar to that of the gray matter, on T2-weighted sequences and were highly suggestive of nodular subependymal heterotopia (Figure 2a, 2c). The same findings were undetectable on T1-weighted and FLAIR sequences. The exam did not reveal the presence of hemorrhagic or ischemic lesions of the brain parenchyma. However, a dysmorphic cerebellum with the right lobe of irregular margins and decreased size was observed (Figure 2b). The transverse cerebellar diameter was below the 10th percentile and was of 21 mm (normal range: 23–34 mm). The images showed a normal-sized cerebellar vermis without any signs of malrotation, with a height of 10 mm (normal range: 10–12 mm) and an anteroposterior diameter of 13 mm (normal range: 8–10 mm). No cardiac, gastrointestinal or other non-CNS (central nervous system) anomalies were detected. Due to the fetal malformations the woman decided to have a termination of pregnancy (TOP). A neuropathological examination of the fetus brain confirmed what reported by MRI, showing bilateral subependymal nodules composed by mature and immature neuronal cells (Figure 2d) and cerebellar lobes asymmetry with irregular vermis. An array CGH test did not show any genomic imbalance demonstrating normal male karyotype (46, XY).
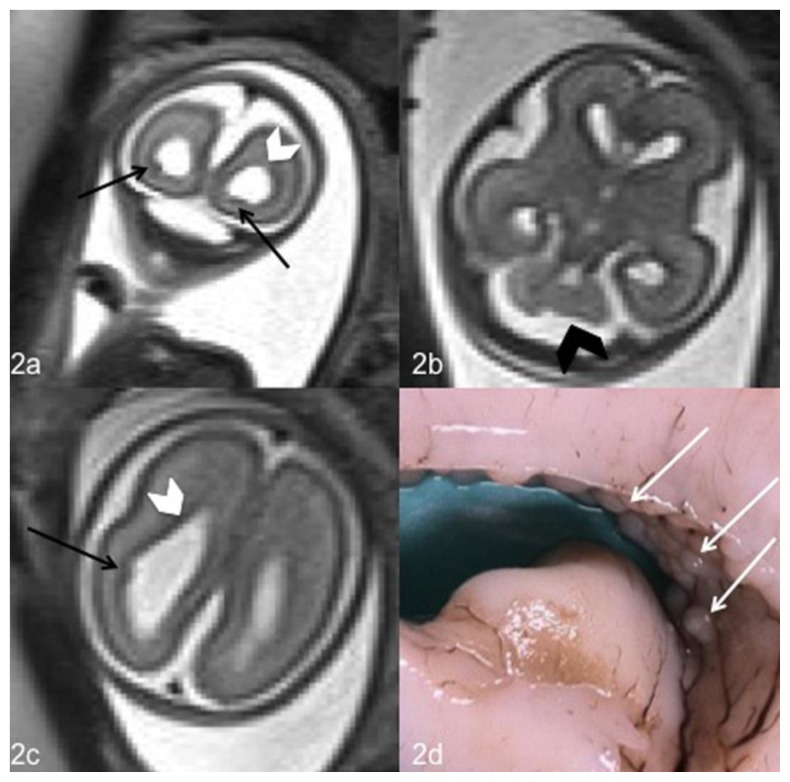
Figure 2.
Fetal brain at 23 weeks of gestation (normal male; 46, XY karyotype) with bilateral subependymal heterotopia, ventriculomegaly and cerebellar asymmetry. Coronal (2a) and axial (2b,2c) T2-weighted magnetic resonance images at the level of the lateral ventricles of the fetal brain show hypointense nodules in the ventricular wall corresponding to heterotopic gray matter (black arrows) (2a,2c), ventriculomegaly (white arrowhead) (2a, 2c) and cerebellar asymmetry (black arrowhead) (2b). A coronal section of the fetal brain obtained for the neuropathological examination confirmed the presence of nodular subependymal heterotopia (2d). (1.5T Magnetic Resonance Imaging. Protocol: TR 1000 msec; TE 149 msec; thickness 3 mm).
CASE 2
A 37-year-old secundigravida primipara woman was referred to our department of radiology because of a finding of triventricular hydrocephalus on morphology ultrasound performed at 18 weeks’ gestation at another institution (Figure 3a, 3b). Maternal serum TORCH screening was negative and she had no relevant medical history. At 20 weeks of gestation we performed a fetal MRI exam on a 1.5-Tesla unit (Somatom Avanto®, Siemens, Erlangen, Germany) including T2-weighted HASTE, FLAIR, T1 FLASH 2D, DWI images in planes that were approximately sagittal, coronal and axial with respect to the head of the fetus. We used a slice thickness of 3 mm to better study the fetal brain. The exam confirmed what reported on prenatal ultrasound, showing bilateral enlargement of the lateral ventricles and of the third ventricle (Figure 4a, 4b). In particular, the lateral ventricular atrial diameter measured at the occipital horns 11 mm at left and 13 mm at right (normal range: below 10 mm). The cerebellar vermis presented decreased size with a height of 7.5 mm (normal range: 6–9 mm) and an anteroposterior diameter of 4.5 mm (normal value = 6 mm) and it was malrotated, showing a wide communication between fourth ventricle and cisterna magna (Figure 4c). All the other parts of the cerebellum were normal and with regular margins and the transverse cerebellar diameter was 17 mm (normal value = 17 mm). Fetal MRI also detected altered sulci on the medial surface of the frontal lobes with multiple, subcentimetric nodular depositions which presented low signal intensity on T2 weighted sequences but were undetectable on T1-weighted and FLAIR sequences. This was highly suggestive of nodular subependymal heterotopia (Figure 4a, 4b). We finally observed an irregular cerebral parenchyma on the medial surface of the frontal right lobe with a mild hyperintense area on T2-weighted (Figure 5a) and DWI images (Figure 5b) and hypointense on the corresponding ADC map (Figure 5c). These findings lead us to suspect the presence of an ischemic area. No cardiac, gastrointestinal or other non-CNS (central nervous system) anomalies were detected. Due to the above-described fetal cerebral malformations the woman decided to have a termination of pregnancy (TOP). A neuropathological examination of the fetus brain confirmed all the findings reported by fetal MRI, including the bilateral subependymal gray matter heterotopic nodules located just beneath the ependymal lining of the frontal horns of the lateral ventricles (Figure 4d) and the malrotated cerebellar vermis (Figure 4e). An array CGH test did not show any genomic imbalance demonstrating normal male karyotype (46, XY).
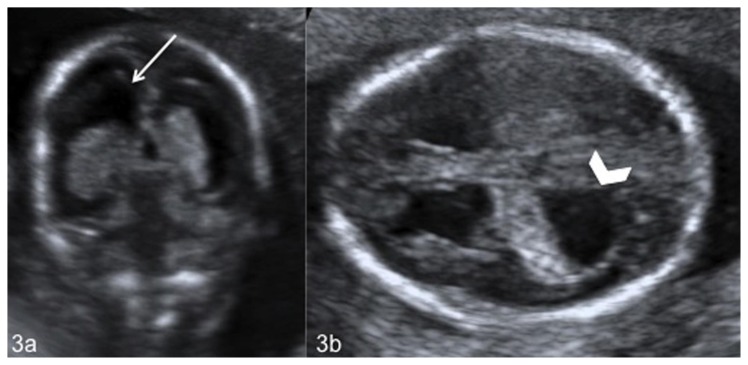
Figure 3.
Fetal brain at 18 weeks of gestation (normal male; 46, XY karyotype) with bilateral subependymal heterotopia, ventriculomegaly and cerebellar asymmetry. Coronal (3a) and axial (3b) US images show enlargement of the frontal (white arrow) (3a) and occipital (white arrowheads) (3b) horns of the lateral ventricles. (GE Voluson 730 Expert, 3.5 MHz convex transducer).
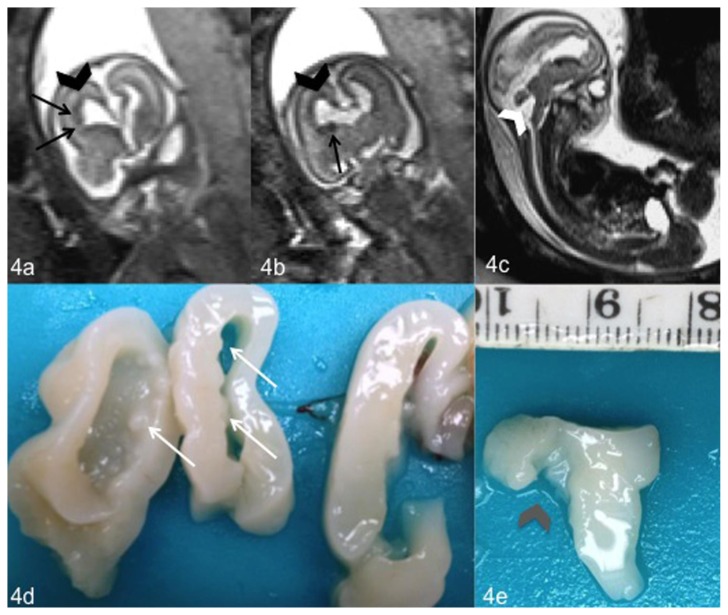
Figure 4.
Fetal brain at 20 weeks of gestation (normal male 46, XY karyotype) with bilateral subependymal heterotopia, ventriculomegaly and cerebellar abnormalities. Coronal (4a, 4b) and sagittal (4c) T2-weighted magnetic resonance images show ventriculomegaly (black arrowhead) (4a, 4b), cerebellar abnormality with a wide communication between fourth ventricle and cisterna magna (white arrowhead) (4c) and nodules of subependymal heterotopia along the walls of the frontal horns of the lateral ventricles (black arrows) (4a, 4b). Coronal and sagittal sections of the fetal brain obtained for the neuropathological examination confirmed the presence of nodules of heterotopic gray matter (white arrows) (4d) and a malrotated cerebellar vermis (gray arrowhead) (4e). (1.5 T Magnetic Resonance Imaging. Protocol: TR 1000 msec; TE 149 msec; thickness 3 mm).
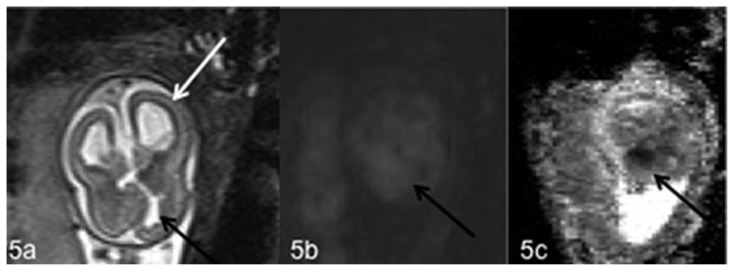
Figure 5.
Fetal brain at 20 weeks of gestation (normal male 46, XY karyotype) with bilateral subependymal heterotopia, ventriculomegaly, cerebellar asymmetry and the presence of an ischemic area. Axial T2-weighted (5a), DWI (5b) magnetic resonance images and corresponding ADC map (5c) show the presence of ventriculomegaly (white arrow) (5a) and an ischemic area on the medial surface of the frontal right lobe with mild hyperintensity on T2-weighted (5a) and DWI images (5b) and hypointensity on the corresponding ADC map (5c) (black arrows). (1.5 T Magnetic Resonance Imaging. DWI sequences Protocol: TR 8000 msec; TE 90 msec; TI 185 msec; thickness 5 mm. T2-weighted sequences Protocol: TR 1000 msec; TE 149 msec; thickness 3 mm).
DISCUSSION
Gray matter heterotopia is a relatively common disorder of cortical development in which collections of cortical neurons are found in an abnormal location [1]. It is related to failure of neuroblasts to undergo apoptosis or to anomalies of neuronal migration from the germinal matrix to the cerebral cortex [2]. Three forms of heterotopia are recognized on the basis of the location and configuration of the ectopic gray matter tissue: subependymal, subcortical, and band heterotopia [1]. The most common type of heterotopic gray matter is the subependymal type (SEH) which consists of small foci of gray matter located in a subependymal location adjacent to the lateral ventricles, bilaterally or unilaterally [3]. The most frequent localizations of SEH are the paratrigonal region and frontal horns of the lateral ventricles [4].
Some studies have reported that SEH has a number of identified genetic causes, particularly the diffuse bilateral form. Most cases are X-linked, with the responsible gene, called filamin 1, localized to Xq28. Its mutation is the most common genetic cause of SEH. The product of the FLNA gene is a phosphoprotein inducing actin reorganization and a mutation induces failure of the neuronal motility causing premature arrest of neuronal migration [5]. Another less frequent mutation in SEH concerns the ARFGEF2 gene which encodes a regulator of neuronal proliferation and causing an unsuccessful migration of later-born neurons [6]. 1p36 deletion [7,8], 7q11.23 deletion [9], 5p15.1 duplication [10,11] and other chromosomal copy number variations are also associated with SEH, especially the bilateral form. In our cases no genomic abnormality or other underlying etiologies could be found.
When heterotopia is isolated, patients typically have normal early development and come to medical attention with the onset of epilepsy, usually in the second decade of life and presenting mostly with partial seizure. Most affected individuals are females and have normal intelligence whereas male patients have widespread cerebral abnormalities and significantly worse clinical outcomes [1]. However, the association with other disorders such as encephalocele [12], cervical meningocele [13], ventriculomegaly, cortical (mostly polymicrogyria), cerebellar and posterior fossa abnormalities, metabolic disorders and anomalies of the corpus callosum have been reported [14]. A high proportion of these patients do not survive into adulthood.
In our cases SEH was associated with ventriculomegaly and cerebellar abnormalities. A dysmorphic right cerebellar hemisphere was found in the first fetus whereas a small-sized and malrotated cerebellar vermis was detected in the other one. The second case had also an ischemic area on the medial surface of the frontal right lobe. Fetal SEH diagnosed with MRI are reported only in few cases [15,16,17] and fetal MRI findings of SEH associated with cerebellar abnormalities and ventriculomegaly haven’t been reported before in literature. Bargallò presented a case of subependymal heterotopia associated with mega cisterna magna in a fetus at 29 weeks’ gestation [16]. Donkol et al. [18] described the association of subependymal heterotopia and ventricular dilation and Srour et al. [14] reported the combination of heterotopia, posterior fossa abnormalities and ventriculomegaly in pediatric patients, but not in fetuses.
Difficulties with fetal movement during early gestation have limited the use of fetal MRI imaging for many years [19]. The development of faster sequences, poorly influenced by fetal movements, and stronger gradients has improved the quality of images that can be obtained in only a few seconds. T2-weighted HASTE sequences allow multiple images to be acquired in a single maternal breath hold and because of the high resolution and short scanning time, are the best imaging sequences for fetal MRI [20]. They offer an excellent visualization of most of the fetal CNS structures, such as sulci, corpus callosum, cerebellum, thalami and brain stem [21]. Also non-CNS organs, such as the heart and the gastrointestinal tract can be optimally visualized with these sequences. However, fetal MRI is not usually performed before 19 weeks because only at this age of gestation most of fetal anatomical structures have reached their adult form and the presence of abnormalities can be evaluated [22].
The differential diagnosis of SEH using fetal MRI, characterized by hypointense nodules on T2-weighted images, includes nodular subependymal hamartomas present in tuberous sclerosis which are hypointense on T2-weighted and hyperintense on T1-weighted images [23]. Sonigo et al. described five fetuses, later confirmed to have tuberous sclerosis, with the US detection of cardiac tumors and normal fetal brain appearance. MRI added further information than US and showed hyperintense periventricular nodules on T1-weighted images, highly suggestive of nodular subependymal hamartomas [24]. In our cases, the nodules of SEH weren’t visualized on T1-weighted images and only the coexistence of other brain anomalies in heterotopia and cardiac abnormalities in tuberous sclerosis along with a search of the family history could be helpful in making this differentiation. Also early subacute subependymal hemorrhage could be characterized by periventricular lesions which are hyperintense to brain tissue in T1-weighted images and marked hypointense on T2-weighted images [25]. However, subependymal hemorrhage is often associated with ventricular bleeding, hydrocephalus and a typical evolution and appearance on US, presenting as hyperechoic lesions in the margins of the lateral ventricles. (Differential Table).
Prenatal SEH could be not easily recognized by fetal ultrasound. It has been described in some cases [26] as hyperechoic nodules close to the ventricular wall of the lateral ventricles and are very similar to those of tuberous sclerosis. In our cases fetal US examinations did not reveal the presence of the subependymal nodules seen at MRI. Thanks to the development of faster sequences, poorly influenced by fetal movements, its multiplanar capabilities and optimal spatial resolution fetal MRI can play a crucial role in evaluation and detection of SEH and other fetal brain abnormalities such as ventriculomegaly, cerebellar anomalies and ischemic areas at early age of gestation. Furthermore, MRI is considered safe in pregnancy and useful for management planning and predicting prognosis. Patients with isolated SEH could be neurologically and developmentally normal or only develop epilepsy. Some cases of medically resistant epilepsy associated with isolated subependymal heterotopia might be even surgically treated [27]. On the contrary, patients affected by SEH with other brain anomalies have significantly worse clinical outcomes with a high incidence of spontaneous abortion and often did not survive infancy.
TEACHING POINT
Fetal MRI, thanks to its multiplanariety and optimal spatial resolution allows an excellent evaluation of fetal brain at early age of gestation (from 19 weeks’ gestation). It is nowadays an always more necessary tool for a correct diagnosis and for a more targeted counseling in case of fetal brain abnormalities such as subependymal heterotopia, characterized by nodules of gray matter along the lateral ventricular walls which present low signal intensity, similar to that of the gray matter, on T2 weighted sequences, and other associated abnormalities.
Table 1.
Summary table for Subependymal Heterotopia associated with other brain anomalies (SEH)
| Etiology | The causes of SEH associated with other brain anomalies are often unknown. However, there are a number of identified genetic causes of SEH, such as a mutation of the filamin 1 or of the ARFGEF2 gene. |
| Incidence | The exact incidence is unknown. |
| Gender ratio | Both the sporadic and the genetic form of SEH are found primarily in females, as affected males have much more severe phenotypes, with a high incidence of spontaneous abortion. However, associated cerebral malformations are found in both males and females. |
| Age predilection | Congenital malformations. Females with isolated SEH usually come to medical attention with the onset of epilepsy, usually in the second decade of life. Male patients and the ones with SEH associated with other brain anomalies often did not survive infancy. |
| Risk factors | Unknown. However, familial cases of SEH have been recognized. |
| Treatment | Only epilepsy associated with subependymal nodular heterotopia could be treated by surgery. |
| Prognosis | Patients, mostly females, with isolated SEH develop epilepsy during the second decade of life. In patients with SEH associated with cerebral malformations there is an elevated perinatal and childhood mortality rate. |
| Imaging findings |
MRI SEH: Multiple nodular depositions located just beneath and abutted the ependymal lining of the occipital horns of the lateral ventricles. The nodules present low signal intensity, similar to that of the gray matter, on T2-weighted sequences. |
Table 2:
Differential diagnosis of Subependymal heterotopia in fetuses (SEH)
| US | MRI T1 | MRI T2 | Further useful information | |
|---|---|---|---|---|
| Subependymal heterotopia | Periventricular nodules:
|
|
Hypointense | SEH could be associated with other brain anomalies |
| Tuberous sclerosis | Periventricular nodules:
|
Hyperintense | Hypointense |
|
| Subependymal Hemorrhage (early subacute) | Periventricular lesions:
|
Hyperintense | Hypointense | Often associated with intraventricular hemorrhage and hydrocephalus |
ABBREVIATIONS
- ADC
Apparent Diffusion Coefficient
- ARFGEF2
ADP-ribosylation factor guanine nucleotide-exchange factor 2
- CGH
Comparative genomic hybridization
- CNS
Central nervous system
- DWI
Diffusion-weighted imaging
- FLAIR
Fluid attenuated inversion recovery
- FLASH
Fast Low Angle Shot
- FLNA
Filamine A
- HASTE
Half-fourier acquisition single-shot turbo spin-echo
- MRI
Magnetic Resonance Imaging
- SEH
Subependymal heterotopia
- TOP
Termination of pregnancy
- TORCH
Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus, and Herpes infections
- US
Ultrasound
REFERENCES
- 1.Barkovich AJ, Kuzniecky RI. Gray matter heterotopia. Neurology. 2000 Dec 12;55(11):1603–1608. doi: 10.1212/wnl.55.11.1603. [DOI] [PubMed] [Google Scholar]
- 2.Barth PG. Disorders of neuronal migration. Can J Neurol Sci. 1987 Feb;14(1):1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- 3.Raymond AA, Fish DR, Stevens JM, Sisodiya SM, Alsanjari N, Shorvon SD. Subependymal heterotopia: a distinct neuronal migration disorder associated with epilepsy. J Neurol Neurosurg Psychol. 1994 Oct;57(10):1195–202. doi: 10.1136/jnnp.57.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zisch R, Artmann W. MR in the diagnosis of heterotopic gray matter. Neuroradiology. 1991;33(6):527–8. doi: 10.1007/BF00588047. [DOI] [PubMed] [Google Scholar]
- 5.Fox JW, Lamperti ED, Ekşioğlu YZ, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998 Dec;21(6):1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferland RJ, Batiz LF, Neal J, et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009 Feb 1;18(3):497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal J, Apse K, Sahin M, Walsh CA, Sheen VL. Deletion of chromosome 1p36 is associated with periventricular nodular heterotopia. Am J Med Genet A. 2006 Aug 1;140(15):1692–1695. doi: 10.1002/ajmg.a.31334. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Kawamura R, Kosho T, et al. Bilateral perisylvian polymicrogyria, periventricular nodular heterotopia, and left ventricular noncompaction in a girl with 10.5–11.1 Mb terminal deletion of 1p36. Am J Med Genet A. 2008 Nov 15;146A(22):2891–2897. doi: 10.1002/ajmg.a.32556. [DOI] [PubMed] [Google Scholar]
- 9.Ferland RJ, Gaitanis JN, Apse K, Tantravahi U, Walsh CA, Sheen VL. Periventricular nodular heterotopia and Williams syndrome. Am J Med Genet A. 2006 Jun 15;140(12):1305–1311. doi: 10.1002/ajmg.a.31259. [DOI] [PubMed] [Google Scholar]
- 10.Sheen VL, Topçu M, Berkovic S, et al. Autosomal recessive form of periventricular heterotopia. Neurology. 2003 Apr 8;60(7):1108–1112. doi: 10.1212/01.wnl.0000055898.00349.02. [DOI] [PubMed] [Google Scholar]
- 11.De Wit MC, de Coo IF, Halley DJ, Lequin MH, Mancini GM. Movement disorder and neuronal migration disorder due to ARFGEF2 mutation. Neurogenetics. 2009 Oct;10(4):333–336. doi: 10.1007/s10048-009-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas L, Melvin JJ, Faeber EN, Valencia I. Anterior encephalocele associated with subependymal nodular heterotopia, cortical dysplasia and epilepsy: case report and review of the literature. Eur J Paediatr Neurol. 2006 Sep-Nov;10(5–6):227–9. doi: 10.1016/j.ejpn.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Lotfi M, Iranpour P, As’adi K. Cervical meningocele associated with subependymal nodular heterotopia. Clin Imaging. 2011 May-Jun;35(3):214–6. doi: 10.1016/j.clinimag.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Srour M, Rioux MF, Varga C, et al. The clinical spectrum of nodular heterotopias in children: report of 31 patients. Epilepsia. 2011 Apr;52(4):728–37. doi: 10.1111/j.1528-1167.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell L, Simon E, Filly R, Barkovich A. The antenatal diagnosis of subependymal heterotopia. AJNR Am J Neuroradiol. 2000 Feb;21:296–300. [PMC free article] [PubMed] [Google Scholar]
- 16.Bargalló N, Puerto B, De Juan C, et al. Hereditary subependymal heterotopia associated with mega cisterna magna: antenatal diagnosis with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2002 Jul;20:86–89. doi: 10.1046/j.1469-0705.2002.00741.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang PH, Bartha AI, Norton ME, Barkovich AJ, Sherr EH, Glenn OA. Agenesis of the corpus callosum: an MR imaging analysis of associated abnormalities in the fetus. AJNR Am J Neuroradiol. 2009 Feb;30(2):257–63. doi: 10.3174/ajnr.A1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donkol RH, Moghazy KM, Abolenin A. Assessment of gray matter heterotopia by magnetic resonance imaging. World J Radiol. 2012 Mar 28;4(3):90–6. doi: 10.4329/wjr.v4.i3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith FW, Adam AH, Phillips WD. NMR imaging in pregnancy (letter) Lancet. 1983 Jan 1;1(8314–5):61–62. doi: 10.1016/s0140-6736(83)91588-x. [DOI] [PubMed] [Google Scholar]
- 20.Simon EM, Goldstein RB, Coakley FV, et al. Fast MR imaging of fetal CNS anomalies in utero. AJNR Am J Neuroradiol. 2000 Oct;21(9):1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 21.Manganaro L, Savelli S, Francioso A, et al. Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med. 2009;114(7):1013–23. doi: 10.1007/s11547-009-0434-2. [DOI] [PubMed] [Google Scholar]
- 22.Triulzi F, Manganaro L, Volpe P. Fetal magnetic resonance imaging: indications, study protocols and safety. Radiol Med. 2011;116(3):337–50. doi: 10.1007/s11547-011-0633-5. [DOI] [PubMed] [Google Scholar]
- 23.Christophe C, Bartholome J, Blum D, et al. Neonatal tuberous sclerosis: US, CT, and MR diagnosis of brain and cardiac lesions. Pediatr Radiol. 1989;19(6–7):446–448. doi: 10.1007/BF02387652. [DOI] [PubMed] [Google Scholar]
- 24.Sonigo P, Elmaleh A, Fermont L, Delezoide AL, Mirlesse V, Brunelle F. Prenatal MRI diagnosis of fetal cerebral tuberous sclerosis. Pediatr Radiol. 1996;26:1–4. doi: 10.1007/BF01403693. [DOI] [PubMed] [Google Scholar]
- 25.Canapicchi R, Cioni G, Strigini FA, Abbruzzese A, Bartalena L, Lencioni G. Prenatal diagnosis of periventricular hemorrhage by fetal brain magnetic resonance imaging. Childs Nerv Syst. 1998 Dec;14(12):689–92. doi: 10.1007/s003810050298. [DOI] [PubMed] [Google Scholar]
- 26.Pellicer A, Cabanas F, Perez-Higueras A, Garcia-Alix A, Quero J. Neural migration disorders studied by cerebral ultrasound and color Doppler flow imaging. Arch Dis Child Fetal Neonatal Ed. 1995;73:F55–F61. doi: 10.1136/fn.73.2.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acar G, Acar F, Oztura I, Baklan B. A case report of surgically treated drug resistant epilepsy associated with subependymal nodular heterotopia. Seizure. 2012 Apr;21(3):223–6. doi: 10.1016/j.seizure.2011.11.002. [DOI] [PubMed] [Google Scholar]