Abstract
Pilomatrixoma or calcifying epithelioma of Malherbe is a rare, benign, skin tumour originating from piliferous follicles; breast localization is considered to be very rare. These lesions can origin from the peri-areolar piliferous bulbs and, due to the clinical and imaging features, be easily misdiagnosed as a breast neoplasm. We present a case of pilomatrixoma of the left breast in a woman of 43 years appearing as a firm, deep nodule in the external quadrants. The lesion had mammographic and sonographic malignant features, but histological analysis on core-needle biopsy and surgical specimens revealed this unusual benign lesion.
Keywords: breast, breast pilomatrixoma, breast calcified nodules, core needle biopsy
CASE REPORT
A 43-year old woman came to our Senology Department to be investigated for a centimetre sized, non-tender breast lump that had a hard consistency and was firm on the deep layers of the breast. The nodule was localized in the peri-areolar area at the confluence of the outer quadrants of the left breast. Physical examination of the axillae was negative.
IMAGING FINDINGS
Mammography in two standard projections, cranio-caudal and medial-lateral-oblique (Fig. 1), showed, in a context of a dense breast, a round nodule containing a cluster of pleomorphic irregular microcalcifications that were classified as ACR BI-RADS IV–V. The size of the nodule was 12×11 mm.
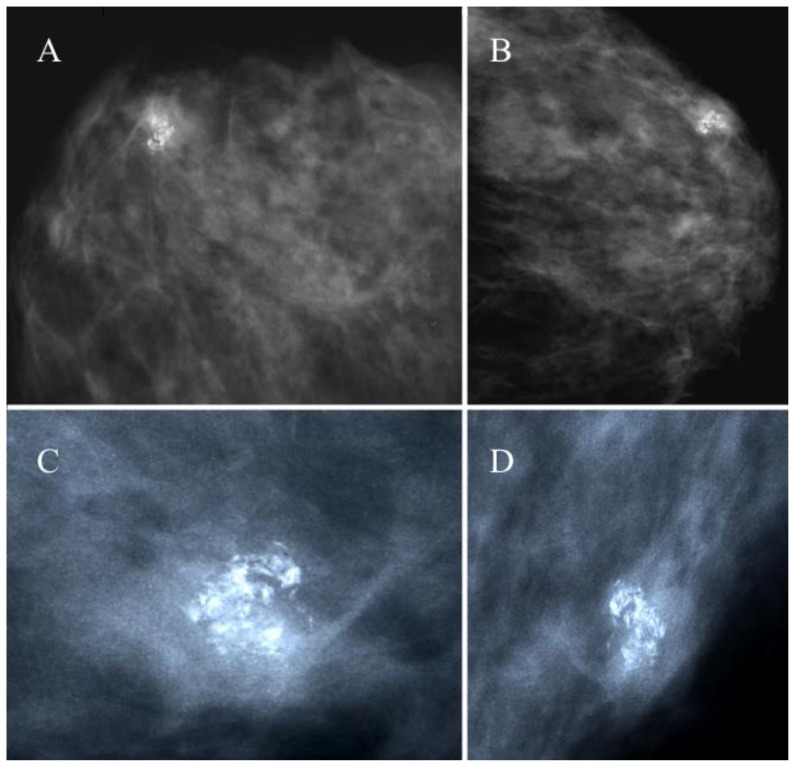
Figure 1.
43 year old female with a hard, firm nodule at the confluence of the external quadrants of the left breast diagnosed as a breast pilomatrixoma after percutaneous and excisional biopsy.
FINDINGS: nodular opacity in the external quadrants of the left breast of 12×11 mm, showing a cluster of pleomorphic irregular microcalcifications (ACR BI-RADS IV–V).
TECHNIQUE: Analogic mammography (28 kV, 100 mAs), cranio-caudal (A) and medio-lateral oblique projections (B) of the left breast. Magnification views of cranio-caudal (C) and medio-lateral oblique (D) projections showing the lesion.
The lesion could not be seen on the previous mammograms performed 12 months earlier (Fig. 2). The ultrasound examination (Fig. 3A) showed a hypoechoic nodule of 13×14×10 mm, with irregular peripheral margins and hyperechoic spots compatible with microcalcifications. There were no signs of intralesional vascularization at the Doppler analysis and no locoregional lymphadenopathy was found.
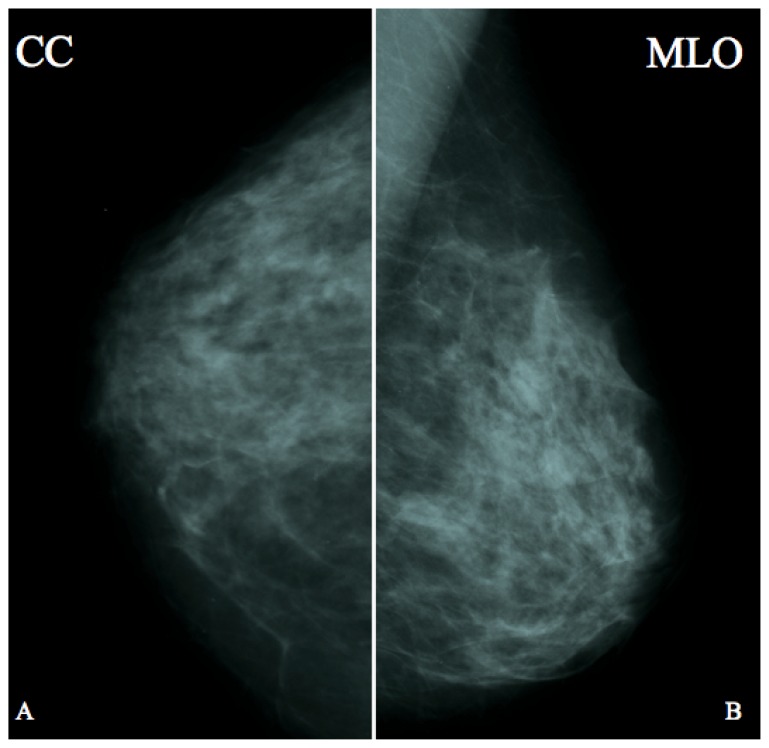
Figure 2.
43 year old female one year before developing a breast pilomatrixoma at the confluence of the external quadrants of the left breast.
FINDINGS: No significant abnormality to report.
TECHNIQUE: Analogic mammography (28 kV, 100 mAs), cranio-caudal (A) and medio-lateral oblique projections of the left breast.
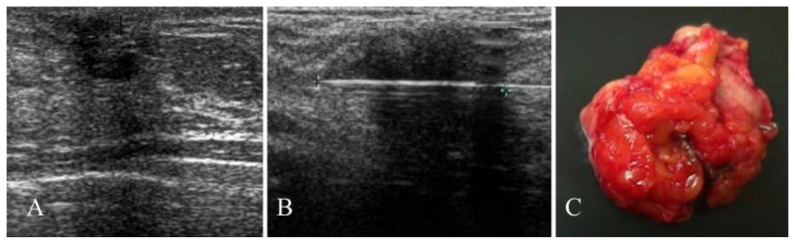
Figure 3.
43 year old female with a hard, firm nodule at the confluence of the external quadrants of the left breast diagnosed as a breast pilomatrixoma after percutaneous and excisional biopsy.
FINDINGS: hypoechogenic nodule (13×14×10 mm) showing irregular margins, hyperechoic spots due to microcalcifications and posterior acoustic shadowing (A). Ultrasound-guided 14-G core-needle biopsy of the lesion (B). Surgical specimen (C).
TECHNIQUE: ultrasound examination using a broadband 10–13 MHz linear transducer.
In consideration of the imaging features and the absence of any abnormality in the previous mammograms, the lesion was highly suspicious for malignancy
MANAGEMENT
A core-needle biopsy (CNB) was performed using a 14-gauge cutting needle with a 22-mm throw (Precisa HS Hospital Service, Rome) sampling three cores of tissue (Fig. 3B). The micro-histological diagnosis was of a pilomatrixoma.
The breast surgical team, considering the imaging findings, decided to perform the excision of the lesion as a precaution. The post-operative histological diagnosis of the surgical specimen confirmed the diagnosis of pilomatrixoma (Fig. 3C).
FOLLOW-UP
No recurrence was seen after 24 months (Fig. 4). Patient was followed-up every year with mammography and ultrasound according to the Italian guidelines and to the American College of Radiology recommendations.
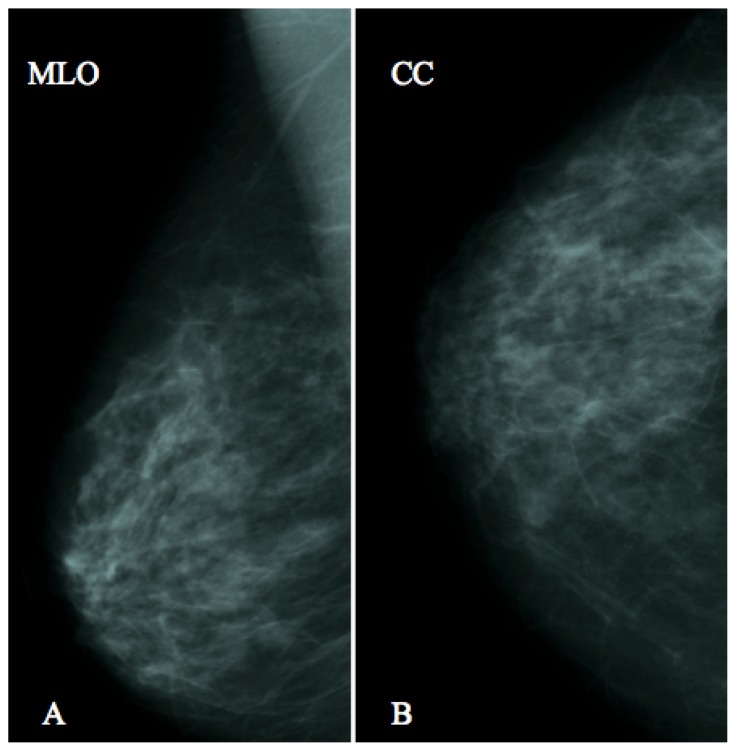
Figure 4.
43 year old female two years after the excision of a breast pilomatrixoma at the confluence of the external quadrants of the left breast.
FINDINGS: No significant abnormality to report.
TECHNIQUE: Analogic mammography, cranio-caudal (A) and medio-lateral oblique projections of the left breast.
DISCUSSION
ETIOLOGY AND DEMOGRAPHICS
Pilomatrixoma is a benign epithelial tumor of the skin originating from piliferous follicles [1]. Originally named as “calcific epithelioma of Malherbe”, it was described for the first time in 1880 by Dr. Chenantals Malherbe who considered it as a calcific epithelioma of the sebaceous glands [2]. Pilomatrixomas usually develop in the subcutaneous tissue from the cellular matrix of hair follicles.
They are usually found in 1st and 2nd decades although they have been described, with a lower incidence, in the 5th and 6th decades.
The etiology has not been established yet but it seems that repeated skin traumas could be the main causes. These stimuli induce an inflammatory response that leads to an overgrowth of hair matrix. Another hypothesis, formuled by Forbis and Helwig, is that pilomatrixomas are hamartomas [3]. Breast localization is very rare (1:100000 people), however, these lesions can origin from the peri-areolar piliferous bulbs and mimic a breast malignancy [2;5–15].
Solitary and multifocal diseases have been reported; in addition these lesions could be sporadic or associated to syndromes such as Gardner syndrome and myotonic dystrophy [4]. Histologically pilomatrixomas are made of epithelial cells organised in nodular aggregates into a connective matrix with scattered inflammatory-like elements. Each nodule is characterized by two types of epithelial cells with different organization: on the peripheral part there are densely packed basophilic cells producing keratin while the center of the nodule is occupied by eosinophilic cells known as “ghost” or “mummified” cells. Moreover, hair, calcifications, foci of necrosis and multinucleated giant cells can be often found [5].
CLINICAL AND IMAGING FINDINGS
Pilomatrixoma is most commonly diagnosed in the head (peri-auricular and juxta-parotid areas), neck, upper and lower extremities and more rarely on the trunk [6]. Breast localization is very rare and only a very few cases have been reported so far [2;5–15]. While lesions in the skin are often easily diagnosed, breast pilomatrixomas require several diagnostic tests due to the difficulty of distinguishing these lesions from breast cancer.
Clinically, breast pilomatrixomas can range in size from 3 to 30 mm. On physical examination they appear as hard nodules with lobulated margins. In addition there may be other signs such as rubor, calor, dolor due to inflammatory phenomena. The skin can be intact, purple or rarely ulcerated [8,16]. In the latter case the differential diagnosis with malignant skin lesions can be difficult. Malignant pilomatrixomas are extremely rare; they have been described the first time in 1980 by Lopansri et al [17] and only a few articles are available reporting this type of disease. [18,19, 20].
The diagnostic management of breast pilomatrixomas should include clinical examination, mammography, ultrasound and ultrasound-guided CNB.
On mammograms, breast pilomatrixomas appear as nodular opacities with pleomorphic coarse irregular calcifications (ACR BI-RADS IV–V) whose number can increase gradually, simulating the microcalcifications often associated with breast cancer. Sonographically, these lesions appear as hypoechoic nodules with irregular margins, hyperechoic spots and a posterior acoustic shadowing (Tab. 1).
Table 1.
Summary table for breast pilomatrixoma
| Etiology | Unknown. Repeated traumas on skin may lead to an overgrowth of hair matrix. |
| Incidence | Very rare (1:100000 people) |
| Gender Ratio | More frequent on women |
| Age Predilection | 1st and 2nd decades |
| Risk Factors | Unknown |
| Treatment | Surgical excision |
| Prognosis | Excellent |
| Findings on Imaging | Mammographic findings: nodular opacity with pleomorphic heterogeneous microcalcifications (ACR BI-RADS IV–V). Ultrasonographic findings: hypoechoic nodule with irregular margins, posterior acoustic shadowing and hyperechoic spots due to microcalcifications. |
TREATMENT AND PROGNOSIS
Imaging findings overlap often those that are characteristic of breast neoplasms. This necessarily leads to an ultrasound-guided CNB which usually proves the biology of the nodule.
The breast pilomatrixoma that came to our observation is unusual and rarely seen. Classic forms of intramammary pilomatrixomas show generally coarse calcification [11], while the lesion we report had granular polymorphic microcalcifications that were considered BI-RADS IV–V and that were not present in the previous examinations.
Despite the percutaneous biopsy, we recommended the surgical excision of the lesion that confirmed the diagnosis of the core-needle biopsy.
Considering the benignity of this lesion, the expected prognosis is excellent.
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of breast pilomatrixoma with both benign and malignant breast lesions can be very challenging. It includes skin calcifying lesions (seborrheic keratosis and inclusion cysts), fibrocystic changes (usual ductal hyperplasia, adenosis, apocrine metaplasia), lobular neoplasias, papillomas, calcified fibroadenomas, fat necrosis and invasive ductal carcinoma as all can appear as nodular opacities with pleomorphic calcifications on mammography (Tab. 2).
Table 2.
Differential diagnosis of breast pilomatrixoma
| Lesion | Imaging modality | ||
|---|---|---|---|
| Mammography | Ultrasound | MRI | |
| Skin calcifying lesions (seborrheic keratosis, inclusion cysts) |
|
|
|
| Fibrocystic changes (usual ductal hyperplasia, adenosis, apocrine metaplasia) |
|
|
|
| Lobular neoplasia |
|
|
|
| Papilloma |
|
|
|
| Fibroadenoma |
|
|
|
| Fat necrosis |
|
|
|
| Invasive ductal carcinoma (IDC) |
|
|
|
| Pilomatrixoma |
|
|
|
Differential diagnosis with ultrasound is also difficult as pilomatrixomas mimic both benign nodules (fibroadenomas, papillomas) and invasive ductal carcinoma (Tab. 2).
MRI features of those more common lesions that can be simulated by a breast pilomatrixoma are well-known, no data are currently available regarding the MRI appearance of this rare disease (Tab. 2).
In addition, despite the presence of cells considered typical of pilomatrixomas (ghost cells and basaloid cells), differential diagnosis with breast cancer by cytology only can be challenging in most cases with a high rate of false positives (75%) [21–24]. On the other hand, core-needle biopsy has proved to be an effective and safe technique for diagnosing breast nodules [25] and should be performed to obtain a pre-surgical diagnosis. In conclusion, breast pilomatrixomas, despite their rarity, should be considered in the differential diagnosis of calcified nodules visible on mammograms and an excisional biopsy for a definitive diagnosis might be advisable.
TEACHING POINT
Pilomatrixomas can have an intramammary localization and, although they are very rare, should be considered in the differential diagnosis of breast nodules that show calcifications on mammograms. Standard examination with mammography and ultrasound should be followed by core-needle biopsy in order to have a precise pre-operative diagnosis.
ABBREVIATIONS
- ACR BI-RADS
American College of Radiology, Breast Imaging Reporting and Data System
- CNB
core needle biopsy
- IDC
invasive ductal carcinoma
- MRI
magnetic resonance imaging
- T1/T2 WI
T1/T2-weighted images
- T2 WI FS
T2-weighted images with fat suppression
- T1 C.E FS
T1-weighted contrast-enhanced images with fat suppression
REFERENCES
- 1.Lever Wf, Griesemer Rd. Calcifying epithelioma of Malherbe; report of 15 cases, with comments on its differentiation from calcified epidermal cyst and on its histogenesis. Arch Derm Syphilol. 1949 May;59(5):506–18. doi: 10.1001/archderm.1949.01520300016003. [DOI] [PubMed] [Google Scholar]
- 2.Hubeny CM, Sykes JB, O’Connell A, Dogra VS. Pilomatrixoma of the adult male breast: a rare tumor with typical ultrasound features. J Clin Imaging Sci. 2011;1:12. doi: 10.4103/2156-7514.76690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbis R, Jr, Helwig Eb. Pilomatrixoma (calcifying epithelioma) Arch Dermatol. 1961 Apr;83:606–18. doi: 10.1001/archderm.1961.01580100070009. [DOI] [PubMed] [Google Scholar]
- 4.Geh JL, Moss AL. Multiple pilomatrixomata and myotonic dystrophy: a familial association. Br J Plast Surg. 1999 Mar;52(2):143–5. doi: 10.1054/bjps.1998.3036. Review. [DOI] [PubMed] [Google Scholar]
- 5.Pascual A, Casado I, Colmenero I, Pelayo A, Asenjo JA. Fine needle aspiration cytology of pilomatrixoma of the breast. Acta Cytol. 2000 Mar-Apr;44(2):274–6. doi: 10.1159/000326375. [DOI] [PubMed] [Google Scholar]
- 6.Rousselot C, Tourasse C, Samimi M, Degand P, Dénier JF, Michenet P. Breast pilomatrixoma manifested as microcalcifications on mammography: report of two cases. J Radiol. 2007 Jul-Aug;88(7–8 Pt 1):978–80. doi: 10.1016/s0221-0363(07)89907-0. [DOI] [PubMed] [Google Scholar]
- 7.Loo CE, van Pel R. Diagnostic image. A woman with an abnormal screening mammogram. Ned Tijdschr Geneeskd. 2009;153:B474. [PubMed] [Google Scholar]
- 8.Reynaud P, Orliaguet T, Robin YM, Buono JP, Darcha C, Suzanne F, Déchelotte P. Mammary pilomatrixoma clinically mimicking carcinoma. Ann Pathol. 1997 Jul;17(3):213–4. [PubMed] [Google Scholar]
- 9.Bhalotra R, Jayaram G. Fine-needle aspiration cytology of pilomatrixoma: a case report. Diagn Cytopathol. 1990;6(4):280–3. doi: 10.1002/dc.2840060411. [DOI] [PubMed] [Google Scholar]
- 10.Gilles R, Guinebretiere JM, Gallay X, Gallay X, Vanel D. Pilomatrixoma mimicking male breast carcinoma on mammography. AJR Am J Roentgenol. 1993 Apr;160(4):895. doi: 10.2214/ajr.160.4.8456689. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton A, Young GI, Davis RI. Pilomatrixoma mimicking breast carcinoma. Br J Dermatol. 1987;116:585–586. doi: 10.1111/j.1365-2133.1987.tb05883.x. [DOI] [PubMed] [Google Scholar]
- 12.Ali MZ, Ali FZ. Pilomatrixoma breast mimicking carcinoma. J Coll Physicians Surg Pak. 2005 Apr;15(4):248–9. [PubMed] [Google Scholar]
- 13.Imperiale A, Calabrese M, Monetti F, Zandrino F. Calcified pilomatrixoma of the breast: mammographic and sonographic findings. Eur Radiol. 2001;11(12):2465–7. doi: 10.1007/s003300000798. [DOI] [PubMed] [Google Scholar]
- 14.Ismail W, Pain S, al-Okati D, al Sewan M. Giant pilomatricoma simulating carcinoma of the male breast. Int J Clin Pract. 2000 Jan-Feb;54(1):55–6. [PubMed] [Google Scholar]
- 15.Becker TS, Moreira MA, Lima LA, de Oliveira EL, Freitas-Júnior R. Pilomatrixoma mimicking breast cancer in man. Breast J. 2010 Jan-Feb;16(1):89–91. doi: 10.1111/j.1524-4741.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Sari A, Yavuzer R, Isik I, Latifoglu O, Ataoglu O. Atypical presentation of pilomatricoma: a case report. Dermatol Surg. 2002 Jul;28(7):603–5. [PubMed] [Google Scholar]
- 17.Lopansri S, Mihm MC., Jr Pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe: a case report and review of literature. Cancer. 1980 May 1;45(9):2368–73. doi: 10.1002/1097-0142(19800501)45:9<2368::aid-cncr2820450922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases. Report of a case. Cancer. 1996 Apr 1;77(7):1311–4. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1311::AID-CNCR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Mikhaeel NG, Spittle MF. Malignant pilomatrixoma with multiple local recurrences and distant metastases: a case report and review of the literature. Clin Oncol (R Coll Radiol) 2001;13(5):386–9. doi: 10.1053/clon.2001.9296. [DOI] [PubMed] [Google Scholar]
- 20.Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993 Apr 15;71(8):2491–8. doi: 10.1002/1097-0142(19930415)71:8<2491::aid-cncr2820710811>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Ma KF, Tsui MS, Chan SK. Fine needle aspiration diagnosis of pilomatrixoma. A monomorphic population of basaloid cells with squamous differentiation not to be mistaken for carcinoma. Acta Cytol. 1991 Sep-Oct;35(5):570–4. [PubMed] [Google Scholar]
- 22.Kinsey W, Coghill SB. A case of pilomatrixoma misdiagnosed as squamous cell carcinoma. Cytopathology. 1993;4(3):167–71. doi: 10.1111/j.1365-2303.1993.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong MP, Yuen ST, Collins RJ. Fine-needle aspiration biopsy of pilomatrixoma: still a diagnostic trap for the unwary. Diagn Cytopathol. 1994;10:365–370. doi: 10.1002/dc.2840100415. [DOI] [PubMed] [Google Scholar]
- 24.Viero RM, Tani E, Skoog L. Fine needle aspiration (FNA) cytology of pilomatrixoma: report of 14 cases and review of the literature. Cytopathology. 1999 Aug;10(4):263–9. doi: 10.1046/j.1365-2303.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi S, Giannotti E, Vanzi E, Marziali M, Abdulcadir D, Boeri C, Livi L, Orzalesi L, Sanchez LJ, Susini T, Vezzosi V, Nori J. Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single-centre and review of the literature. Breast. 2012 Apr;21(2):159–64. doi: 10.1016/j.breast.2011.09.005. [DOI] [PubMed] [Google Scholar]