Abstract
Incomplete endothelialization, blood cell adhesion to vascular stents, and inflammation of arteries can result in acute stent thromboses. The systemic administration of acetylsalicylic acid decreases endothelial dysfunction, potentially reducing thrombus, enhancing vasodilatation, and inhibiting the progression of atherosclerosis; but, this is weakened by upper gastrointestinal bleeding. This study proposes a hybrid stent with biodegradable nanofibers, for the local, sustained delivery of acetylsalicylic acid to injured artery walls. Biodegradable nanofibers are prepared by first dissolving poly(D,L)-lactide-co-glycolide and acetylsalicylic acid in 1,1,1,3,3,3-hexafluoro-2-propanol. The solution is then electrospun into nanofibrous tubes, which are then mounted onto commercially available bare-metal stents. In vitro release rates of pharmaceuticals from nanofibers are characterized using an elution method, and a highperformance liquid chromatography assay. The experimental results suggest that biodegradable nanofibers release high concentrations of acetylsalicylic acid for three weeks. The in vivo efficacy of local delivery of acetylsalicylic acid in reducing platelet and monocyte adhesion, and the minimum tissue inflammatory reaction caused by the hybrid stents in treating denuded rabbit arteries, are documented. The proposed hybrid stent, with biodegradable acetylsalicylic acid-loaded nanofibers, substantially contributed to local, sustained delivery of drugs to promote re-endothelialization and reduce thrombogenicity in the injured artery. The stents may have potential applications in the local delivery of cardiovascular drugs. Furthermore, the use of hybrid stents with acetylsalicylic acid-loaded nanofibers that have high drug loadings may provide insight into the treatment of patients with high risk of acute stent thromboses.
Keywords: biodegradable drug-eluting nanofibers, acetylsalicylic acid, release characteristics, cell adhesion to vascular stents
Introduction
Acute coronary syndrome, including non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), often leads to emergent treatment, using coronary angiography.1 Despite the use of drug-eluting and bare-metal stents (BMS) in millions of patients worldwide, to reduce mortality and morbidity associated with acute symptoms, dysfunctional endothelium, in combination with a lack of endothelial coverage, still leads to the occurrence of stent thrombosis after the treatment intervention.2 The risk of acute stent thrombosis results from multifactorial prothrombotic and mechanical conditions: 1) local thrombus burden at the site of plaque rupture, 2) impaired flow due to distal embolization, with obstruction of microcirculation, and 3) incomplete deployment of the stent in the coronary artery (ie, incorrectly sized and expanded stent).3
Endothelial plaque disruption is the main pathophysiological mechanism responsible for the majority of acute coronary syndromes, followed by subsequent blood cell adhesion, activation, and thrombus formation.4 Ultimately, thrombus forms within a coronary artery, leading to occlusion of the artery, with NSTEMI and STEMI. The clinical outcome may be catastrophic, with mortality in the range 7%–25% for patients with acute stent thromboses.5–9
Endothelial dysfunction, in response to a vascular injury, is associated with a high incidence of cardiovascular events, which are characterized by reduced bioavailability of prostacyclin and nitric oxide, followed by binding of platelets, by specific membrane receptors, to cellular and extracellular matrix constituents within vascular tissues.10,11 In pre-atherosclerotic vascular disease, impaired endogenous platelet inhibition causes platelet activation, resulting in enhanced susceptibility of platelets to agonists released from the inflamed endothelium. The adhesion of platelets to inflamed and dysfunctional endothelia precedes leukocyte adhesion. Leukocyte recruitment in the early phases of atherosclerosis is through adherent, activated platelets in arterial flow. Among patients with advanced atherosclerotic coronary artery disease, the formation of arterial thrombi through platelet aggregation is generally viewed as a biological end-point in contribution to the thrombogenic mechanism.12
Acetylsalicylic acid is the single most cost-effective and widely prescribed drug used to prevent atherothrombotic ischemic events. It exerts an antithrombotic effect by irreversibly acetylating cyclooxygenase-1 (COX-1), thereby reducing the thromboxane A2 produced by platelets.13 Acetylsalicylic acid also improves endothelial function, which may enhance vasodilatation, reduce thrombus, and inhibit progression of atherosclerosis.14 However, despite the proven benefits of the above therapies, in preventing cardiovascular complications, the systemic administration of acetylsalicylic acid is limited, mainly due to concern over the potential development of upper gastrointestinal symptoms and bleeding; also due to differences in the absorption pharmacokinetics of available medications.15,16 Delivery of an insufficient concentration of the drug at the location of the stent might increase the rate of restenosis after percutaneous coronary intervention. Santos et al found that only high-dose acetylsalicylic acid prevents the synergism between red cells and platelets in promoting thrombosis. The inference from these investigations is that patients who are treated with acetylsalicylic acid may not be completely protected from arterial thrombosis.17,18
Among the advantages that drug-eluting stents hold over systemic pharmacotherapy are higher drug concentrations at the injury site, with minimal systemic side effects. A possible solution to achieving an effective drug concentration is direct and local delivery into the diseased vessels, by stenting.19,20 Local delivery of high-dose acetylsalicylic acid, which enhances endothelialization and decreases blood cell adhesion to diseased arterial walls, is highly desirable.
In this study, we developed a novel hybrid stent, with poly(D,L)-lactide-co-glycolide (PLGA) nanofibers, for local delivery of sustainable high-dose acetylsalicylic acid into a rabbit denuded artery. The in vitro release characteristics of the pharmaceuticals, the in vivo efficacy of local delivery of acetylsalicylic acid in reducing blood cell adhesion, and the tissue reaction caused by the hybrid stent were evaluated.
Materials and methods
Fabrication of acetylsalicylic acid-loaded nanofibrous tubes
The PLGA we used is a commercially available material (Resomer® RG 503, Boehringer Ingelheim, Ingelheim am Rhein Germany). It has a lactide:glycolide ratio of 50:50 and a molecular weight of approximately 33,000 Da, as measured by a gel permeation chromatograph (Waters Corp, Milford, MA, USA) equipped with a Waters 2414 Refractive Index Detector (Waters Corp). Acetylsalicylic acid and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) were purchased from Bayer HealthCare (Berlin, Germany) and Sigma-Aldrich (Saint Louis, MO, USA), respectively. The electrospinning setup used in this study consisted of a syringe and needle (internal diameter: 0.42 mm), a ground electrode, a metallic pin (diameter: 0.95 mm) mounted on a motor, and a high voltage supply.21 The needle and the metallic pin were connected to the high voltage supply, which generated positive direct current voltages up to 35 kV, and currents up to 4.16 mA/125 W. The rotational speed of the motor was 300 rpm. To electrospin the nanofibers, a predetermined weight ratio of PLGA to acetylsalicylic acid (240 mg/40 mg, w/w) was first dissolved into 1 mL of HFIP. The solution was then delivered and electrospun by a syringe pump, at a volumetric flow rate of 3.6 mL per hour, to obtain nanofibrous tubes on the metallic pin (Figure 1A). The distance between needle tip and ground electrode was 10 cm. The positive voltage applied to the polymer solution was 17 kV. All electrospinning experiments were performed at room temperature. After electrospinning, the nanofibrous tube was hand-crimped on to the outside of a 3.5 mm × 20 mm bare-metal stent (316 L stainless steel, balloon-expandable) (Liberté®, Boston Scientific, Natick, MA, USA) (Figure 1B). Thus, a hybrid acetylsalicylic acid nanofibrous mounted stent was obtained (Figure 1C). All stents were placed in a vacuum oven at 40°C for 72 hours, for evaporation of solvents.
Figure 1.
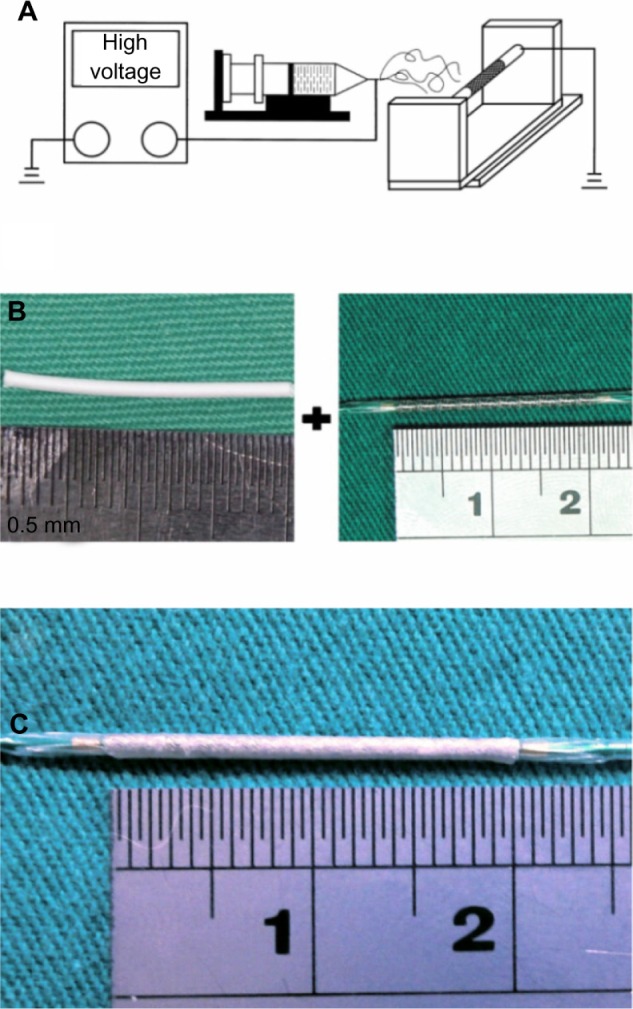
Setup for electrospinning.
Notes: (A) Electrospinning setup consists of a power supply (35 kV, 4.16 mA/125 W), a syringe pump, and a metallic pin (with a diameter of 0.95 mm) that is mounted on a motor with a rotational speed of 300 rpm. (B) Electrospun nanofibrous tube and bare-metal stent (BMS) (3.5 × 20 mm). (C) The tube was mounted onto a commercially available BMS.
Effect of acetylsalicylic acid loading on platelet adhesion
Blood was drawn from a healthy rabbit and mixed with 3.2% sodium citrate at a volumetric ratio of 1:9. Platelet-rich plasma (PRP) was obtained by centrifugation at 150 g for 10 minutes. The sample was maintained at 22°C before PRP was separated. The quantity of platelets in PRP was 2 × 105 cells/μL, determined using a semi-automated hematology analyzer (Sysmex F820, Sysmex Corp, Kobe, Japan). Fifty microliters (107 platelets) of the platelet suspension was then placed on the surfaces of nanofibers with three doses (1 μg/mm2, 5 μg/mm2, and 25 μg/mm2), then incubated at 37°C for 1 or 3 hours. After incubation with PRP, the platelets were washed three times in phosphate buffered solution (PBS). Platelets that adhered to fiber surfaces were mixed with 1% glutaraldehyde, then immersed in PBS, before being allowed to stand in fixative for 60 minutes at 4°C.
In vitro release of pharmaceuticals
The in vitro release characteristics of acetylsalicylic acid from nanofibers were examined by an elution method. The samples (3.5 mm in diameter and 20 mm long), with two acetylsalicylic acid loadings (5 μg/mm2 and 25 μg/mm2), were placed in glass test tubes (one sample per test tube; total number: 3 per loading) with 1 mL of PBS (0.15 mol/L; pH: 7.4) in each tube. Fresh PBS (1 mL) was added then the eluent was allowed to stand for 24 hours. The test tubes were then incubated at 37°C for 24 hours, before the eluent was collected and analyzed. This procedure was repeated for 28 days.
The drug concentrations in the eluents were determined using a high-performance liquid chromatography (HPLC) assay. These analyses were conducted using a Hitachi L-2200 Multisolvent Delivery System (Hitachi Medical Corp, Tokyo, Japan). A Symmetry C8 3.9 cm × 150 mm HPLC column (Waters Corp) was used for the separation of acetylsalicylic acid. The mobile phase contained 0.01 mol of heptanesulphonic acid (Fisher Scientific UK, Loughborough, UK) and acetonitrile (Mallinckrodt Pharmaceuticals, St Louis, MO, USA.) in 85:15 ratio by volume. The absorbency was monitored at a wavelength of 254 nm, while the flow rate was 1.4 mL per minute. All experiments were undertaken in triplicate. Sample dilutions were performed to bring unknown concentrations into the range of the assay standard curve. A calibration curve was then made for each set of measurements (correlation coefficient >0.99). The elution product can be identified and quantified with a high degree of sensitivity using the HPLC system.
Surgical procedure and animal care
Adult male New Zealand White rabbits with a mean weight of 3.5 ± 0.3 kg were utilized in the animal study. They were housed in individual cages in a temperature- and light-controlled room, and given standard rabbit chow ad libitum, with free access to sterilized drinking water. All animal procedures were approved by the Institutional Animal Care and Use Committee of Chang Gung University, and all of the animals studied were cared for according to the regulations of the National Institute of Health of Taiwan, under the supervision of a licensed veterinarian.
Rabbits were sedated and anesthetized through a muscular injection of xylazine (9.3 mg/kg), and the administration of tiletamine-zolazepam (10 mg/kg) (Zoletil® 50, Virbac Group, Carros Cedex, France), and oxygen (2 L per minute), through a face mask. A 5F sheath was inserted into the femoral artery, using the puncture technique. Hybrid stents, on which were mounted nanofibers that had two acetylsalicylic acid loadings (5 μg/mm2 and 25 μg/mm2, with total doses of 250 μg and 1,250 μg, respectively) were used in vivo. A total of 24 rabbits were separated into two groups: Group A comprised 12 rabbits, in which were deployed hybrid stents with a drug loading of 25 μg/mm2; Group B comprised 12 rabbits that received hybrid stents with a drug loading of 5 μg/mm2. All of the rabbits received a second hybrid stent, with no drug loading (ie, stent plus virgin nanofibers), to act as a control (Group C; n=24). During the procedure, the rabbits first underwent endothelial denudation of the descending abdominal aorta, achieved by using a 3.5 × 20 mm Maverick® angioplastic balloon (Boston Scientific, Natick, MA, USA) to cause an injury. The balloon was passed three times over a 0.014-inch guide wire into the aorta, inflated to a nominal pressure (8 bar) with 50% (v/v) contrast/saline, then withdrawn, in a retrograde manner, into the lower descending abdominal aorta. Briefly, two hybrid stents were deployed in each rabbit: one stent (Group C) was used in the upper descending abdominal aorta, and the other (Groups A and B) was deployed in the lower descending abdominal aorta. When the stents had reached the target sites, they were expanded for 15 seconds (pressure: 8 bar) to a diameter of 3.5 mm, obtaining a stent-to-artery diameter ratio of 1.2:1. The distance between the two stents was about 8 mm, due mainly to the length of the lower descending abdominal aorta (30–35 mm). Following stent implantation, post-procedural angiography was performed to document vessel patency before the animals were allowed to recover. The rabbits received anticoagulation therapy with acetylsalicylic acid (40 mg per day), administered orally 24 hours before catheterization, and with a continuous dosage throughout the in-life phase of the study (following institutional recommendations). Furthermore, single-dose intra-arterial heparin (150 IU/kg) was administered upon catheterization.
After stent deployment, stented vessels, including those with drug-loaded stents (Groups A and B) and non-drug-loaded stents (Group C), were collected from three animals weekly for 4 weeks, for microscopic observation, as well as for histological examination of both the inflammatory and the injury responses. Anaesthetized animals were exsanguinated by left ventricular puncture, perfused with lactated Ringer’s solution, and given a lethal injection of lidocaine (100 mg/kg). The explanted vessels were flushed with lactated Ringer’s solution before fixation. The regions close to the distal non-acetylsalicylic acid-loaded and acetylsalicylic acid-loaded stented vessels were sectioned transversely, for immunofluorescence assay.
Microscopic observation
Intact stented vessels for microscopic observation were longitudinally bisected. to expose the lumen surface, and were photographed. Specimens were rinsed in sodium phosphate buffer (0.1 mmol/L; pH 7.2 ± 0.1) and subsequently post-fixed in 1% osmium tetroxide for about 30 minutes. They were then dehydrated in a graded series of ethanol concentrations (50%, 60%, 70%, 80%, 90%, 100%). After critical point drying, the tissue samples were mounted and sputter coated with gold then observed through a scanning electron microscope (SEM) (Hitachi S-3000N, Hitachi High Technologies Corp, Tokyo, Japan).
Low-power photographs of the lumen surface at a magnification of 35× were taken, to estimate the degree of endothelialization of the implant. Composites of serial en face SEM images, obtained at low power (35×), were digitally assembled to generate a complete view of the entire luminal (stented) surface. The images were further enlarged (200×), allowing direct visualization of the endothelial cells. The extent of endothelial surface coverage above the stent struts was traced and measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).22 The degrees of cell coverage on randomly selected areas were reported as endothelial coverage percentages.
Levels of platelet and monocyte adhesion to the vessels were determined by counting the number of adhered cells in the SEM photographs. For each surface of the cell count, 20 rectangular fields were chosen at random. Adhered cells were counted manually as the photographs were observed (enlargement: 1,000×; area: 11,136 μm2). Based on these data, average densities of adhered platelets per square millimeter were calculated.
Histological examination
Semiquantitative histopathological approaches were used including inflammation and vascular injury scores.23,24 Briefly, the inflammation scores used were: 0 (none); 1 (mild with minimal infiltrated inflammatory cells); 2 (moderate); and 3 (severe, with large clusters of inflammatory cells with granulomatous morphology). The vascular injury scores used were: 0 (strut not in contact with internal elastic lamina [IEL]); 1 (strut in contact with IEL and profile in neointima); 2 (strut penetrates IEL with profile in media); 3 (strut penetrates media and is in contact with external elastic lamina); and 4 (strut is in adventitia). The scores for each strut were averaged to obtain the mean score for each of the 72 histological sections from stented arteries (in 12 animals, at both 2 and 4-weeks).
Immunofluorescence
The chemicals used in the assay were obtained from Sigma-Aldrich Corp (St Louis, MO, USA). Fluorescent dyes were purchased from Molecular Probes (Eugene, OR, USA). The tissue samples were embedded in optimal cutting temperature compound prior to being frozen sectioned using a microtome-cryostat. For immunostaining, frozen sections were washed in PBS and blocked with 2% Bovine Serum Albumin for 30 minutes, at room temperature. Sections were then incubated for 1 hour, at room temperature, with primary antibodies against type I collagen, diluted in blocking solution. Nuclei were visualized through DAPI-staining. The experiments were conducted in triplicate.
Statistics and data analysis
All data are presented as means ± standard deviation. One-way ANOVA was used to compare the data for statistical significance. Within ANOVA, a post hoc Bonferroni procedure for multiple comparisons was applied to detect significant differences between pairs. Differences were considered statistically significant for P-values less than 0.05. SPSS version 17.0 statistical software (IBM Corp, Armonk, NY, USA) was used for data analysis.
Results
In vitro assessment of hybrid stent/biodegradable nanofibers
Hybrid stents, with acetylsalicylic acid-loaded nanofibers, were successfully fabricated using an electrospinning method (Figure 1). Figure 2 shows SEM micrographs of the electrospun nanofibrous membrane, before (Figure 2A) and after (Figure 2B) expansion by a balloon (magnification: 3,000×). The diameters of the electrospun PLGA/acetylsalicylic acid nanofibers ranged from 50–8,720 nm. The pore size of the membrane tube was approximately 5 μm before balloon expansion; it later increased to 10 μm after expansion. Additionally, after immersion in PBS for 24 hours, the nanofibrous tubes swelled and became sponge-like. It is estimated that the pore size of the nanofibrous tube increased to more than 10 μm owing to pore swelling.
Figure 2.
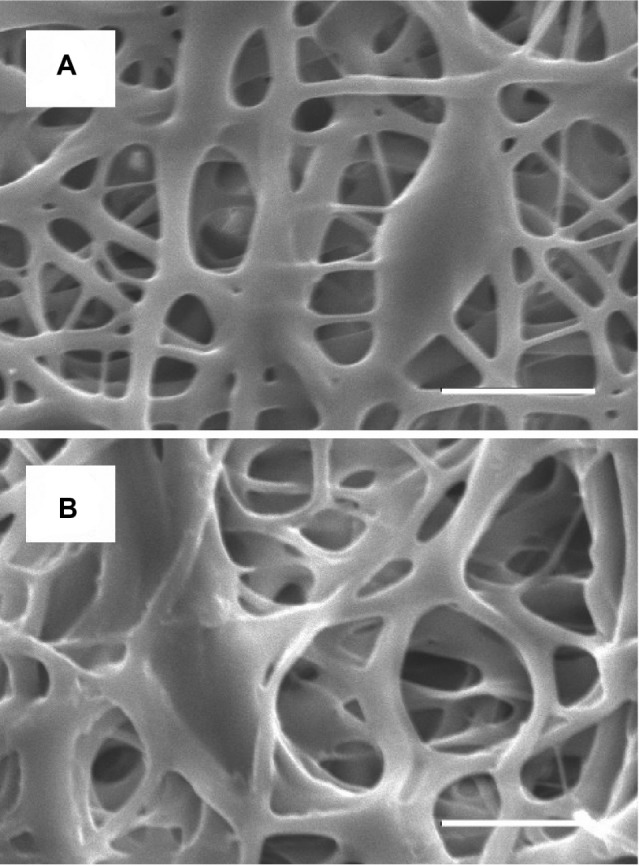
Morphology of nanofibrous membrane elucidated by scanning electron microscopy.
Notes: Images of acetylsalicylic acid-loaded nanofibers within the diameter range 50–8,720 nm, (A) before expansion (the pore size is around 5 μm), and (B) after expansion by a balloon (the pore size is around 10 μm). Scale bar: 10 μm.
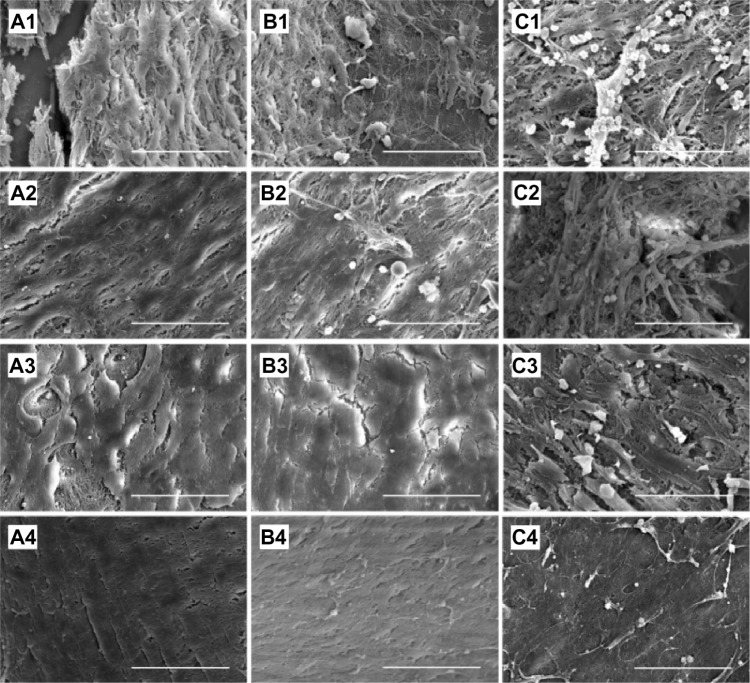
The influence of acetylsalicylic acid loading on platelet adhesion was examined in vitro. Fabricated nanofibers, with three different acetylsalicylic acid loadings (1 μg/mm2, 5 μg/mm2, and 25 μg/mm2), were immersed in PRP for 1–3 hours. Platelets that had adhered to the fibers were then counted. Figure 3 shows a photograph of the nanofibers with, adherent platelets, while Table 1 lists the numbers of adherent platelets. Significantly fewer platelets adhered onto the nanofibers with 5 μg/mm2and 25 μg/mm2 acetylsalicylic acid loadings (Figure 3A1, A2, B1 and B2) than to the fibers with 1 μg/mm2 loading (Figure 3C1 and C2), at both 1 and 3 hours. Furthermore, at 3 hours, significantly fewer platelets adhered to the nanofibers with 25 μg/mm2 loading than to those with 5 μg/mm2 loading. Larger platelet aggregates, and more extensive platelet pseudopod formation, were observed at 1 and 3 hours on nanofibers with 1 μg/mm2 drug loading (Figure 3C1 and C2). These experimental results suggest that the number of adhering platelets and monoctyes decreases as the acetylsalicylic acid drug loading increases. This characteristic provides a significantly advantageous high acetylsalicylic acid concentration at the vessel wall, while maintaining the minimum systemic level of the drug.
Figure 3.
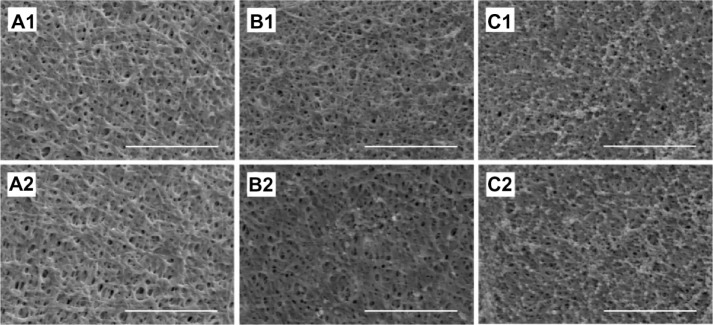
Platelet adhesion test in vitro.
Notes: After immersion of acetylsalicylic acid-loaded nanofibrous membranes, (A) (25 μg/mm2) and (B) (5 μg/mm2) in platelet-rich plasma. No significant difference existed between platelet adhesion at 1 hour (A1 and B1). At 3 hours, significantly fewer platelets adhered to the nanofibers with 25 μg/mm2 than with 5 μg/mm2 acetylsalicylic acid loading (A2 and B2). Significantly more platelets adhered on the nanofibrous membranes with the lowest acetylsalicylic acid loading (1 μg/mm2) at both 1 and 3 hours (C1 and C2). Scale bar: 10 μm.
Table 1.
Numbers of adherent platelets in vitro (per mm2)
| Elapsed time | Drug loadings
|
ANOVA P-value | ||
|---|---|---|---|---|
| 25 μg/mm2 | 5 μg/mm2 | 1 μg/mm2 | ||
| One hour | 60 ± 22 | 110 ± 64 | 41,200 ± 1,320 | <0.001a,b |
| Three hours | 65 ± 31 | 150 ± 67 | 63,400 ± 2,430 | <0.001a,b,c |
Notes:
P<0.05 Group A versus Group C in post hoc analysis
P<0.05 Group B versus Group C in post hoc analysis
P<0.05 Group A versus Group B in post hoc analysis.
The in vitro release behavior of the hybrid stent with drug-eluting nanofibers, with two high drug loadings (5 μg/mm2 and 25 μg/mm2) was determined by an elution method and HPLC assay. Figure 4 plots the daily and accumulated release curves of acetylsalicylic acid from the nanofibers. The measurements suggest that the hybrid stents exhibited biphasic release patterns, with an initial burst release for the first few days, followed by a rather stable release of acetylsalicylic acid for up to 3 weeks (20 μg/mL and 8 μg/mL, respectively). After the electrospinning procedure for the 25 μg/mm2 and 5 μg/mm2 acetylsalicylic acid loaded stents, most pharmaceuticals were dispersed in the bulk of PLGA matrix. However, some drugs might have located on the surface of the nanofibers, leading to the initial burst of drug release. Following this initial burst, drug release was controlled solely by degradation of the polymeric materials.25,26 Thus, for 3 weeks, the nanofibers exhibited a rather stable local release of high concentration acetylsalicylic acid which is compatible with the required systemic concentration of 0.5–10 μg/mL, for the inhibition of thromboxane generation.27,28
Figure 4.
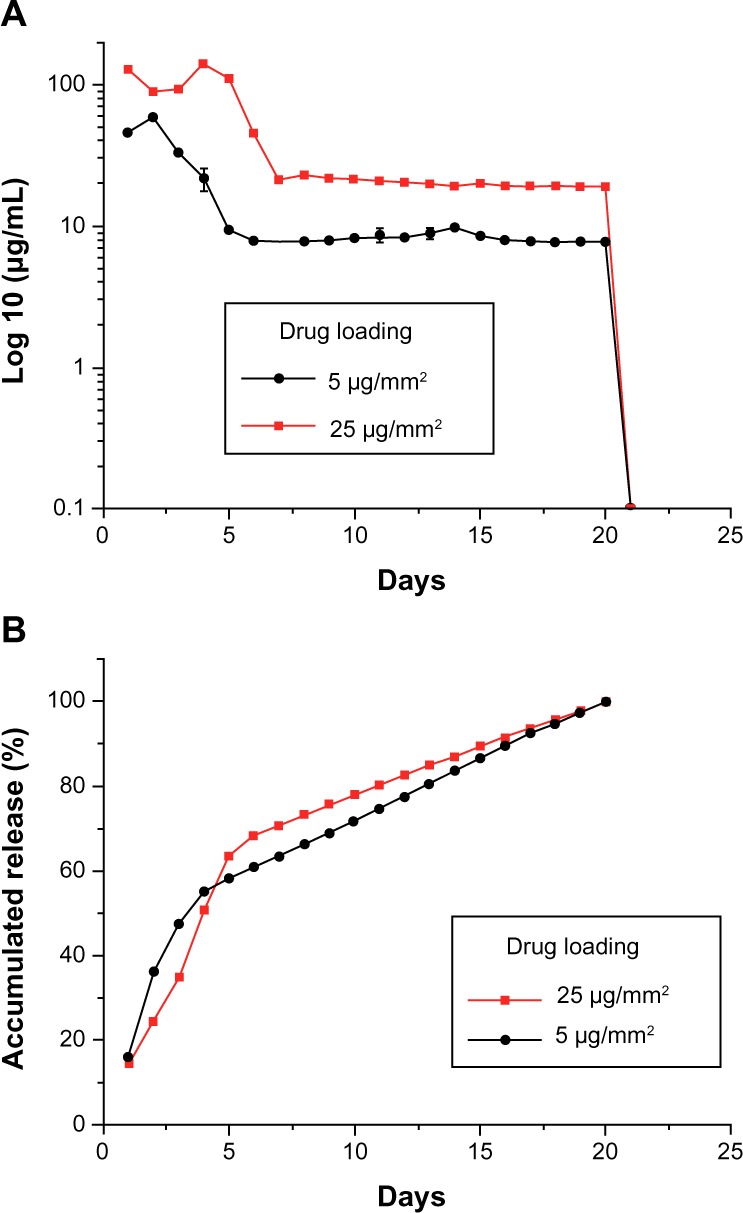
In vitro release of acetylsalicylic acid.
Notes: In vitro (A) daily and (B) accumulated release behaviors of acetylsalicylic acid from nanofibrous membranes. A biphasic release pattern was found, with an initial burst of release for the first few days, followed by a rather stable release for up to 3 weeks.
In vivo animal studies
In vivo studies were performed using 24 animals, which received hybrid stents with acetylsalicylic acid-loaded biodegradable nanofibers, fitted within the descending abdominal aorta (Figure 5A). Animal variability was accounted for by using a hybrid stent, without drug loadings, as a control within each animal, and performing a paired analysis. Vessel position and stent deployment were verified after the procedure (Figure 5B). All animals survived the in-life phase of the study, and no evidence of infection was observed. Furthermore, the position of the branch vessel (Figure 6A), the position of stent deployment (Figure 6B), and post-implanted patency (Figure 6C) were confirmed via angiography in each animal. No compromise of the branch vessels was observed after implantation of the hybrid stent, probably because the pore size of the nanofibers exceeded 10 μm, allowing red blood cells to pass to the branch vessels. The drug-loaded nanofibers did not affect blood circulation after deployment of the stent.
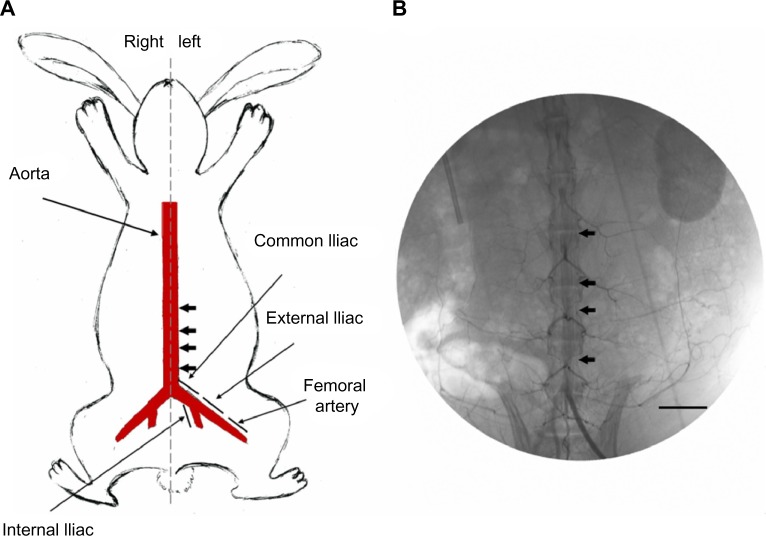
Figure 5.
Stent implantation.
Notes: (A) Schematic locations of implantation of two stents in rabbit descending abdominal aorta (arrow). (B) Angiograms of rabbit vasculature following injection of contrast dye, showing location of stented area (arrow). Scale bar: 20 mm.
Figure 6.
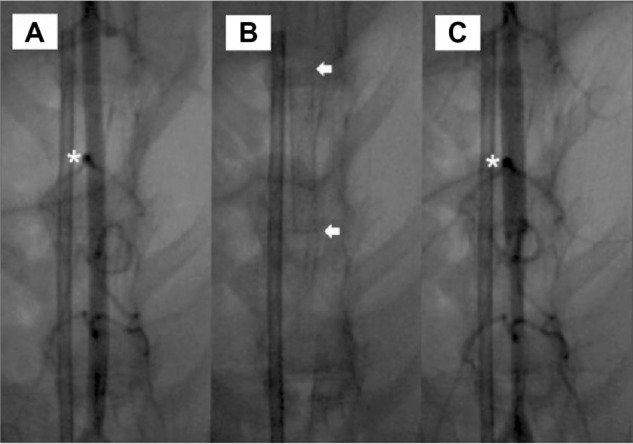
Angiograms of rabbit vasculature.
Notes: (A) Following injection of contrast dye. (B) stented descending abdominal aorta. (C) showing patent branch vessels following implantation. Asterisks indicate abdominal aorta branch. Arrows indicate implantation part of stent. Scale bar: 10 mm.
Endothelial coverage and adhesion of blood cells
Endothelial coverage and the adhesion of blood cells were observed (Figure 7). At 14 days (Figure 7A1, B1 and C1), re-endothelialization onto the surface of struts was variable among groups (Figure 8), with significantly greater coverage in Group A (89.9% ± 7.8%) and Group B (88.2% ± 8.7%), than in the control Group C (80.3% ± 6.5%). At 28 days, areas above struts showed more than 95% surface area coverage, for all groups. Group A exhibited more complete coverage (99.2% ± 0.7%) than Group C (95.0% ± 3.0%), with borderline significance between Group B (97.1% ± 2.6%) and Group C (P=0.05). Microscopic results (Figure 7) also suggest that areas lacking endothelial coverage, such as those in Group C, generally showed focal aggregates of platelets and inflammatory cells. Additionally, adherent inflammatory cells and platelets were found on endothelial surfaces.
Figure 7.
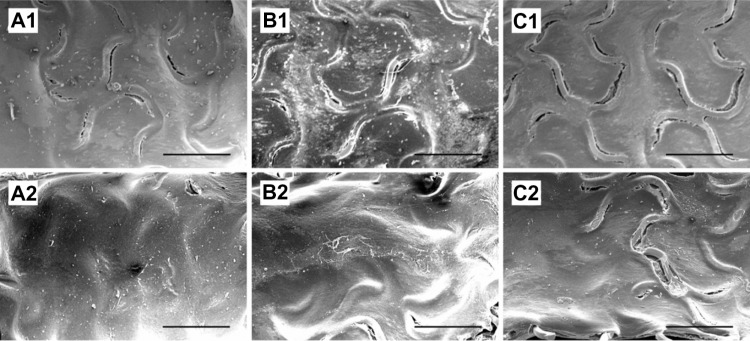
Quantitative analysis of endothelial coverage.
Notes: Endothelial coverage above struts at 2 weeks (A1, B1 and C1) and 4 weeks (A2, B2 and C2) in Groups A, B, and C. Group A exhibited the most complete coverage at both 2 and 4 weeks.
Figure 8.
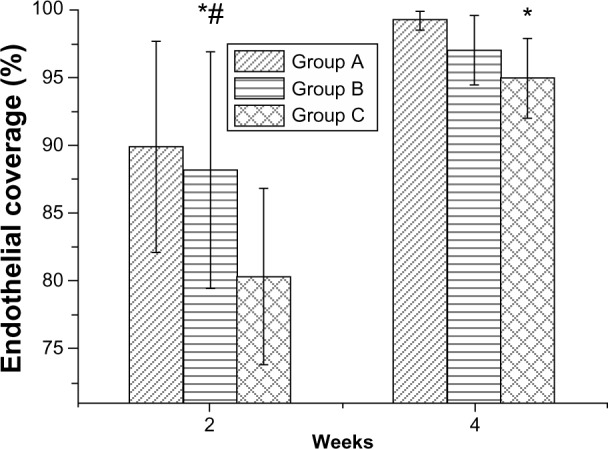
Percentage of endothelial coverage on stent struts.
Notes: Acetylsalicylic acid-loaded (Groups A and B), and non-acetylsalicylic acid-loaded (Group C) nanofibrous membrane stent. *P<0.05 Group A versus Group C in post hoc analysis; #P<0.05 Group B versus Group C in post hoc analysis.
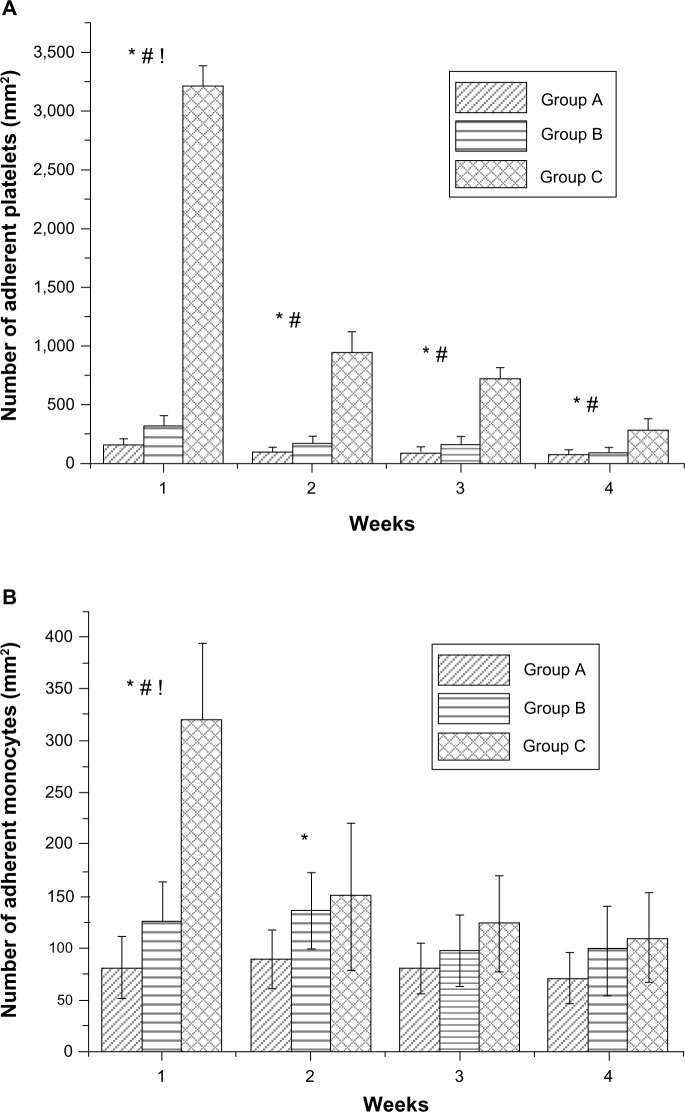
The adhesion of platelets and monocytes to the surface of stented arterial vessels was assessed at 1, 2, 3, and 4 weeks, using SEM. The photographs (Figure 9) suggest that the hybrid stent with acetylsalicylic acid-loaded biodegradable nanofibers can effectively inhibit the adhesion of platelets and monocytes to the endothelial surface of blood vessels. Figure 10A shows the calculated platelets adhesion densities for the endothelial surfaces in Groups A, B and C at 1, 2, 3, and 4 weeks. Hybrid stents can significantly reduce the adhesion of platelets after deployment. The number of platelet adhered to vessel walls in Group A was lower, at Day 7, (141 ± 69 per mm2) than in Group B (325 ± 83 per mm2) and Group C (3,209 ± 174 per mm2). Post hoc testing also indicated that Groups A and B exhibited significantly less platelet adhesion than Group C at 2, 3, and 4 weeks. This suggests that the acetylsalicylic acid loaded nanofibers in the hybrid stents help to reduce the adhesion of platelets to endovascularized surfaces. Additionally, despite platelet adhesion densities decreasing over time in all groups, the numbers of adhered platelets in Group A (81 ± 45 per/mm2) and Group B (99 ± 45 per mm2) were still significantly lower than the numbers in Group C (285 ± 96 per mm2), after 4 weeks. Figure 10B also shows that the number of adhered monocytes in Group A was significantly lower than in Groups B and C, at both 1 and 2 weeks.
Figure 9.
Adherent platelets and monocytes at various times.
Notes: Adhesion of platelets and monocytes on endothelial surface of stented arterial vessels in Groups A, B, and C at 1, 2, 3, and 4 weeks by scanning electron microscopy. Acetylsalicylic acid-loaded hybrid stents can inhibit the adhesion of platelets and monocytes on the endothelial surface of blood vessels. Scale bar: 50 μm.
Figure 10.
Number of adherent blood cells on stent struts.
Notes: (A) Number of adherent platelets on stent struts. Acetylsalicylic acid-loaded (Groups A and B) and non-acetylsalicylic acid-loaded (Group C) nanofibrous membrane stent. (B) Number of monocytes that adhered on stent struts. *P<0.05 Group A versus Group C in post hoc analysis. #P<0.05 Group B versus Group C in post hoc analysis; !P<0.05 Group A versus Group B in post hoc analysis.
While the nanofibers could release a high concentration of acetylsalicylic acid for only 3 weeks in vitro, the results of in vivo investigation indicate that acetylsalicylic acid release could effectively reduce platelet agglomerations for longer than 4 weeks. For all pharmaceuticals, the in vivo environment appears to provide a slower metabolic rate than the in vitro environment.29 This phenomenon may explain why the effective period of in vivo drug release is longer than it is in vitro. In addition, the in vivo antiplatelet property of acetylsalicylic acid is known to continue for some time after discontinuation of therapy. This might also account for the longer effective release of acetylsalicylic acid in vivo.
Histological examination
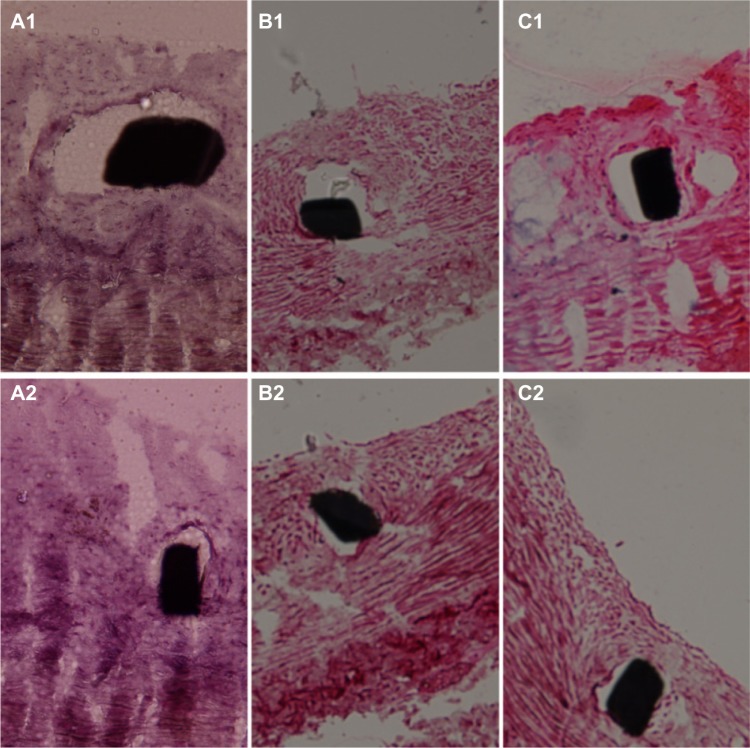
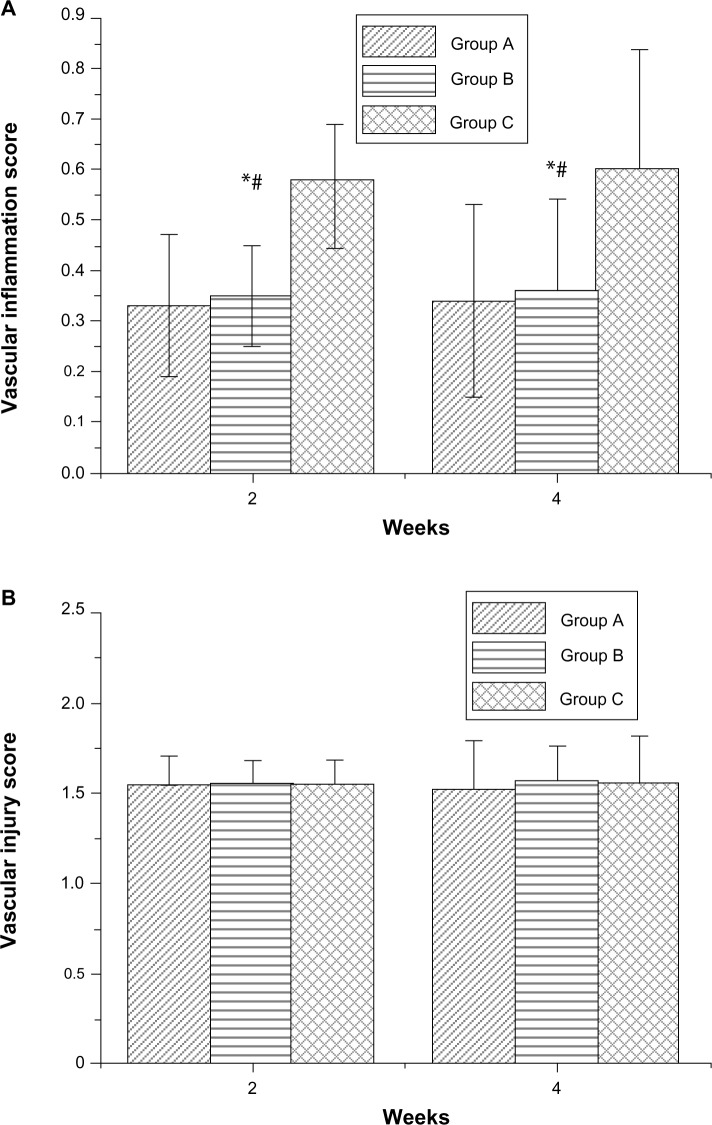
Figure 11 shows pathological sections of arterial lesions, stained with hematoxylin-eosin, in different groups, at 2 weeks (Figure 11A1, B1 and C1), and at 4 weeks (Figure 11A2, B2 and C2). Figure 12 shows the vascular inflammation scores and vascular injury scores calculated. Various groups exhibited significant differences for inflammation response. At 2 weeks, the vascular inflammation score was lower for vessels in Group A (0.33 ± 0.14) and Group B (0.35 ± 0.10) than in Group C (0.58 ± 0.11). At 4 weeks, the scores of Group A (0.34 ± 0.19) and Group B (0.36 ± 0.18) were still lower than those of Group C (0.60 ± 0.24).
Figure 11.
Pathology of Groups A, B, and C at 2 and 4 weeks.
Notes: Two-week (A1, B1 and C1) and 4-week (A2, B2 and C2) high-power photomicrographs (H&E staining; 200 × magnification) of pathology of Groups A, B, and C. The inflammation response was significant lower in Groups A and B than in Group C.
Figure 12.
Vascular inflammation and injury scores.
Notes: (A) Vascular inflammation score. (B) Vascular injury score. *P<0.05 Group A versus Group C in post hoc analysis; #P<0.05 Group B versus Group C in post hoc analysis.
Vascular injury scores were comparable in all groups at 2 weeks (Group A: 1.55 ± 0.15; Group B: 1.56 ± 0.2; Group C: 1.55 ± 0.13) and 4 weeks (Group A: 1.52 ± 0.28; Group B: 1.57 ± 0.20; Group C: 1.56 ± 0.27). The biodegradable PLGA nanofibers gradually degraded after operative implantation, and were almost diminished after 4 weeks.
Detection of collagen Type 1 after four weeks of response-to-injury
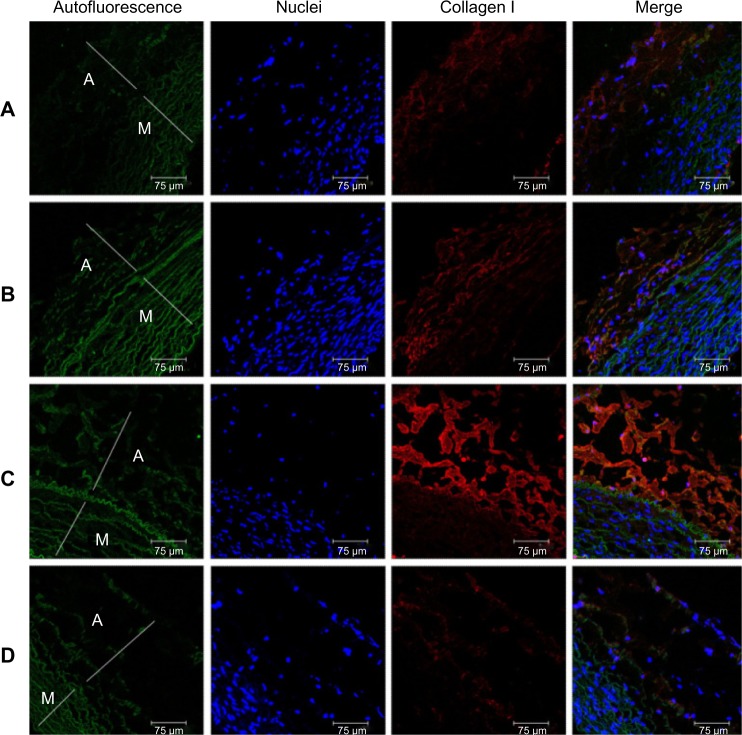
Immunofluorescent labeling of collagen type I, an arterial fibrosis marker, was observed on stented arteries by confocal fluorescence microscopy. The specimens were also co-stained with DAPI, to reveal cell nuclei. Samples of untreated rabbit aorta were used as controls for immunofluorescent labeling (Figure 13D). Collagen type I was present at the tunica adventitia of the aorta. For all samples, less collagen I-positive labeling was observed close to the distal acetylsalicylic acid-loaded stented vessels (Figure 13A and B) than the proximal non-acetylsalicylic acid-loaded stented vessels (Figure 13C). Shekhonin et al reported that collagen type I, which interstitial collagens contain, may be relevant to the subendothelium of local intimal thickenings, to the thickened intima of the aged, and the fibrosis process.30 The acetylsalicylic acid-loaded nanofibers developed in this study can therefore be used to minimize atherogenesis in response to injury after stent deployment.
Figure 13.
Immunofluorescence of collagen I close to stented arteries.
Notes: Collagen type I immunostaining (orange) of acetylsalicylic acid-loaded (Groups A and B) and non-acetylsalicylic acid-loaded (Group C) nanofibrous membrane stent. (Group D) normal vessels. Autofluorescence on tunica media (green), and DAPI-stained nuclei are also shown. Less collagen type I-positive labeling was observed close to the acetylsalicylic acid-loaded stent vessels. scale bar: 75 μm.
Abbreviations: A, tunica adventitia; M, tunica media; D, control group.
Discussion
This study has developed biodegradable acetylsalicylic acid-loaded nanofibers from PLGA materials using an electrospinning process. In vitro and in vivo analysis of the hybrid stent with acetylsalicylic acid-loaded PLGA nanofibers has demonstrated local, sustained, high-dose acetylsalicylic acid delivery, efficacy in reducing platelet and monocyte adhesion, and less tissue reaction from stenting. Compared to nano sprayed (or nano coated) stents that require a complex manufacturing process, the hybrid stent in this study was prepared using a simple electrospinning process, and the drug-loading efficiency is high (40/(240 + 40) =14.3%). Furthermore, the hybrid stent is a robust platform for drug delivery, and has been shown to cause no branch vessel compromise after implantation.
Endothelial cells play a central role in vascular homeostasis, including the regulation of the interaction of circulating cells with the vessel wall.31 Platelets and leukocytes have been implicated in the pathogenesis of atherothrombosis and inflammation after vascular injury. Leukocyte activation, with platelet adherence, occurs after coronary angioplasty. The magnitude of leukocyte activation and platelet adherence appears to be higher in patients experiencing late clinical events.32–35 During incomplete re-endothelialization at the inner surface of a stent, platelets adhering to the nonendothelialized arterial surface pose a life-threatening phenomenon in stent thrombosis after implantation.36 Acetylsalicylic acid is the “gold standard” antiplatelet agent for preventing arterial thrombosis; it has been demonstrated that dosages as low as 50 mg can sufficiently block thromboxane synthesis by platelets, in patients with angina.37 Acetylsalicylic acid also inhibits monocyte adhesion, in stimulated human endothelial cells.38 However, the inhibition by acetylsalicylic acid of COX-1 in gastric mucosal cells decreases the production of cytoprotective prostaglandins, possibly increasing the risk of gastrointestinal bleeding. Other studies have established that major bleeding is associated with higher rates of mortality in several large-scale observational analyses.39,40 Therefore, the avoidance of major bleeding is essential in managing acute coronary syndrome. Local acetylsalicylic acid delivery, with a nanofibrous membrane via stent, decreased platelet adhesion during the early stage of incomplete re-endothelialization, and promoted endothelialization for about 4 weeks. The vascular injury scores in this study may be higher than those of previous studies,41,42 which possibly results from differences in denudation procedures and animal arterial models. The inflammation scores were significantly lower than the scores of previous studies, which raises the possibility that decreased inflammatory response can lead to improved endothelial function at, and adjacent to, the stent site.36
Collagen type I, the principle content of interstitial collagens, was significantly increased in the subendothelium of local intimal thickenings, and in a thickened intima of the aged. This fact, considering the thrombogenicity of interstitial collagens, may be relevant to atherogenesis, through the injury-response mechanism.30 Dose-dependent platelet aggregation induced by collagen is strongly potentiated by the anti-aggregating effect of antiplatelet therapy.43 This study found local, sustained delivery of acetylsalicylic acid, via a hybrid stent with biodegradable nanofibers, reduces collagen type I in denuded rabbit abdominal aortas.
Finkelstein et al suggest that current methods of local drug delivery from vascular stents can be viewed in a perspective analogous to progress in developing systemically administered drugs.44 Previously, systemic medication therapy was limited by the fixed pharmacokinetics of specific drugs. Combining drugs with a controlled release method to create flexible hybrid delivery platforms (thereby decoupling chemical and kinetic properties) has revolutionized systemic therapy. Furthermore, programmable, controlled-release stent technology suggests that similar approaches may be feasible for treatment interventions for cardiovascular diseases.
After mounting on the BMS, nanofibers can provide a sustained release of high concentration acetylsalicylic acid for 3 weeks in vitro, exhibiting biphasic release characteristics. After the electrospinning procedure, most pharmaceuticals were dispersed in the bulk of the PLGA matrix. However, some drugs might have been located on the surface of the nanofibers, leading to the initial burst of drug release. Following the initial burst, drug release was controlled solely by degradation of the polymeric materials.25,26 Thus, for 3 weeks, the nanofibers exhibited a rather stable local release of high concentration acetylsalicylic acid greater than 20 μg/mL and 8 μg/mL for the 25 μg/mm2 and 5 μg/mm2 drug loaded stents, respectively, which is compatible with the required systemic concentration of 0.5–10 μg/mL for the inhibition of thromboxane generation.27,28 The numbers of adhered platelets and monoctyes decreased with the drug loadings of acetylsalicylic acid. This feature represents a significant advantage, in terms of providing high acetylsalicylic acid concentration at the vessel wall, while maintaining the minimum systemic drug level. Additionally, while the nanofibers could release a high concentration of acetylsalicylic acid for only 3 weeks in vitro, the in vivo results indicated that the released drug still reduces platelet agglomeration after 4 weeks. For all pharmaceuticals, the in vivo environment appears to provide a slower metabolic rate than the in vitro environment. This phenomenon may explain why the effective period of in vivo drug release is longer than it is in vitro.
Previous studies have identified side branch occlusion, after stent implantation in patients with coronary artery disease, as contributing to an increase of post-interventional cardiac enzymes; even a minimal increase is associated with increased incidence of cardiac events during the first year.45–47 Experimental results from this study found no compromise of the branch vessels after hybrid stent implantation. This avoids complications, including side branch occlusion and flow deterioration, and provides local, as well as sustained, high concentration acetylsalicylic acid therapy.
As a potential material for arterial revascularization, biodegradable polymers can exist safely in the human body, eventually absorbing, without causing harm or adverse interactions. Their ability to remain in the human body safely for extended lengths of time makes biodegradable polymers especially appropriate for medical applications – an alternative to traditional polymers. The development of newer devices focuses on attaining optimal anti-restenotic efficacy, at a minimum of arterial wall toxicity and inflammation, for endothelium regrowth.48 Biodegradable polymers allow the controlled elution of active drugs from the stent backbone through a biocompatible polymer coating, which degrades slowly, to form inert organic monomers, after the completion of its useful function, thus reducing risk associated with the long-term presence of durable polymers in the arterial vessel wall.49–51 PLGA is non-toxic, elicits minimal inflammatory response, and can be absorbed eventually, without any accumulation in vital organs.52 PLGA belongs to a class of synthesized biodegradable and biocompatible copolymers, from which absorbable sutures, absorbable surgical clips, and controlled-release implants have been made. Different forms of PLGA can be obtained, depending on the ratio of lactide to glycolide used in polymerization. The 50:50 ratio copolymer exhibits the fastest degradation (about 2 months), and falls within the class of materials used for implantable, and injectable, controlled-release drug delivery systems. With a history of safe use, these copolymers have been approved for human use.53 Following its introduction into the human body, PLGA material induces only a minimal inflammatory response; it biodegrades through the hydrolysis of its ester linkages to yield biocompatible lactic and glycolic acids.54
Previous studies show that the electrospinning process denaturalizes the biological and structural properties of a natural protein, such as collagen.55 The experimental results of this study demonstrated that the PLGA with acetylsalicylic acid nanofibers remained intact during our procedure, and could function as an excellent vehicle for drug delivery. Furthermore, the antiplatelet function of of acetylsalicylic acid remains high after the electrospinning process. Our findings suggest that electrospun PLGA with acetylsalicylic acid-loaded nanofibers is a promising candidate for drug release, to prevent platelet and monocyte aggregation after stent implantation.
Study limitations
Despite its contributions, this study has several limitations. First, the assessment of sustained release of acetylsalicylic acid in an animal model, using nonatherosclerotic arteries, may have underestimated the effect of thrombogenic responses on arterial vasculature; atherosclerotic tissue intensifies platelet adhesion. However, the number of platelets adhered to injured arterial walls was still significantly greater in the non-drug-loaded group. Second, the animal models used currently for the assessment of stents are limited with respect to their ability to replicate human conditions. Results with the rabbit model have been generally representative of human responses, although with a different time-course of healing. We recommend that future studies should investigate atherosclerotic coronary models, to confirm the analytical results of this study.
Conclusions
This study demonstrated the use of hybrid stents with biodegradable acetylsalicylic acid-loaded nanofibers (fabricated by the electrospinning process) for vessel revascularization-related applications. Acetylsalicylic acid-loaded nanofibers were functionally active, highly effective as inhibitors of platelet and monocyte adhesion, and promoted re-endothelialization. Furthermore, the nanofibers induced only a minimal inflammatory reaction of the vascular tissues, and were completely absorbed in 4 weeks. Importantly, hybrid stents with biodegradable drug-eluting nanofibers support local, sustained drug delivery, and may have potential applications in the delivery of cardiovascular drugs. Finally, hybrid stents with acetylsalicylic acid-loaded nanofibers and high drug loadings (25 μg/mm2) effectively reduce adhesion of blood cells and promote re-endothelialization, which may provide insight into treating patients with a high risk of acute stent thromboses.
Acknowledgments
The authors would like to thank the National Science Council of Taiwan (Contract No NMRPG5C0041) and Chang Gung Memorial Hospital (Contract No CMRPD2A0082) for financially supporting this research. Ted Knoy and Yichia Lin are appreciated for their editorial assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52(5):333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Cook S, Windecker S. Early stent thrombosis: past, present, and future. Circulation. 2009;119(5):657–659. doi: 10.1161/CIRCULATIONAHA.108.842757. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Orford JL, Lennon R, Melby S, et al. Frequency and correlates of coronary stent thrombosis in the modern era: analysis of a single center registry. J Am Coll Cardiol. 2002;40(9):1567–1572. doi: 10.1016/s0735-1097(02)02374-4. [DOI] [PubMed] [Google Scholar]
- 6.Schuhlen H, Kastrati A, Dirschinger J, et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation. 1998;98(2):104–111. doi: 10.1161/01.cir.98.2.104. [DOI] [PubMed] [Google Scholar]
- 7.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316(12):701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Morimoto T, Kozuma K, et al. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART) Circulation. 2010;122(1):52–61. doi: 10.1161/CIRCULATIONAHA.109.903955. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Lansky AJ, Mehran R, et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009;119(5):687–698. doi: 10.1161/CIRCULATIONAHA.108.804203. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer TL, Becker RC. Inhibitors of platelet adhesion. Circulation. 2009;120(24):2488–2495. doi: 10.1161/CIRCULATIONAHA.109.886895. [DOI] [PubMed] [Google Scholar]
- 11.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Steele PM, Chesebro JH. Role of platelets and thrombosis in coronary atherosclerotic disease and sudden death. J Am Coll Cardiol. 1985;5(Suppl 6):175B–184B. doi: 10.1016/s0735-1097(85)80552-0. [DOI] [PubMed] [Google Scholar]
- 13.Roth GJ, Calverley DC. Aspirin, platelets, and thrombosis: theory and practice. Blood. 1994;83(4):885–898. [PubMed] [Google Scholar]
- 14.Kharbanda RK, Walton B, Allen M, et al. Prevention of inflammation-induced endothelial dysfunction: a novel vasculo-protective action of aspirin. Circulation. 2002;105(22):2600–2604. doi: 10.1161/01.cir.0000017863.52347.6c. [DOI] [PubMed] [Google Scholar]
- 15.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Conejero R, Rivera J, Corral J, Acuna C, Guerrero JA, Vicente V. Biological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure? Stroke. 2005;36(2):276–280. doi: 10.1161/01.STR.0000151362.65339.f9. [DOI] [PubMed] [Google Scholar]
- 17.Santos MT, Valles J, Aznar J, Marcus AJ, Broekman MJ, Safer LB. Prothrombotic effects of erythrocytes on platelet reactivity. Reduction by aspirin. Circulation. 1997;95(1):63–68. doi: 10.1161/01.cir.95.1.63. [DOI] [PubMed] [Google Scholar]
- 18.Altman R, Scazziota A. Why aspirin cannot prevent arterial thrombosis. Circulation. 1996;94(11):3002–3003. [PubMed] [Google Scholar]
- 19.Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002;106(21):2734–2740. doi: 10.1161/01.cir.0000038982.49640.70. [DOI] [PubMed] [Google Scholar]
- 20.Lu F, Shen YY, Shen YQ, Hou JW, Wang ZM, Guo SR. Treatments of paclitaxel with poly(vinyl pyrrolidone) to improve drug release from poly(varepsilon-caprolactone) matrix for film-based stent. Int J Pharm. 2012;434(1–2):161–168. doi: 10.1016/j.ijpharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17(14):R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa G, Granada JF, Alviar CL, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3(1):68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31(1):224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19(2):267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 25.Ramchandani M, Robinson D. In vitro and in vivo release of ciprofloxacin from PLGA 50:50 implants. J Control Release. 1998;54(2):167–175. doi: 10.1016/s0168-3659(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 26.Amann LC, Gandal MJ, Lin R, Liang Y, Siegel SJ. In vitro-in vivo correlations of scalable PLGA-risperidone implants for the treatment of schizophrenia. Pharm Res. 2010;27(8):1730–1737. doi: 10.1007/s11095-010-0152-4. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984;311(19):1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 28.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabara R, Pendyala L, Geva S, Chen J, Chronos N, Robinson K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention. 2009;5(Suppl F):F58–F64. doi: 10.4244/EIJV5IFA10. [DOI] [PubMed] [Google Scholar]
- 30.Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. 1985;5(4):355–368. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- 31.Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- 32.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28(2):345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 33.Beekhuizen H, van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993;54(4):363–378. [PubMed] [Google Scholar]
- 34.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Jennings LK. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103(Suppl 3):4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 37.Montalescot G, Maclouf J, Drobinski G, Salloum J, Grosgogeat Y, Thomas D. Eicosanoid biosynthesis in patients with stable angina: beneficial effects of very low dose aspirin. J Am Coll Cardiol. 1994;24(1):33–38. doi: 10.1016/0735-1097(94)90538-x. [DOI] [PubMed] [Google Scholar]
- 38.Weber C, Erl W, Pietsch A, Weber PC. Aspirin inhibits nuclear factor-kappa B mobilization and monocyte adhesion in stimulated human endothelial cells. Circulation. 1995;91(7):1914–1917. doi: 10.1161/01.cir.91.7.1914. [DOI] [PubMed] [Google Scholar]
- 39.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 40.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49(12):1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Miyauchi K, Kasai T, Yokayama T, et al. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J. 2008;72(5):832–838. doi: 10.1253/circj.72.832. [DOI] [PubMed] [Google Scholar]
- 42.Kong JY, Wang TQ, Jiang GH, Li L, Wang FP. Urinary trypsin inhibitor reduced inflammatory response after stent injury in minipig. Pathol Res Pract. 2012;208(6):344–349. doi: 10.1016/j.prp.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Moshfegh K, Redondo M, Julmy F, et al. Antiplatelet effects of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effects of combination therapy. J Am Coll Cardiol. 2000;36(3):699–705. doi: 10.1016/s0735-1097(00)00817-2. [DOI] [PubMed] [Google Scholar]
- 44.Finkelstein A, McClean D, Kar S, et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003;107(5):777–784. doi: 10.1161/01.cir.0000050367.65079.71. [DOI] [PubMed] [Google Scholar]
- 45.Mazur W, Grinstead WC, Hakim AH, et al. Fate of side branches after intracoronary implantation of the Gianturco-Roubin flex-stent for acute or threatened closure after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1994;74(12):1207–1210. doi: 10.1016/0002-9149(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Hahn JY, Song YB, et al. Carina shift versus plaque shift for aggravation of side branch ostial stenosis in bifurcation lesions: volumetric intravascular ultrasound analysis of both branches. Circ Cardiovasc Interv. 2012;5(5):657–662. doi: 10.1161/CIRCINTERVENTIONS.112.969089. [DOI] [PubMed] [Google Scholar]
- 47.Toyofuku M, Kimura T, Morimoto T, et al. Three-year outcomes after sirolimus-eluting stent implantation for unprotected left main coronary artery disease: insights from the j-Cypher registry. Circulation. 2009;120(19):1866–1874. doi: 10.1161/CIRCULATIONAHA.109.873349. [DOI] [PubMed] [Google Scholar]
- 48.Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33(10):1214–1222. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 49.Wessely R. New drug-eluting stent concepts. Nat Rev Cardiol. 2010;7(4):194–203. doi: 10.1038/nrcardio.2010.14. [DOI] [PubMed] [Google Scholar]
- 50.Byrne RA, Kastrati A, Kufner S, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30(20):2441–2449. doi: 10.1093/eurheartj/ehp352. [DOI] [PubMed] [Google Scholar]
- 51.Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372(9644):1163–1173. doi: 10.1016/S0140-6736(08)61244-1. [DOI] [PubMed] [Google Scholar]
- 52.Lu L, Peter SJ, Lyman MD, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21(18):1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 53.Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29(30):4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali SA, Doherty PJ, Williams DF. Mechanisms of polymer degradation in implantable devices. 2. Poly(DL-lactic acid) J Biomed Mater Res. 1993;27(11):1409–1418. doi: 10.1002/jbm.820271108. [DOI] [PubMed] [Google Scholar]
- 55.Powell HM, Supp DM, Boyce ST. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials. 2008;29(7):834–843. doi: 10.1016/j.biomaterials.2007.10.036. [DOI] [PubMed] [Google Scholar]