Abstract
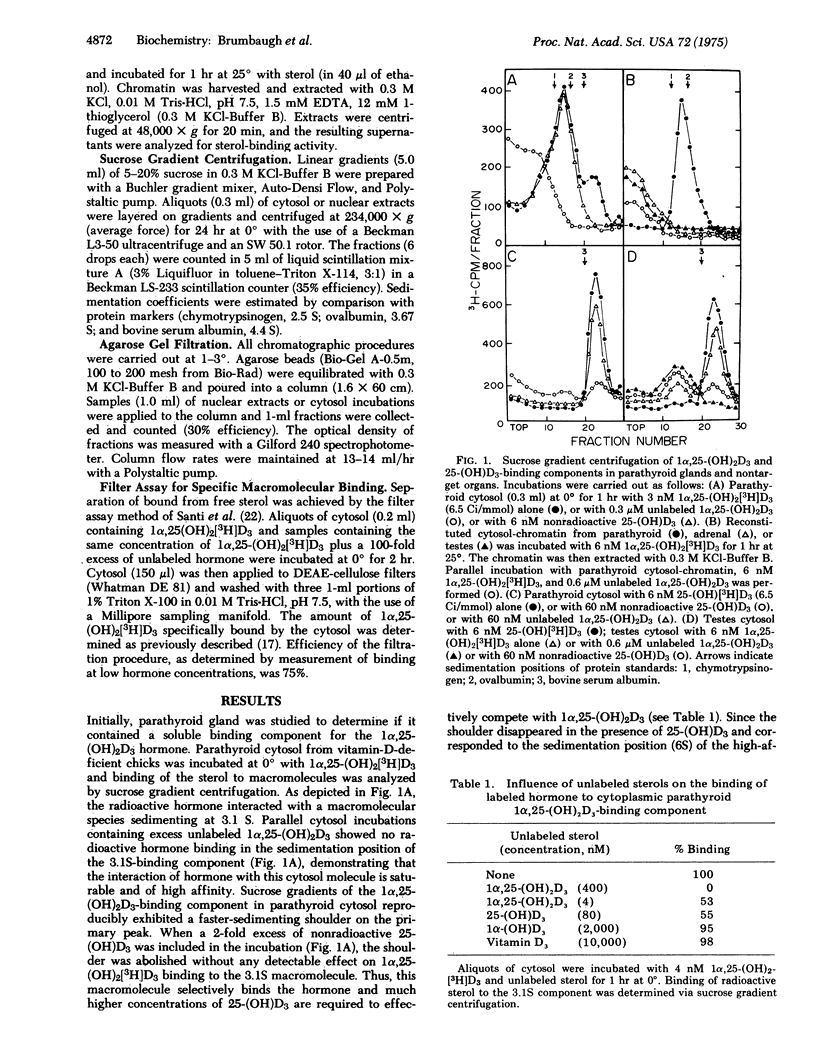
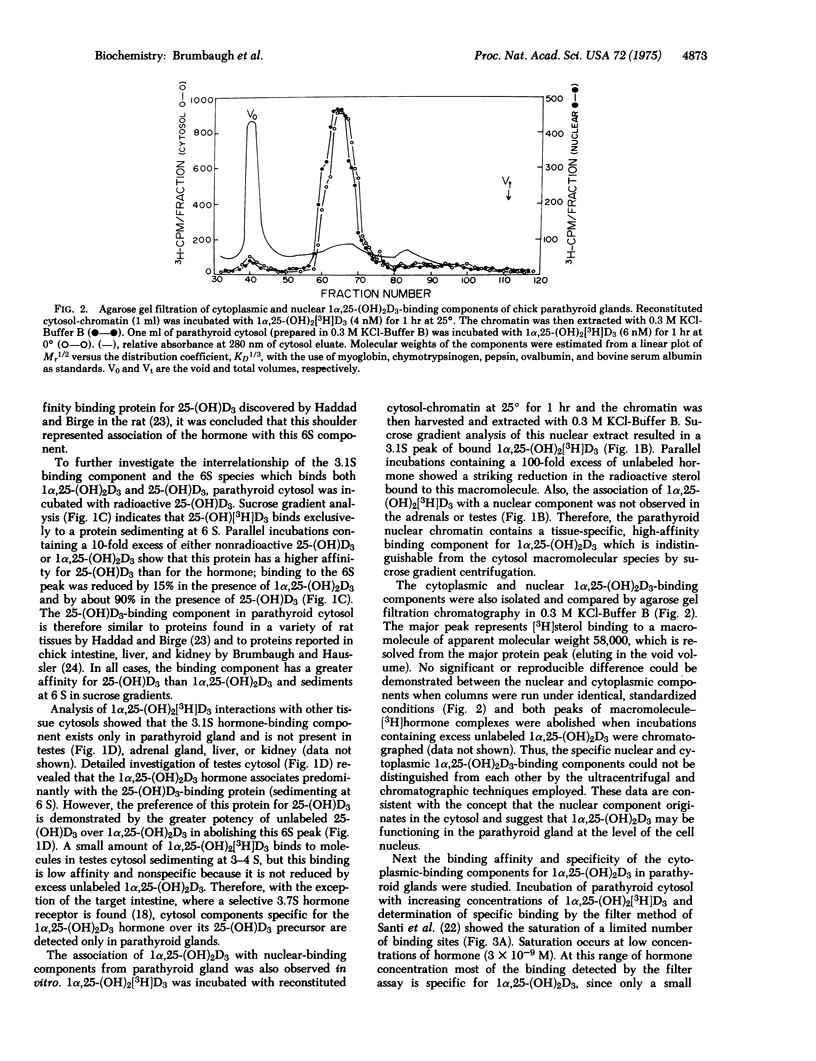
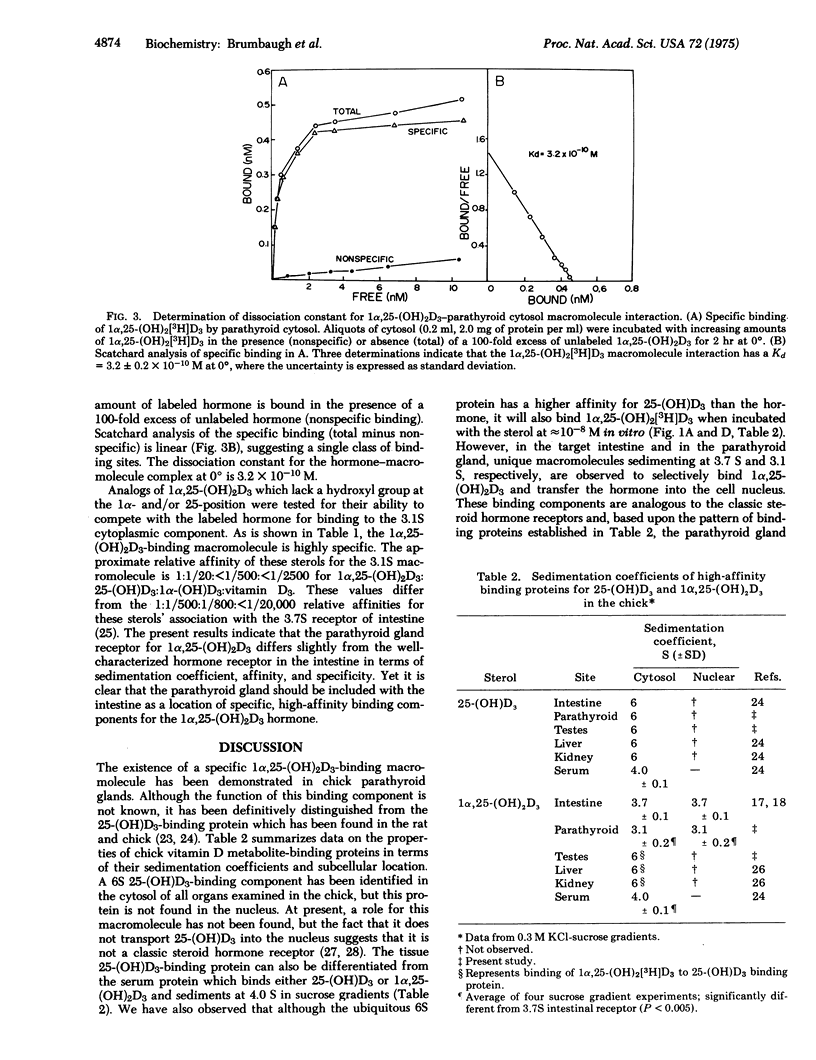
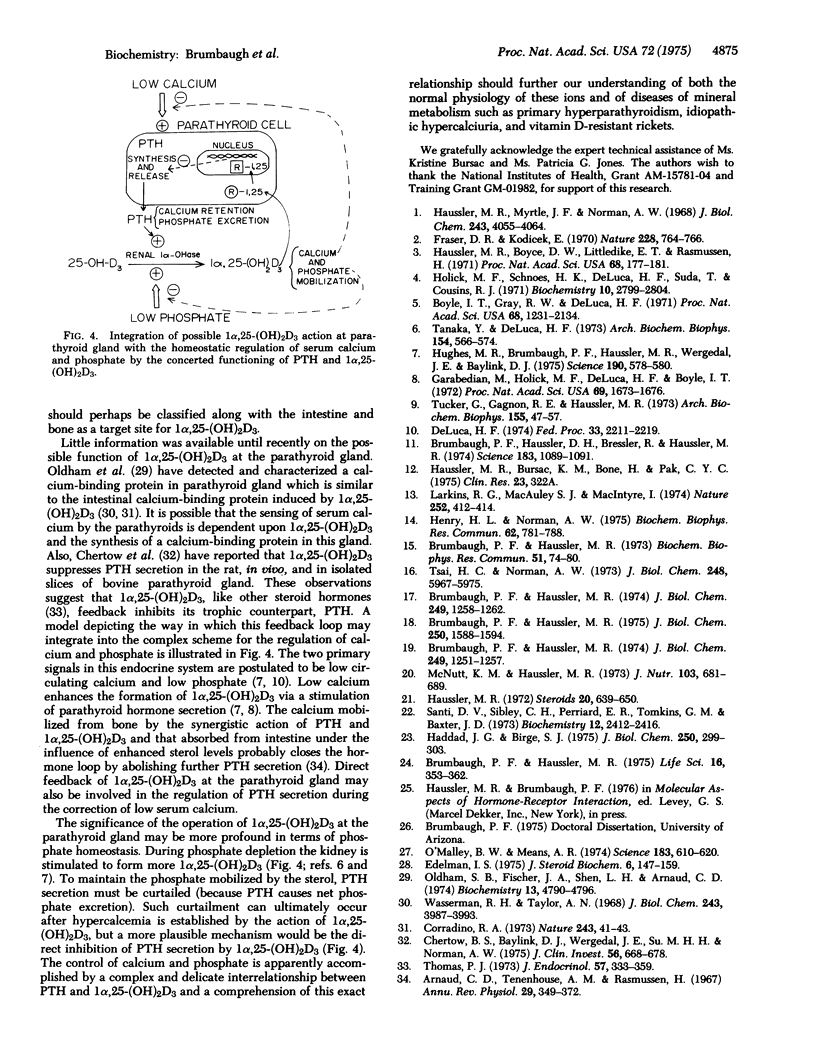
Specific binding of 1 alpha,25-dihydroxyvitamin D3 [1alpha,25-(OH)2D3] to macromolecular components in the cytoplasm and nucleus is demonstrated in parathyroid glands of vitamin-D-deficient chicks. The interaction of 1alpha,25-(OH)2D3 with the cytoplasmic binding component is of high affinity (Kd = 3.2 X 10(-10) M) and high specificity [1alpha,25-(OH)2D3 greater than 25-hydroxyvitamin D3 greater than 1alpha-hydroxyvitamin D3 greater than vitamin D3 in competing with radioactive 1alpha,25-(OH)2D3]. Both cytoplasmic and nuclear hormone-macromolecular complexes sediment at 3.1 S in 0.3 M KC1-sucrose gradients, and agarose gel filtration of the components indicates an apparent molecular weight of 58,000. The 3.1S binding molecules are not observed in adrenal gland, testes, liver, or kidney, but similar receptors for 1alpha,25-(OH)2D3 have been found previously in intestine. Macromolecular species with a high affinity and preference for 25-hydroxyvitamin D3 [25-(OH)D3] are also identified in parathyroid cytosol and differ from the parathyroid 1alpha,25-(OH)2D3-binding component in that: (1) they sediment at 6 S in 0.3 M KC1-sucrose gradients, (2) they are observed in all tissues examined, (3) they have a higher affinity for 25-(OH)D3 than 1alpha,25-(OH)2d3, and (4) they are not found in the nucleus of the parathyroid glands, in vitro. The discovery of unique 1alpha,25-(OH)2D3-binding components in the parathyroid glands is consistent with the sterol hormone's action at this endocrin site and possible involvement in the regulation of parathyroid hormone synthesis and secretion.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud C. D., Jr, Tenenhouse A. M., Rasmussen H. Parathyroid hormone. Annu Rev Physiol. 1967;29:349–372. doi: 10.1146/annurev.ph.29.030167.002025. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler D. H., Bressler R., Haussler M. R. Radioreceptor assay for 1 alpha,25-dihydroxyvitamin D3. Science. 1974 Mar 15;183(4129):1089–1091. doi: 10.1126/science.183.4129.1089. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974 Feb 25;249(4):1251–1257. [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. Nuclear and cytoplasmic binding components for vitamin D metabolites. Life Sci. 1975 Feb 1;16(3):353–362. doi: 10.1016/0024-3205(75)90256-8. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. Nuclear and cytoplasmic receptors for 1,25-dihydroxycholecalciferol in intestinal mucosa. Biochem Biophys Res Commun. 1973 Mar 5;51(1):74–80. doi: 10.1016/0006-291x(73)90509-3. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. Specific binding of 1alpha,25-dihydroxycholecalciferol to nuclear components of chick intestine. J Biol Chem. 1975 Feb 25;250(4):1588–1594. [PubMed] [Google Scholar]
- Chertow B. S., Baylink D. J., Wergedal J. E., Su M. H., Norman A. W. Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretion in vitro by 1,25-dihydroxycholecalciferol. J Clin Invest. 1975 Sep;56(3):668–678. doi: 10.1172/JCI108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradino R. A. 1,25-Dihydroxycholecalciferol: inhibition of action in organ-cultured intestine by actinomycin D and alpha-amanitin. Nature. 1973 May 4;243(5401):41–43. doi: 10.1038/243041a0. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D: the vitamin and the hormone. Fed Proc. 1974 Nov;33(11):2211–2219. [PubMed] [Google Scholar]
- Edelman I. S. Mechanism of action of steroid hormones. J Steroid Biochem. 1975 Mar-Apr;6(3-4):147–159. doi: 10.1016/0022-4731(75)90125-9. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Birge S. J. Widespread, specific binding of 25-hydroxycholecalciferol in rat tissues. J Biol Chem. 1975 Jan 10;250(1):299–303. [PubMed] [Google Scholar]
- Haussler M. R., Boyce D. W., Littledike E. T., Rasmussen H. A rapidly acting metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1971 Jan;68(1):177–181. doi: 10.1073/pnas.68.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R. Characterization of the metabolites of vitamin D 3 in the chick. Steroids. 1972 Nov;20(5):639–650. doi: 10.1016/0039-128x(72)90021-9. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Studies on the mechanism of action of calciferol VII. Localization of 1,25-dihydroxy-vitamin D3 in chick parathyroid glands. Biochem Biophys Res Commun. 1975 Feb 17;62(4):781–788. doi: 10.1016/0006-291x(75)90391-5. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Suda T., Cousins R. J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971 Jul 6;10(14):2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., MacIntyre I. Feedback control of vitamin D metabolism by a nuclear action of 1,25-dihydroxycholecalciferol on the kidney. Nature. 1974 Nov 29;252(5482):412–414. doi: 10.1038/252412a0. [DOI] [PubMed] [Google Scholar]
- McNutt K. W., Haussler M. R. Nutritional effectiveness of 1,25-dihydroxycholecalciferol in preventing rickets in chicks. J Nutr. 1973 May;103(5):681–689. doi: 10.1093/jn/103.5.681. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Oldham S. B., Fischer J. A., Shen L. H., Arnaud C. D. Isolation and properties of a calcium-binding protein from porcine parathyroid glands. Biochemistry. 1974 Nov 5;13(23):4790–4796. doi: 10.1021/bi00720a017. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Sibley C. H., Perriard E. R., Tomkins G. M., Baxter J. D. A filter assay for steroid hormone receptors. Biochemistry. 1973 Jun 19;12(13):2412–2416. doi: 10.1021/bi00737a007. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. J. Steroid hormones and their receptors. J Endocrinol. 1973 May;57(2):333–359. doi: 10.1677/joe.0.0570333. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Norman A. W. Studies on calciferol metabolism. 8. Evidence for a cytoplasmic receptor for 1,25-dihydroxy-vitamin D3 in the intestinal mucosa. J Biol Chem. 1973 Sep 10;248(17):5967–5975. [PubMed] [Google Scholar]
- Tucker G., 3rd, Gagnon R. E., Haussler M. R. Vitamin D 3 -25-hydroxylase: tissue occurrence and apparent lack of regulation. Arch Biochem Biophys. 1973 Mar;155(1):47–57. doi: 10.1016/s0003-9861(73)80008-6. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J Biol Chem. 1968 Jul 25;243(14):3987–3993. [PubMed] [Google Scholar]


