Abstract
Sclerosing encapsulating peritonitis, previously referred to as cocoon bowel, is a rare cause of intestinal obstruction that often results in obstruction due to the development of a fibrous enhancing membrane that encases multiple small bowel loops. We present a case of a patient who presented to our institution with abdominal distension and guarding. Computed tomography was obtained which revealed findings concerning for sclerosing encapsulating peritonitis. Sonographic imaging was also obtained and provides correlative imaging.
Keywords: Sclerosing Encapsulating Peritonitis, Cocoon bowel, Peritonitis, Peritoneal Dialysis
CASE REPORT
A 49-year-old male with a past medical history significant for malnutrition, cirrhosis secondary to non-alcoholic steatohepatitis, and end-stage renal disease requiring hemodialysis presented to our institution with persistent serosanguineous discharge from a prior paracentesis site. Despite not having a liver biopsy, Hepatology felt that the patient’s cirrhosis was secondary to non-alcoholic steatohepatitis because the patient was at one time morbidly obese (over 350 pounds) and had negative hepatitis B and C serologies. On physical exam the patient’s abdomen was distended with tenderness to deep palpation within the left upper abdominal quadrant. Guarding was present although the patient had normal bowel sounds. A Jackson-Pratt drain was present in the left upper quadrant that was placed from a prior admission during which the patient developed splenic abscesses positive for Klebsiella pneumonia. He had also developed spontaneous bacterial peritonitis with vancomycin-resistant enterococcus isolated as the causative agent. An exploratory laparotomy, splenectomy, and prolonged antibiotics were necessary to treat these complications.
Once re-admitted, a CT scan of the patient’s abdomen and pelvis was obtained that showed diffuse peritoneal thickening and enhancement, which was likely consistent with the patient’s history of spontaneous bacterial peritonitis diagnosed one-month prior by ascitic fluid culture. In addition, the CT showed encapsulation of the entire small bowel by a thin fibrous membrane (Figures 1 and 2). The image findings were concerning for sclerosing encapsulating peritonitis with possible closed loop small bowel obstruction. Surgery was consulted but opted for medical management since the patient did not demonstrate clinical signs of small bowel obstruction. Due to the patient’s recurring ascites, ultrasound-guided paracentesis was obtained which showed numerous septations and loculations throughout the abdomen (Figure 3). Paracentesis yielded around 1.75 liters of reddish ascitic fluid. The patient remained clinically stable and afebrile, so he was discharged a few days later to a local rehabilitation center.
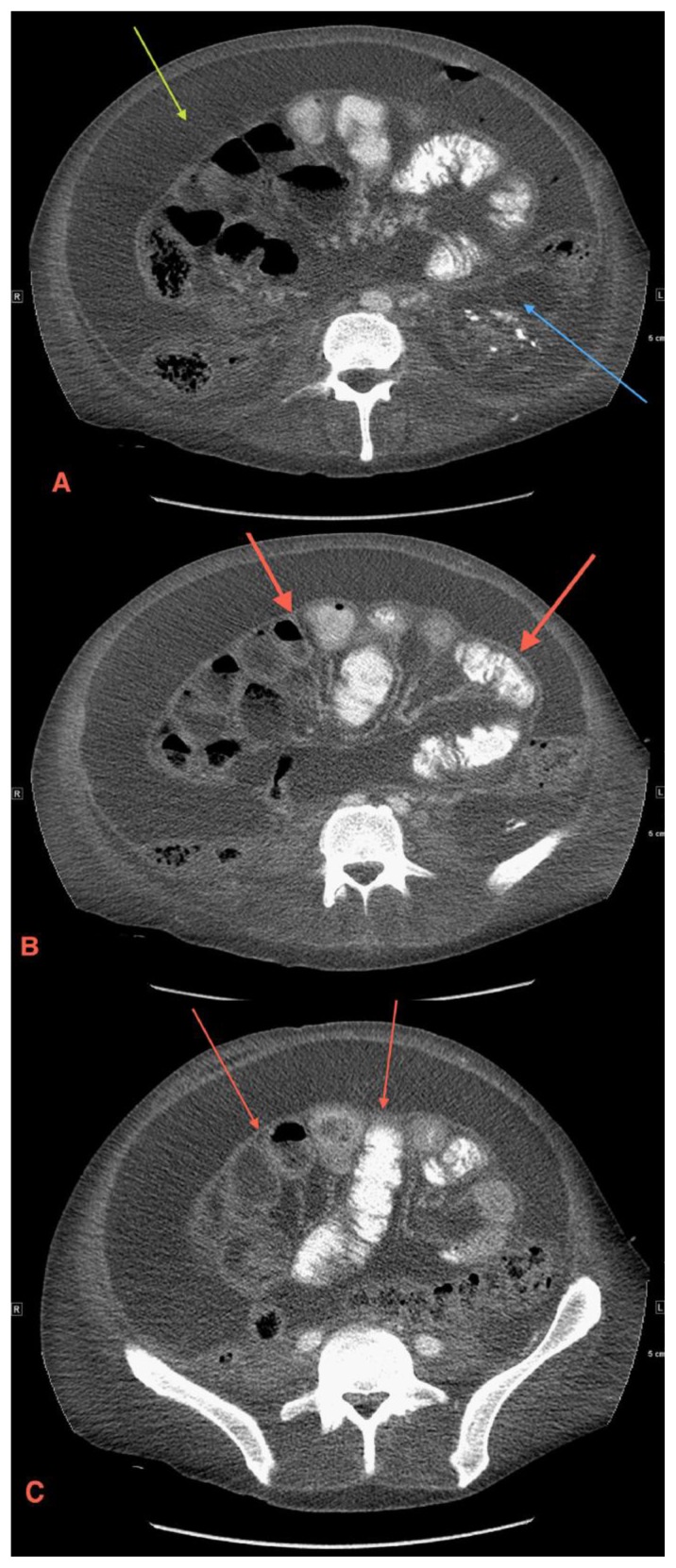
Figure 1.
49 year old male with sclerosing encapsulating peritonitis. Axial contrast-enhanced CT scan through different abdominal levels shows diffuse abdominal ascites (1a green arrow), a polycystic left kidney (1a blue arrow), and a thin fibrous membrane encasing the small bowel (1b and 1c red arrows). (Protocol: GE LightSpeed VCT 64 slice CT scanner, kvp 120, mA 772, 5 mm slice thickness, Pitch 1.375:1, 100 cc of Optiray 350 IV, in venous phase)
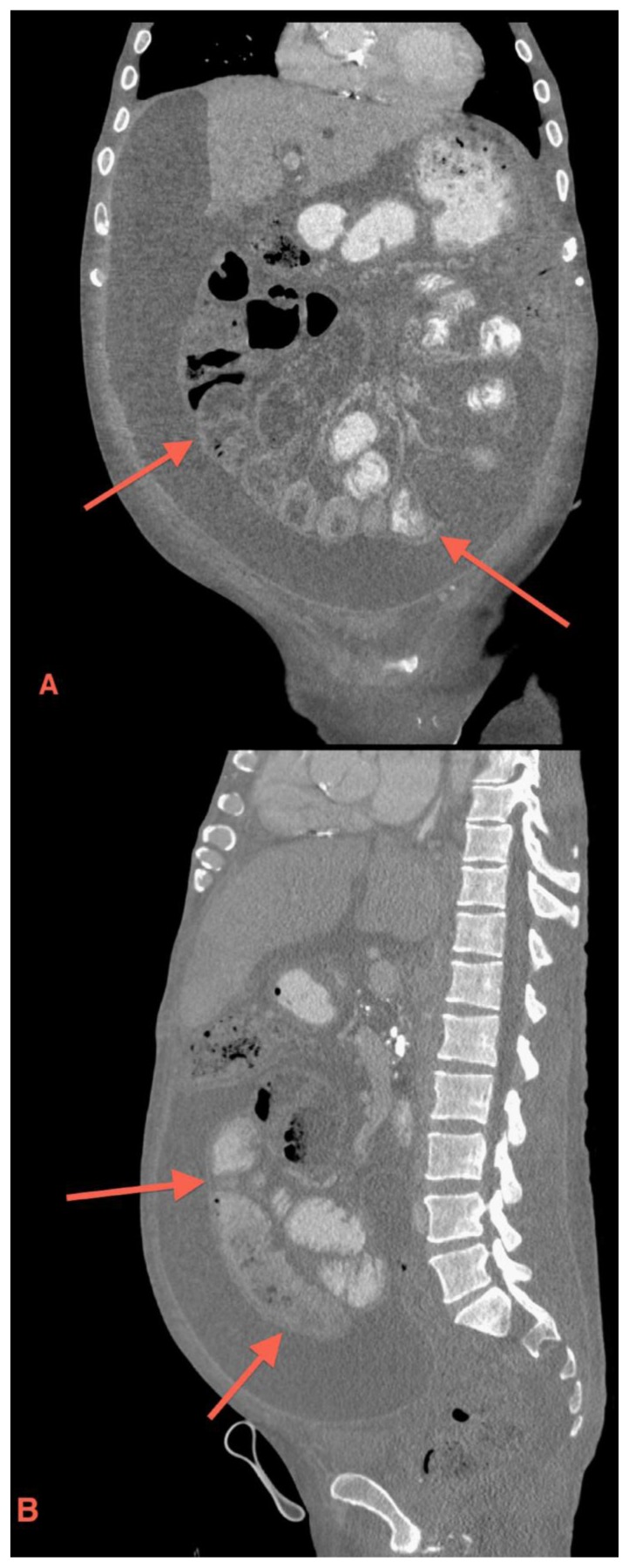
Figure 2.
49 year old male with sclerosing encapsulating peritonitis. Coronal (2a) and sagittal (2b) contrast-enhanced CT reformats demonstrate cirrhosis, diffuse ascites and a fibrous membrane (red arrows) encasing the small bowel. (Protocol: GE LightSpeed VCT 64 slice CT scanner, kvp 120, mA 772, 5 mm slice thickness, Pitch 1.375:1, 100 cc of Optiray 350 IV, in venous phase)
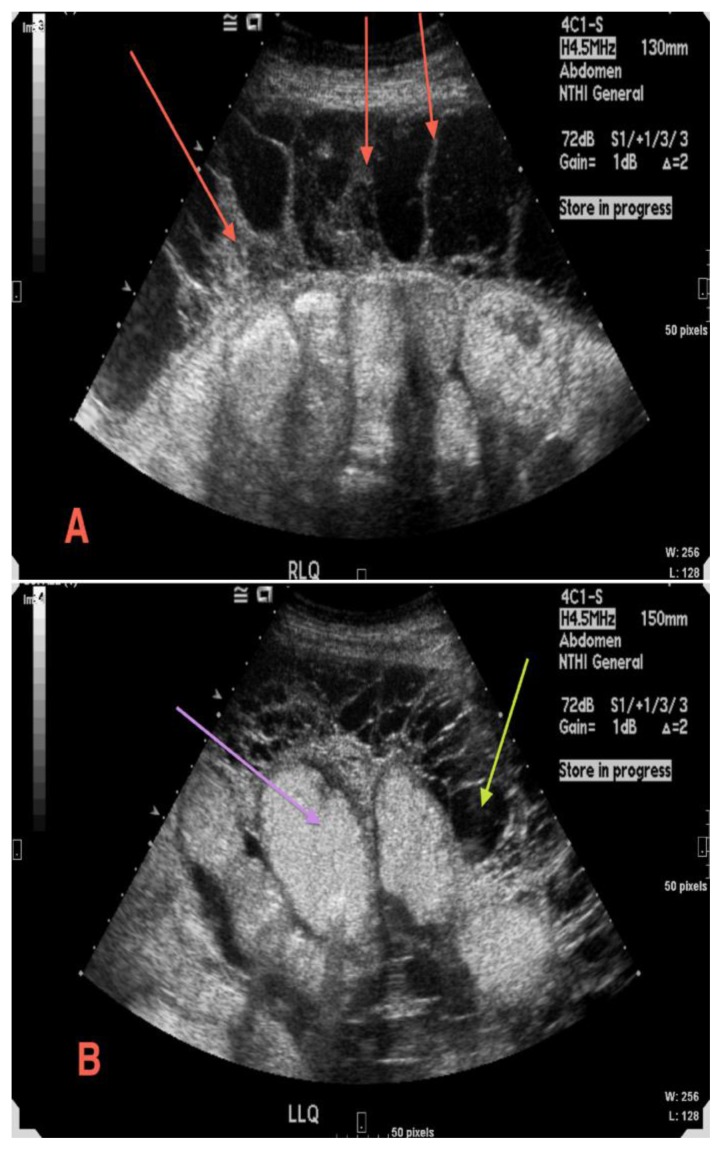
Figure 3.
49 year old male with sclerosing encapsulating peritonitis. Multiple gray-scale images demonstrate thick septations (3a red arrows) and loculated ascites (3b yellow arrow) encasing the small bowel (3b purple arrow). (Protocol: Siemens Acuson Sequoia 512 Ultrasound, 4CI - 4.0/3.0/1.75 MHz -abdominal curved array transducer)
The patient was readmitted six days later complaining of bleeding from his splenectomy incision site. Once re-admitted, the patient was found to have a systolic blood pressure of 70 and a heart rate in the 120s. A fluid culture from the incision site was positive for Klebsiella pneumonia. Abdominal fluid culture from paracentesis was positive for both Klebsiella pneumonia and vancomycin-resistant enterococcus. The patient was started on aztreonam and tigecycline due to concerns of sepsis from continued intraabdominal infection. Laboratory data showed a platelet count of 4,000 (normal range is 150,000–450,000/mmol) and an INR of 2.2 (normal range is 0.8–1.0). A repeat CT scan again showed centrally encapsulated small bowel surrounded by loculated ascites. General surgery was consulted but deemed the patient not to be a surgical candidate due to his chronic cachectic, malnourished state. The patient continued to deteriorate despite medical management and later developed hypothermia. Despite supportive measures the patient passed away twelve days following readmission.
DISCUSSION
Sclerosing encapsulating peritonitis, previously termed cocoon bowel, is characterized by an abnormally thickened, fibrotic peritoneum that encapsulates the small bowel loops resulting in obstruction. Although many risk factors exist for sclerosing encapsulating peritonitis, the exact etiology and underlying pathophysiology of this disease process are not well understood. One proposed mechanism pertains to patients with cirrhosis who have peritoneovenous shunts [1]. Since these patients tend to have a faster ascitic fluid circulation, it is believed that the release of fibrogenic cytokines causes increased deposition of fibrin on the peritoneum, which converts fibrinous adhesions to generalized peritoneal fibrosis [2]. Histologically, mononuclear inflammatory cells infiltrate the peritoneal membrane [3]. The incidence of sclerosing encapsulating peritonitis ranges from 0.5–4.4%, and the incidence increases with the duration of peritoneal dialysis. Furthermore, there appears to be a direct relationship between duration of peritoneal dialysis, mortality, and the incidence of sclerosing encapsulating peritonitis [4]. A prospective, multicenter study in Japan performed from 1999 to 2003 by Kawanishi et al. involved 1,958 peritoneal dialysis patients across 57 facilities [4]. Results of this study showed both an incidence and mortality of 0% at 3 years [4]. Incidence and mortality rose to 2.1% and 8.3%, respectively, at 8 years [4]. After 15 years of peritoneal dialysis, the incidence and mortality were 17.2% and 100%, respectively [5,6]. The overall mortality of sclerosing encapsulating peritonitis is high at 56% with a range of 38–83%. Currently, no established age predilection or gender ratio exists for sclerosing encapsulating peritonitis.
Many risk factors exist for sclerosing encapsulating peritonitis [1,2,7–10]. Several case studies list long term peritoneal dialysis, prior abdominal surgery, retrograde peritonitis, practolol therapy, sarcoid, liver transplantation, peritoneal tuberculosis, ventriculoperitoneal shunts, and peritoneovenous shunts as potential risk factors [9]. Many cases are also idiopathic [8,10]. The idiopathic form is commonly seen in adolescent females and is thought to result from retrograde menstruation that leads to a subclinical peritoneal infection [11]. Treatment options for sclerosing encapsulating peritonitis consist of surgical intervention or medical management depending on the clinical presentation [11]. Medical options include supportive measures such as bowel rest and nutritional support [11]. Medications that recently have been used to treat sclerosing encapsulating peritonitis include: corticosteroids, tamoxifen, and immunosuppressants. Each of these medications treat the condition by reducing the inflammation and fibrosis of sclerosing encapsulating peritonitis [11]. Surgical intervention consists of lysis of intestinal adhesions. Diagnosis of sclerosing encapsulating peritonitis is usually confirmed by laparotomy, which reveals a fibrous membrane surrounding multiple small bowel loops [9,10]. Key computed tomography findings seen in sclerosing encapsulating peritonitis include peritoneal calcification and a thick fibrous, enhancing membrane that encases several small bowel loops or the entire small bowel. Ascites is often seen as well and can be diffuse or loculated [8]. Key sonographic features include internal septations, thick fibrous membranes, and loculated ascites surrounding the small bowel [8]. Additional ultrasound features include the small-bowel loops having the overall appearance of a cauliflower or concertina due to their arrangement within the fibrous membrane sac.
Sclerosing encapsulating peritonitis often has a vague clinical presentation that can lead to a delay in diagnosis and treatment. Physical exam and laboratory testing will not raise clinical suspicion for this rare disease process [2]. Symptoms of sclerosing encapsulating peritonitis are nonspecific and usually consist of anorexia, bloody ascites, nausea, diarrhea, and abdominal pain [7]. Sclerosing encapsulating peritonitis can lead to serious complications such as severe malnutrition, sepsis, and death if not promptly diagnosed and treated. Abdominal plain films can often show dilated small bowel loops typical of obstruction; however, the fibrosing, encapsulating membrane typical of sclerosing encapsulating peritonitis will not be seen on this imaging modality. Ultrasound and computed tomography are two easily accessible imaging modalities with relatively short study times that can be key in making a diagnosis. Since surgery is often needed to decompress the encased small bowel loops, CT and ultrasound imaging can lead to prompt diagnosis, treatment, and improved patient outcomes [8,10].
The differential diagnosis for sclerosing encapsulating peritonitis includes disease processes that could result in similar imaging features such as peritoneal enhancement, peritoneal calcification, ascites, or possible small bowel obstruction [8,10]. Similar disease processes to consider in the differential diagnosis would include peritoneal carcinomatosis, pseudomyxoma peritonei, peritoneal mesothelioma, and tuberculous peritonitis. Clinical history and CT findings are critical in differentiating these respective disease entities, although overlap of CT features is commonly seen [4].
Differentiating sclerosing encapsulating peritonitis from peritoneal carcinomatosis: the latter is commonly seen in patients with a history of ovarian cancer or gastrointestinal tract malignancy [4]. Presenting symptoms are nonspecific and usually consist of ascites, abdominal pain, nausea, and vomiting. Important CT features of peritoneal carcinomatosis include thickening, nodularity, and enhancement of the peritoneum and diffuse tumor infiltration of the mesentery producing a pleated or stellate pattern [4]. Small bowel obstruction is often seen in peritoneal carcinomatosis from colorectal carcinoma [4]. Omental caking is another key CT feature of peritoneal carcinomatosis due to replacement of the omental fat by tumor and fibrosis [12]. Sonographically, peritoneal carcinomatosis can present with hypoechoic nodules that are commonly seen in Morison’s pouch, the pouch of Douglas, and the right subphrenic region [12]. Peritoneal nodules can also appear hyperechoic with acoustic shadowing from psammomatous calcification [12]. Septated ascites is often present as well.
Differentiating sclerosing encapsulating peritonitis from pseudomyxoma peritonei: the latter is commonly seen with mucin-producing tumors, most commonly appendiceal, pancreas, gallbladder or ovarian in origin [4]. Pseudomyxoma peritonei is more commonly seen in women and has a mean age at diagnosis of 49 [4]. Pseudomyxoma peritonei has nonspecific presenting symptoms such as weight loss, abdominal pain, and increasing abdominal girth [4]. Common CT features seen in pseudomyxoma peritonei include diffuse low attenuation mucinous ascites containing curvilinear or amorphous calcifications [4]. Scalloped margins of abdominal visceral organs, mainly the liver and spleen, are commonly seen as well [4]. Sonographically, pseudomyomxa peritonei can present with nonmobile echoes and ascites, suggesting gelatinous fluid [4]. Septations are often present and appear echogenic [4]. The gelatinous fluid appears hypoechoic and displaces the intestines centrally, leading to a starburst or star-like configuration due to echogenic bowel being surrounded by hypoechoic, gelantinous fluid.
Differentiating sclerosing encapsulating peritonitis from peritoneal mesothelioma: the latter is more commonly seen in males with an age range of 50–69 years and a history of prior asbestos exposure is seen in approximately 50% of peritoneal mesothelioma cases [13,14]. Common presenting symptoms of peritoneal mesothelioma include anorexia, weight loss, abdominal pain, and abdominal swelling from ascites. Three distinct CT types of peritoneal mesothelioma have been described in the literature: dry-painful, wet, and mixed [13]. The dry-painful type is most common and defined by a single large mass or multiple small peritoneal masses located in a single abdominal quadrant without ascites [13]. The wet type presents with diffuse nodules and plaques along with ascites and intestinal distention [14]. The mixed type contains features of both the dry-painful and wet types. Additional CT features of peritoneal mesothelioma include: omental masses, scalloped margins of abdominal visceral organs, and direct invasion of abdominal organs [15]. Sonographically, peritoneal mesothelioma can present with hypoechoic, sheetlike or nodular masses that cause peritoneal thickening [15]. Omental invasion can occur and presents as a hypoechoic mass with scattered hyperechoic areas suggesting entrapped omental fat [15]. Ascites can be seen but is usually minimal compared to the amount tumor dissemination [15]. Intestinal wall and solid organ invasion can also occur.
Differentiating sclerosing-encapsulating peritonitis from tuberculosis peritonitis: the latter is commonly seen in immunocompromised individuals, usually with a history AIDS or prior transplant. Tuberculosis peritonitis commonly presents with abdominal pain, fever, night sweats, weight loss, and anorexia [16,17]. Three CT types of tuberculosis peritonitis have been described: wet, fibrotic, and dry [16]. The wet type is the most common occurring in 90% of cases and consists of diffuse ascites [4,16]. The ascites in the wet type can be diffuse or loculated and is slightly hyperattenuating due to its high cellular or protein content [16]. The fibrotic type is by characterized by matted bowel loops along with low attenuating omental and mesenteric nodular masses [16]. The dry type commonly presents with caseous nodules, mesentery thickening, or fibrous adhesions. Although three distinct subtypes of tuberculosis peritonitis have been described, the features of each subtype often overlap [4]. Abdominal lymphadenopathy involving the mesenteric, retroperitoneal, periportal, and peripancreatic lymph nodes can also be seen in tuberculosis peritonitis [12]. Sonographically, tuberculosis peritonitis often presents with ascites [12]. The ascites can be free or loculated containing mobile septa that can produce a latticelike pattern [12]. Sonographic findings of tuberculosis peritonitis are not pathognomonic but hypoechoic or nodular peritoneum thickening can be seen [12]. Omental and mesenteric thickening with enlarged mesenteric lymph nodes can also be seen.
TEACHING POINT
Sclerosing encapsulating peritonitis, previously termed cocoon bowel, causes intestinal obstruction due to development of a fibrous membrane that encases multiple small bowel loops. Since surgery is often needed to decompress encased small bowel loops, CT and ultrasound imaging can lead to a prompt diagnosis.
Table 1.
Differential diagnosis for causes of peritoneal thickening, enhancement and ascites.
| Diagnosis | History/Clinical Features | CT Findings | Ultrasound Findings |
|---|---|---|---|
| Sclerosing encapsulating peritonitis | History of long-term peritoneal dialysis, prior abdominal surgery, liver transplant, retrograde peritonitis, ventriculoperitoneal or peritoneovenous shunts. Symptoms are nonspecific and usually consist of anorexia, bloody ascites, nausea, diarrhea, and abdominal pain | Thick fibrous, enhancing membrane that encases the small bowel, ascites often present with loculations | Internal septations, thick fibrous membranes, and loculated ascites surrounding the small bowel. Concertina arrangement of small bowel within fibrous membrane sac. |
| Peritoneal carcinomatosis | History of ovarian cancer or gastrointestinal tract malignancy, nonspecific symptoms usually consisting of ascites, abdominal pain, nausea, and vomiting | Thickening, nodularity, and enhancement of the peritoneum, omental caking, peritoneal implants, ascites, stellate mesentery, small bowel obstruction | Echogenic nodules with acoustic shadowing from psammomatous calcification. Nodules can also appear hypoechoic and are commonly seen in Morison’s pouch, the pouch of Douglas, and the right subphrenic region. Septated ascites often present as well. |
| Pseudomyxoma peritonei | Mucin-producing tumor, commonly appendiceal, gallbladder, pancreas or ovarian in origin. Occurs more frequently in women, mean age at diagnosis is 49 (range 23–83 yrs). Nonspecific presenting symptoms of weight loss, abdominal pain, and increasing abdominal girth | Thickened gelatinous material on the peritoneal surface, low-attenuation mucinous ascites, scalloped margins of abdominal visceral organs, displaced bowel and mesentery, curvilinear or amorphous calcifications within the ascites | Ascites with nonmobile echoes, suggesting gelatinous fluid. Gelatinous fluid can appear hypoechoic and displace the intestines centrally leading to a starburst or star-like configuration due to echogenic bowel being surrounded by hypoechoic, gelatinous fluid. Echogenic septations often present. |
| Peritoneal mesothelioma | Most common age range is 50–69, more common in men, nonspecific presenting symptoms such as anorexia, weight loss, abdominal pain, abdominal swelling from ascites. History of asbestos exposure reported in 50% of cases. | Three types: dry-painful, wet, and mixed. Variable degrees of ascites. Enhancing soft tissue masses or small nodules and plaques may be present along the peritoneum. Scalloped margins of abdominal visceral organs and direct invasion of abdominal organs can be seen. Omental masses can also be seen. | Hypoechoic sheet like or nodular masses that cause peritoneal thickening. Intestinal wall and solid organ invasion can also occur. Ascites can be seen but is usually minimal compared to the amount of tumor dissemination. |
| Tuberculosis peritonitis | Immunocompromised patient (AIDS or transplant recipients), abdominal pain, fever, night sweats, weight loss, anorexia | Three types: wet, fibrotic and dry. Variable amount of proteinaceous ascites, omental and mesentery caseous nodules, abdominal lymphadenopathy, caseating necrosis within lymph nodes | Ascites is frequently present and can be free or loculated and contain mobile septa. Hypoechoic or nodular peritoneum thickening can be seen. Omental and mesenteric thickening with enlarged mesenteric lymph nodes can also be seen. |
Table 2.
Summary table of sclerosing encapsulating peritonitis
| Etiology | No clear etiology known |
| Incidence | 0.5–4.4% in patients receiving peritoneal dialysis |
| Gender Ratio | No established gender ratio |
| Age Predilection | No established age predilection |
| Risk Factors | Long term peritoneal dialysis, prior abdominal surgery, retrograde peritonitis, practolol therapy, sarcoid, liver transplantation, peritoneal tuberculosis, ventriculoperitoneal shunts, and peritoneovenous shunts |
| Treatment | Bowel rest and nutritional support, lysis of intestinal adhesions, corticosteroids, tamoxifen, and immunosuppressants |
| Prognosis | 56% mortality rate with a range of 38–83% |
| Findings on Imaging |
|
ABBREVIATIONS
- AIDS
Acquired Immunodeficiency Syndrome
- CT
Computed Tomography
- INR
International Normalized Ratio
- IV
Intravenous
- mmol
micromole
- US
Ultrasound
- Yrs
Years
REFERENCES
- 1.Gupta S, Shirahatti RG, Anand J. CT findings of an abdominal cocoon: a case report. American Journal of Radiology. 2004 Dec;183(6):1658–60. doi: 10.2214/ajr.183.6.01831658. [DOI] [PubMed] [Google Scholar]
- 2.Nakamota H. Encapsulating peritoneal sclerosis-a clinician’s approach to diagnosis and medical treatment. Peritoneal Dialysis International. 2005 Apr;25( Suppl 4):S30–38. [PubMed] [Google Scholar]
- 3.Gayomali C, Hussein U, Cameron SF, Protopapas Z, Finkelstein FO. Incidence of encapsulating peritoneal sclerosis: a single-center experience with long-term peritoneal dialysis in the United States. Peritoneal Dialysis International. 2011 May-Jun;31(3):279–86. doi: 10.3747/pdi.2010.00196. [DOI] [PubMed] [Google Scholar]
- 4.Levy A, Shaw J, Sobin L. Secondary tumors and tumor-like lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009 Mar-Apr;29(2):347–73. doi: 10.1148/rg.292085189. [DOI] [PubMed] [Google Scholar]
- 5.Kawanishi H, Kawaguchi Y, Fukui H, et al. Encapsulating peritoneal sclerosis in Japan: a Prospective, Controlled, Multicenter study. American Journal of Kidney Diseases. 2004 Oct;44(4):729–37. [PubMed] [Google Scholar]
- 6.Ti JP, Al-Aradi A, Conlon PJ, Lee MJ, Morrin MM. Imaging features of encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. American Journal of Roentgenology. 2010 Jul;195(1):W50. doi: 10.2214/AJR.09.3175. [DOI] [PubMed] [Google Scholar]
- 7.Vlijm A, Stoker J, Bipat S, et al. Computed tomographic findings characteristic for Encapsulating Peritoneal Sclerosis: a case-control study. Peritoneal Dialysis International. 2009 Sep-Oct;29(5):517–22. [PubMed] [Google Scholar]
- 8.Vijayaraghavan SB, Palanivelu C, Sendhilkumar K, Parthasarathi R. Abdominal cocoon sonographic features: a case report. Journal of Ultrasound in Med. 2003 Jul;22(7):719–21. doi: 10.7863/jum.2003.22.7.719. [DOI] [PubMed] [Google Scholar]
- 9.Menassa-Moussa L, Bleibel L, Sader-Ghorra C, Smayra T, Aoun NJ. MRI findings in intestinal cocoon. American Journal of Radiology. 2006 Mar;186(3):905–6. doi: 10.2214/AJR.05.0252. [DOI] [PubMed] [Google Scholar]
- 10.Tombak MC, Apaydin FD, Colak T, et al. An Unusual Cause of Intestinal Obstruction: Abdominal Cocoon. American Journal of Roentgenology. 2010 Feb;194(2):W176–8. doi: 10.2214/AJR.09.3083. [DOI] [PubMed] [Google Scholar]
- 11.Harel Z, Bargman J. Noninfectious Complications of Peritoneal Dialysis. In: Himmelfarb J, Sayegh M, editors. Chronic Kidney Disease, Dialysis, and Transplantation: A Companion to Brenner & Rector’s the Kidney. 3rd ed. Philadelphia: Saunders Elsevier; 2010. pp. 468–473. [Google Scholar]
- 12.Hanbidge AE, Lynch D, Wilson SR. Ultrasound of the Peritoneum. Radiographics. 2003 May-Jun;23(3):663–84. doi: 10.1148/rg.233025712. [DOI] [PubMed] [Google Scholar]
- 13.Bridda A, Padoan I, Mencarelli R, Frego M. Peritoneal mesothelioma: a review. MedGenMed. 2007 May 10;9(2):32. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1994863/doa:12/27/2012. [PMC free article] [PubMed] [Google Scholar]
- 14.Park JY, Kim KW, Kwon HJ, et al. Peritoneal mesotheliomas: clinicopathologic features, CT findings, and differential diagnosis. American Journal of Roentgenology. 2008 Sep;191(3):814–25. doi: 10.2214/AJR.07.3628. [DOI] [PubMed] [Google Scholar]
- 15.Akhan O, Kalyoncu F, Ozmen MN, et al. Peritoneal mesothelioma: sonographic findings in nine cases. Abdominal Imaging. 1993;18(3):280–2. doi: 10.1007/BF00198123. [DOI] [PubMed] [Google Scholar]
- 16.Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics. 2007 Sep-Oct;27(5):1255–73. doi: 10.1148/rg.275065176. [DOI] [PubMed] [Google Scholar]
- 17.Manohar A, Simjee AE, Haffejee AA, Pettengell KE. Symptoms and investigative findings in 145 patients with tuberculous peritonitis diagnosed by peritoneoscopy and biopsy over a five year period. Gut. 1990 Oct;31(10):1130–2. doi: 10.1136/gut.31.10.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]