Abstract
We report the neuroimaging findings of a 26-year-old female patient with a biopsy-proven dysembryoplastic neuroepithelial tumor (DNET). DNETs are an uncommon, usually benign, glial-neural cortical neoplasm of children and young adults who typically present with intractable seizures. DNETs may occur in any region of the supratentorial cortex, but have a predilection for the temporal lobes. Accurate neuroimaging diagnosis is essential since patients with DNET benefit from complete resection. However, accurate differentiation from other cortical lesions may be challenging. Typical conventional Magnetic Resonance Imaging (MRI) features can help in the differentiation from other similar cortical tumors. Diffusion tensor imaging can also provide important additional diagnostic information regarding the degree of involvement of adjacent parenchyma and white matter tracts. In this case, tractography and fractional anisotropy maps demonstrated that fiber tracts surrounding the lesion were displaced, but fiber integrity was maintained, which is more suggestive of a DNET rather than a more aggressive neoplasm. Accurate identification of DNETs is essential for the purpose of rendering a timely diagnosis and start appropriate treatment.
Keywords: Dysembryoplastic neuroepithelial tumor (DNET), Diffusion tensor imaging, Neuroimaging, Tractography
CASE REPORT
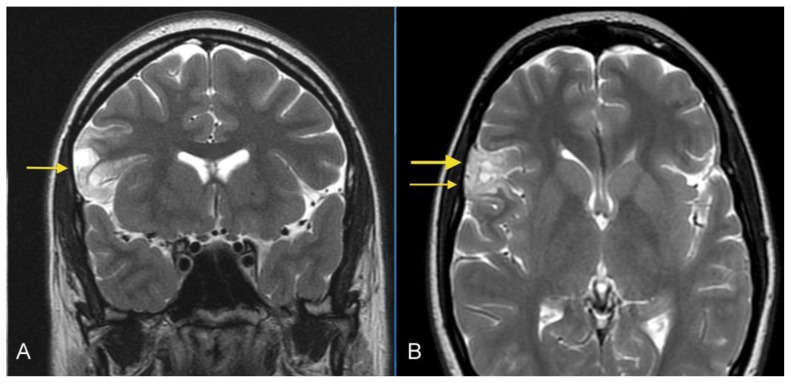
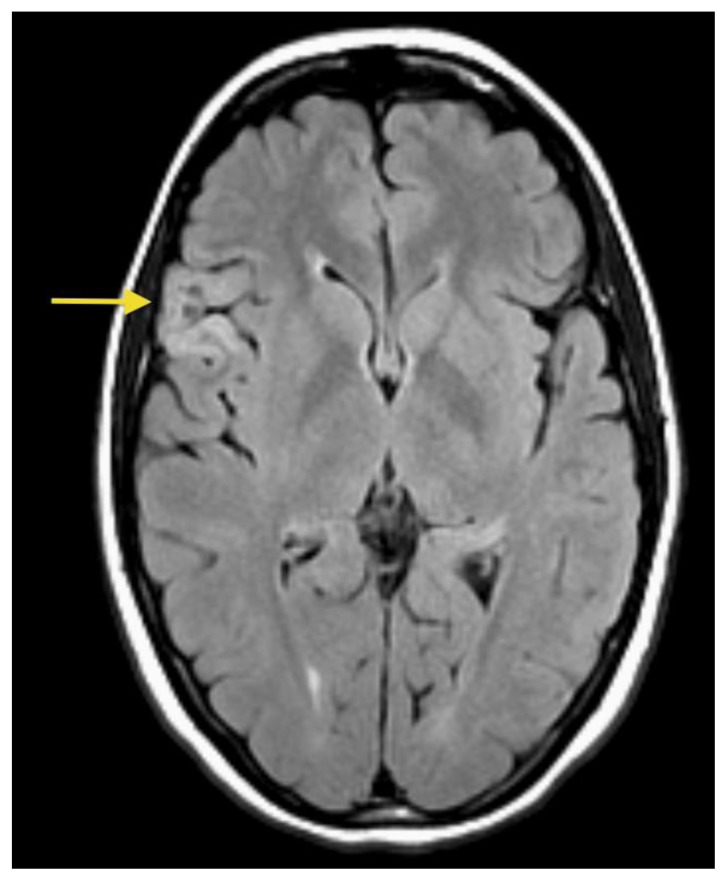
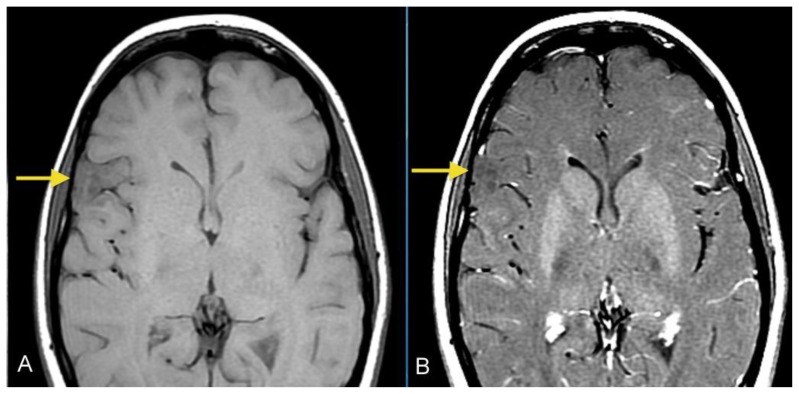
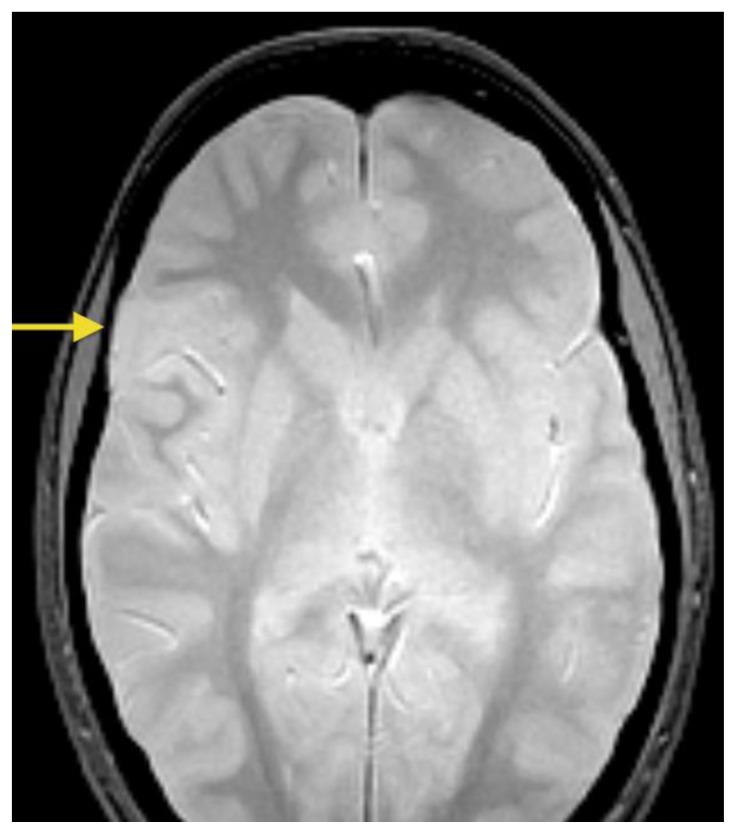
A 26- year-old female presented with a history of a prior seizure occurring several months previously and maintained on antiepileptic medication. The patient underwent conventional MRI scanning including diffusion tensor imaging (DTI). MRI data was processed to obtain tractography and fractional anisotropy (FA) maps using General Electric (GE) Functools software. MR images were analyzed for location, extension of the mass lesion as well as displacement or destruction of brain parenchyma and white matter tracts. Brain MR imaging revealed a well-circumscribed, cortical based, non-enhancing, lobulated, intra-axial mass lesion in the superficial right frontal lobe (Figures 1A, 1B) measuring approximately 2.0 × 2.4 × 3.5 cm. The T2-weighted MR images also showed rounded focal areas of marked high signal intensity within the mass, which extended to the normal cortical margin (“soap bubble” appearance) (Figures 1A, 1B). There was scalloping of the adjacent inner table of the right frontal bone, indicating slow-growing lesion (Figure 1B). The FLAIR images showed a slightly hyperintense cortex and decreased signal in the nodular areas (Figure 2), with no evidence of hyperintense rim sign. Compared to normal white matter, the lesion had low-signal intensity on T1-weighted images and high signal intensity on T2-weighted images, without prominent mass effect or surrounding parenchymal edema (Figures 1, 3A). Contrast enhancement was not present (Figures 3A, 3B). No evidence of calcifications was noted on gradient echo images (Figure 4). Tractography demonstrated that the fiber tracts surrounding the lesion were displaced away from the center of the cortical based lesion, resembling a “comet-tail” (Figures 5A, 5B). Fractional anisotropy maps showed decreased fractional anisotropy within the lesion but no significant change in the perilesional white matter (Figure 6).
Figure 1.
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. A: Coronal T2-weighted MR image shows a cortical-based superficial lesion in right frontal lobe extending to the cortical margin. There are multiple small rounded internal focal regions of high signal intensity compatible with a “soap bubble” appearance (arrow). There is no evidence of associated mass effect or vasogenic edema. (Protocol: 1.5 Tesla MRI (GE system), Repetition time/Echo time TR/TE: 3750/76.9, 3mm slice thickness, non-contrast, field of view 24 cm). B: Axial T2-weighted MR image demonstrates superficial lesion in right frontal lobe extending to cortical margin. There are internal focal regions of high signal intensity, compatible with a “soap bubble” appearance (thin arrow). There is also narrowing and remodeling of the adjacent inner table of the skull, compatible with slow growth (wide arrow). (Protocol: 1.5 Tesla MRI (GE system), TR/TE: 3200/256, 5mm slice thickness, field of view 24 cm, non-contrast).
Figure 2.
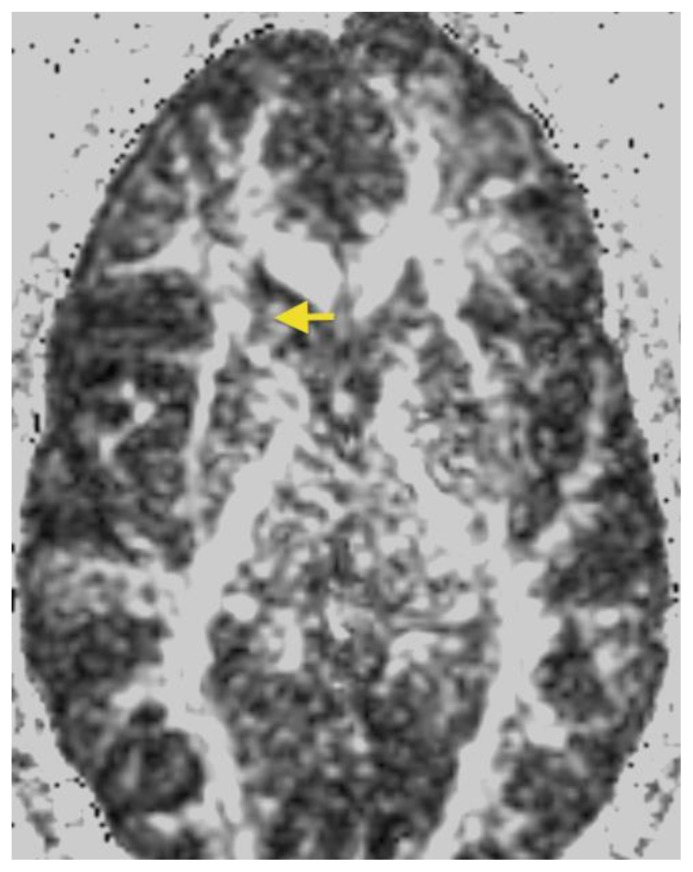
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Axial FLAIR image show a slightly hyperintense cortex and slightly decreased signal in the small focal nodular areas (arrow). (Protocol: 1.5 Tesla MRI (GE system), TR/TE: 8002/123, 5mm slice thickness, field of view 24 cm, non-contrast).
Figure 3.
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Axial pre (A) and post-contrast (B) T1-weighted images image show a slightly hypointense cortex in the right lateral frontal lobe with no definite enhancement (Protocol: 1.5 Tesla MRI (GE system), TR/TE: 767/16, 5mm slice thickness, field of view 24 cm).
Figure 4.
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Axial gradient echo image shows no signal loss or ‘blooming’ within the mass, indicating no evidence of calcifications or hemorrhage within the mass (arrow). (Protocol: 1.5 Tesla MRI (GE system), TR/TE: 500/9, 5mm slice thickness, field of view 24 cm, non-contrast).
Figure 5.
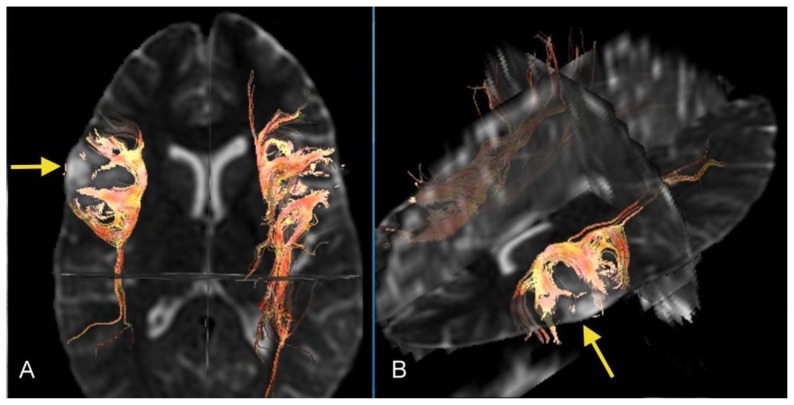
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Axial Diffusion tensor imaging (DTI) with axial (A) and oblique (B) 3-dimensional images demonstrates deformed and displaced but intact fiber tracts curving around and surrounding the lesion (arrows). The red color is present for visual demonstration of the tracts. (Protocol: DTI was processed with GE Functools software after acquiring data from 1.5 Tesla MRI, GE system, TR/TE: 8500/91.8, 5 mm slice thickness, field of view 24 cm, b-value 1000 s/mm2, number of directions 25)
Figure 6.
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Axial Fractional Anisotropy (FA) map corresponding to Figure 1B shows that the signal in the white matter surrounding the lesion is preserved, suggesting intact fiber integrity (arrow). (Protocol: GE system 1.5 Tesla MRI, TR/TE: 8500/91.8, 5 mm slice thickness, field of view 24 cm, b-value 1000 s/mm2, number of directions 25).
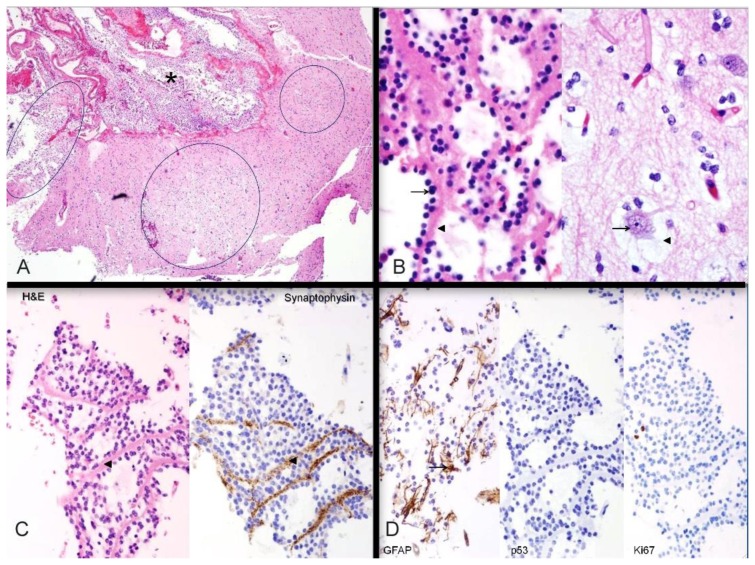
The patient underwent right frontal craniotomy for resection of the mass. Histopathology showed findings typical of a mixed glial-neural neoplasm, with a multinodular architecture and a heterogeneous cellular composition, and confirmed a DNET. A section of neocortex showed interruption by multiple tumor nodules, some extending into the subarachnoid space (Figure 7A). The nodules showed a distinctive arrangement of oligodendroglia-like cells (OLC) lined up around bundles of neuropil filaments (Figure 7B, left). In close proximity were normal cortical neurons suspended in pale myxoid material (floating neurons, Figure 7B, right). The two features collectively made up the “specific glioneuronal element” that characterizes DNETs. The neuropil bundles satellited by the oligodendroglia-like cells showed strong synaptophysin immunopositivity, supporting the notion that these bundles are composed of axons from cortical neurons (Figure 7C). Scattered reactive astrocytes were identified in the specific glioneuronal elements (Figure 7D, left). The tumor was negative for mutant p53 protein expression (Figure 7D, center) and demonstrated a low proliferation index by Ki-67 immunostaining (Figure 7D, right). A follow-up MRI performed one month after resection demonstrated expected postoperative changes, and the patient had complete resolution of seizures and was doing very well.
Figure 7.
26- year-old female with a dysembryoplastic neuroepithelial tumor (DNET) in the right frontal lobe. Histochemical and immunohistochemical stains of a dysembryoplastic neuroepithelial tumor (DNET) in a 26-year-old male. A: Haematoxylin- and eosin-stained section through the tumor demonstrates discrete intracortical tumor nodules (ovals); the nodule on the left extends into subarachnoid space (asterisk). Hematoxylin and eosin (H&E), high power (40×). B: Histologic features. LEFT: Oligodendroglia-like cells (arrow) lined up along neuropil bundles (arrowhead). RIGHT: Cortical neurons (arrow) floating in myxoid pools (arrowhead). These two features comprise the “specific glioneuronal element” of dysembryoplastic neuroepithelial tumor. Both images H&E, high power (400×). C: Axonal nature of neuropil bundles. The neuropil bundles (arrowheads) in the specific glioneuronal element are immunopositive (brown) for synaptophysin, supporting their axonal nature. LEFT: Hematoxylin and eosin (H& E), high power (200×); RIGHT: synaptophysin immunostain, high power (200×). D: Additional immunohistochemical findings. LEFT: GFAP-immunopositive reactive astrocytes (arrow) are scattered in the specific glioneuronal element. CENTER: Mutant p53 protein is not expressed in the nuclei of oligodendroglia-like cells. RIGHT: Neoplastic cells show a low proliferation index with Ki-67 immunostain. Left: Hematoxylin and eosin (H&E), glial fibrillary acidic protein (GFAP) immunostain, 200×; Center: p53 protein immunostain, 200×; Right; Ki-67 immunostain, 200
DISCUSSION
This case presents with atypical findings, as DNETs are less common in the frontal regions and less often seen in this age range. Dysembryoplastic neuroepithelial tumors (DNETs) are rare, usually benign tumors of neuroepithelial origin arising from the cortical gray matter. The temporal lobe is the most common site (62%), followed by the frontal lobe (31%) [1]. Although the vast majority of DNETs are confined to the cortical gray matter, they may also arise within the caudate nucleus, cerebellum, or pons [2]. The vast majority of patients are younger than 20 years, and males are more commonly affected with a 23:16 male to female ratio [1]. Large variations are noted in the incidence of DNET’s depending on the criteria used, but most (80%) tend to occur in patients with ages less than 30 years. In one report of DNETs in patients with partial complex epilepsy, 1.2% of patients were less than 20 years of age and 0.24% were more than 20 years old [1]. In terms of clinical symptoms, the usual presentation is intractable seizures, as in this case. Other neurological deficits are not common with DNETs unless they occur in the presence of complex congenital abnormalities, in contrast to other brain neoplasms [1]. Cortical dysplasias are commonly seen with DNETs, occurring in nearly 50 % of cases [3].
On MR imaging, DNET most commonly manifest as intracortical masses that are hypointense on T1-weighted images and hyperintense on T2-weighted images without surrounding perilesional edema or signs of mass effect. Some lesions may appear as an enlarged heterogeneous gyrus, with delicate septa-like structures that are visible within the lesion, producing a soap-bubble appearance at the cortical margins [1, 4, 5]. The conventional MRI imaging appearance of DNET is similar to those of other low-grade glial tumors, and in some cases it may be difficult to distinguish this tumor from other cortical tumors such as oligodendrogliomas, gangliogliomas, and astrocytomas. Ostertun et al noted that DNETs could show a multicystic appearance more commonly than gangliogliomas [6]. Fernandez et al analyzed the MR images and CT scans of 16 patients with complex partial epilepsy and DNET and found that in all cases, the lesion exceeded the thickness of the normal cortex and involved the adjacent white matter. In 8 cases, the tumor width was maximal at the cortex and decreased toward the ventricles, leading to a triangular pattern of distribution best seen on coronal images [7]. In some cases, the lesions had a cortical base and an apex pointing toward the lateral ventricle giving the appearance of a comet-tail [5]. The FLAIR hyperintense rim sign [5] was described as a thin rim of well-defined hyperintensity at the borders of the DNET, separating it from the surrounding normal brain. The pathologic correlate of this imaging sign was thought to be loosely packed glioneuronal elements at the margin of the lesion [5]. However, in our case a FLAIR hyperintense ring sign was not demonstrated. Since DNETs are slow growing, adjacent skull remodeling is often present. The distinction of this tumor from other brain tumors is important because patients with DNET benefit from complete resection, and the presence of residual tumor is a risk factor for relapse of seizures [8].
Diffusion tensor imaging (DTI) is a relatively new MR imaging technique that provides information on brain parenchyma and can be used for the preoperative assessment of white matter tracts in patients with brain tumors. Tractography and FA values have been used to classify a tumor’s effect on fiber tracts, such as disruption, displacement, infiltration, or edema [9,10,11]. This can be used to improve presurgical planning and classify the histopathologic and behavioral characteristics of the tumor. In our case, DTI indicated displacement rather than disruption of fiber tracts, with fiber integrity (fractional anisotropy) being maintained, suggestive of a lower grade, less aggressive neoplasm.
The differential diagnosis of DNET includes oligodendrogliomas, low-grade gliomas, gangliogliomas and pleomorphic xanthoastrocytomas (PXA). Clinical features, imaging findings and histologic findings are key in making the diagnosis. Oligodendrogliomas usually occur in the frontal lobes as in this case, but they do not demonstrate a soap-bubble appearance, and usually displace or disrupt fiber tracts [9]. Both oligodendrogliomas and gangliogliomas are usually calcified, and no calcifications were present in this case. A cystic supratentorial mass containing a mural nodule that is adjacent to the peripheral leptomeninges is the classic imaging appearance of a pleomorphic xanthoastrocytoma [12] with the solid portions of the tumor enhancing intensely following intravenous administration of contrast material. MRI with diffusion tensor imaging can help to differentiate lower-grade from higher-grade tumors [10]. Aggressive intra-axial tumors such as glioblastomas can cause infiltration and destruction of adjacent white matter tracts [7]. Generally, DNETs appear to be remarkably stable in terms of biologic behavior, and surgical resection is the preferred method of treatment. Despite only partial resection of many of these lesions, complete cessation of all seizure activity is a common outcome following neurosurgical intervention [13].
In terms of gross pathology, DNETs may show wide variations in size and shape, but are usually mucinous, multinodular or muticystic lesions of friable consistency [14]. Microscopically, DNETs are composed of multiple nodules containing both neuronal and glial components. They are characterized by an admixture of astrocytes and oligodendroglial elements, in association with “floating neurons” and mucinous degeneration [12]. The multinodular pattern and areas of mucinous degeneration observed on histopathology may contribute to a soap-bubble appearance on neuroimaging. Other common histological features include “specific glioneuronal elements” oriented in a columnar pattern, perpendicular to the cortical surface and focal areas of cortical dysplasia [14].
Though recurrence is very rare, there is one recent report in the literature of malignant transformation of a DNET that demonstrated a higher degree of mitotic activity than is commonly seen in these tumors [12]. This case emphasizes the need for long-term follow-up of patients with atypical DNETs [12]. In our case, no atypical cells were demonstrated.
TEACHING POINT
Diagnosis of dysembryoplastic neuroepithelial tumors (DNETs) is based on a combination of findings: a cortical-based mass in a young patient presenting with intractable seizures with a typical soap-bubble appearance, and without mass effect or perilesional edema on MRI. Diffusion Tensor Imaging (DTI) with tractography and fractional anisotropy can provide information on adjacent white matter tracts for preoperative assessment and surgical planning. Displacement, rather than disruption of fiber bundles on DTI was a feature in this example of a DNET, and is more commonly seen in low- grade tumors. Recognition of DNETs is important because surgical resection is curative with a good prognosis.
Table 1.
Summary table for dysembryoplastic neuroepithelial tumor
| Etiology | Glioneuronal neoplasm often associated with cortical dysplasia. Originate from abnormal dysplastic germinal matrix cells |
| Incidence | Dysembryoplastic neuroepithelial tumor (DNET) represents 1.2% of all neuroepithelial tumors in patients under age 20 and 0.2% of those aged more than 20 years |
| Gender ratio | M:F=23:16 |
| Age predilection | Peak incidence in first two decades |
| Treatment | Surgical Resection |
| Prognosis | Excellent. Grade I WHO |
| Findings on imaging | MRI: usually supratentorial, intracortical, T1 hypointense, T2 hyperintense, non-enhancing lesion. Commonly shows a soap bubble, “bubbly” or septated appearance. Adjacent cortical dysplasia may be seen. More common in temporal rather than frontal lobes. |
Table 2.
Differential diagnosis for dysembryoplastic neuroepithelial tumor
| Entity | CT findings | MRI findings | Diffusion tensor imaging (DTI) findings |
|---|---|---|---|
| Dysembryoplastic neuroepithelial tumor (DNET) |
|
|
Displacement of fiber bundles |
| Oligodendroglioma |
|
|
Displacement or infiltration of fiber tracts |
| Ganglioglioma |
|
|
Displacement of fiber bundles |
| Pleomorphic xanthoastrocytoma |
|
|
Displacement of fiber bundles |
| Glioblastoma multiforme( GBM) |
|
|
Infiltration and destruction of the adjacent white matter tracts |
ABBREVIATIONS
- CT
computed tomography
- DNET
dysembryoplastic neuroepithelial tumor
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- GBM
glioblastoma multiforme
- GFAP
glial fibrillary acidic protein
- H&E
Hematoxylin and eosin
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OLC
oligodendroglia-like cells
- PXA
pleomorphic xanthoastrocytoma
- TE
echo time
- TR
repetition time
REFERENCES
- 1.Daumas-Duport C, Pietsch T, Lantos PL. Dysembryoplastic neuroepithelialtumour. In: Kleihues P, Cavenee WK, editors. Pathology and genetics of tumours of the nervous system. Lyon, France: IARC; 2000. pp. 103–106. [Google Scholar]
- 2.Cervera-Pierot P, Varlet P, Chodkiewicz JP, Daumas-Duport C. Dysembryoplastic neuroepithelial tumors located in the caudate nucleus area: report of four cases. Neurosurgery. 1997 May;40(5):1065–1070. doi: 10.1097/00006123-199705000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Frater JL, Prayson RA, Morris HH, Bingaman WE. Surgical pathological findings of extratemporal-based intractable epilepsy: a study of 133 consecutive resections. Arch Pathol Lab Med. 2000 Apr;124:545–549. doi: 10.5858/2000-124-0545-SPFOEB. [DOI] [PubMed] [Google Scholar]
- 4.Kuroiwa T, Kishikawa T, Kato A, Ueno M, Kudo S, Tabuci K. Dysembryoplastic neuroepithelial tumors: MR findings. J Comput Assist Tomogr. 1994 May-Jun;18(3):352–356. doi: 10.1097/00004728-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Parmar HA, Hawkins C, Ozelame R, Chuang S, Rutka J, Blaser S. Fluid-attenuated inversion recovery ring sign as a marker of dysembryoplastic neuroepithelial tumors. J Comput Assist Tomogr. 2007 May-Jun;31(3):348–353. doi: 10.1097/01.rct.0000243453.33610.9d. [DOI] [PubMed] [Google Scholar]
- 6.Ostertun B, Wolf HK, Campos MG, et al. Dysembryoplastic neuroepithelial tumors: MR and CTevaluation. Am J Neuroradiol. 1996 Mar;17(3):419–430. [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez C, Girard N, Paz Paredes A, Bouvier-Labit C, Lena G, Figarella-Branger D. The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR. 2003 May;24(5):829–34. [PMC free article] [PubMed] [Google Scholar]
- 8.Raz E, Kapilamoorthy TR, Gupta AK, Fiorelli M. Dysembryoplastic Neuroepithelial Tumor. Radiology. 2012 Oct;265(1):317–320. doi: 10.1148/radiol.12100118. [DOI] [PubMed] [Google Scholar]
- 9.Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J of neurosurgery. 2002 Sep;97(3):568–75. doi: 10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]
- 10.Yen PS, Teo BT, Chiu CH, Chen SC, Chiu TC, Su CF. White matter tract involvement in brain tumors: a diffusion tensor imaging analysis. Surg neurol. 2009 Nov;72(5):464–9. doi: 10.1016/j.surneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Wei CW, Guo G, Mikulis DJ. Tumor effects on cerebral white matter as characterized by diffusion tensor tractography. Can J Neurol sci. 2007 Feb;34(1):62–8. doi: 10.1017/s0317167100005801. [DOI] [PubMed] [Google Scholar]
- 12.Koeller KK, Henry JM. Superficial Gliomas: Radiologic- Pathologic Correlation. RadioGraphics. 2001 Nov-Dec;21(6):1533–56. doi: 10.1148/radiographics.21.6.g01nv051533. [DOI] [PubMed] [Google Scholar]
- 13.Chan CH, Bittar RG, Davis GA, Kalnins RM, Fabinyi GC. Long-term seizure outcome following surgery for dysembryoplastic neuroepithelial tumor. J Neurosurg. 2006 Jan;104(1):62–9. doi: 10.3171/jns.2006.104.1.62. [DOI] [PubMed] [Google Scholar]
- 14.Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumour: a surgically curable tumor of young patients with intractable partial seizures. Neurosurgery. 1988 Nov;23(5):545–56. doi: 10.1227/00006123-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hammond RR, Duggal N, Woulfe JM, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor. J Neurosurg. 2000 Apr;92(4):722–5. doi: 10.3171/jns.2000.92.4.0722. [DOI] [PubMed] [Google Scholar]