Abstract
BACKGROUND
It has been widely reported that individuals with a light phenotype (i.e., light hair color, light base skin color, and propensity to burn) have more nevi and are at greater risk for developing skin cancer. No studies have systematically investigated how phenotypic traits may interact in relation to nevus development.
OBJECTIVE
We sought to systematically examine whether any combinations of phenotype are associated with a greater or lesser risk for nevus development in white children.
METHODS
In the summer of 2007, 654 children were examined to determine full body nevus counts, skin color by colorimetry, and hair and eye color by comparison to charts. Interviews of parents were conducted to capture sun sensitivity, sun exposure and sun protection practices.
RESULTS
Among 9-year-old children with sun sensitivity rating type 2 (painful burn/light tan), those with light hair had lower nevus counts than did those with dark hair (p-value for interaction = 0.03). This relationship was independent of eye color, presence of freckling, gender, usual daily sun exposure, sunburn in 2004–2007, sun protection index and waterside vacation sun exposure. The difference in nevus counts was further determined to be specific to small nevi (less than 2 mm) and nevi in intermittently exposed body sites.
LIMITATIONS
Geographic and genetic differences in other study populations may produce different results.
CONCLUSION
The standard acceptance that dark phenotype is a marker for low melanoma risk and light phenotype a marker for high risk may need to be reevaluated. In non-Hispanic white children, dark haired individuals who burn readily and then tan slightly are more prone to nevus development, and may therefore be a previously under-recognized high risk group for melanoma.
Keywords: children, nevus, phenotype, hair color, sun sensitivity, interaction, epidemiology
INTRODUCTION
In 2008, over 62,480 new melanomas were diagnosed in the United States, and there were over 8,000 deaths.1 The latest national data based on cases diagnosed in 2008 place melanoma sixth in incidence among all cancers for men and seventh for women.2 Based on rates from 2003–2005, 1.8% (or 1 in 55) of men and women born today will be diagnosed with melanoma at some time during their lifetime.3
Melanoma incidence is highest in whites, up to 20 times higher than for other racial or ethnic groups, and is strongly associated with the presence of numerous melanocytic nevi.4–5 The total number of nevi on the whole body is thought to be the most important independent risk factor and the risk of melanoma increases almost linearly with rising numbers of nevi.6 The role of benign melanocytic nevi as precursors and not only as risk markers for the development of melanoma is controversial.7 A history of a preexisting nevus at the site of a melanoma is recorded in between 19% 8 and 85%9 of cases. A more recent histopathological analysis of melanomas by Skender-Kalnenas et al7 suggests that greater than 50% of melanomas are associated with melanocytic nevi either of the benign or atypical type. Additionally, the presence of numerous nevi and melanoma share several common risk factors (light skin, light hair, light eyes, inability to tan, history of sunburns etc.). 10–19 Nevi are therefore considered reasonable markers for estimating melanoma risk.
The study of melanocytic nevi in young children is important for skin cancer prevention efforts because childhood is a time when nevi are acquired rapidly.12 Although children are rarely diagnosed with melanoma, the number of nevi in childhood is often used as a proxy in determining their melanoma risk.
Many studies have reported that children who are of a lighter phenotype acquire more nevi, even when adjusting for factors such as sunburn history and gender. 13,17,19,20–23 However, none of the previous studies that have investigated the relationship between phenotype and childhood nevus development systematically analyzed whether the five major phenotypic factors (freckling, hair color, eye color, base skin color, and sun sensitivity) interact in relation to nevus development. Studies have usually focused on the main effects of phenotype on nevus development and only occasionally report interaction effects as incidental findings. 13,18,24–26 In a recent study by our group,26 we found that very light-skinned children who tan had more nevi than those who did not tan, while in darker-skinned white children the relationship between tanning and nevi was not present. This suggests that the relationship between sun exposure and nevus development is modified by degrees of skin color.
The present analysis was motivated by the clinical observation of our team of skin examiners, over the past five years, of a subset of blonde children who appeared to tan readily and had low numbers of nevi. This suggested there is a subset of children who do not conform to the expectation that light hair color is associated with an inability to tan and higher nevus counts. These observations led us to believe that the relationship between these phenotypic characteristics and nevus development may not be as straightforward as generally assumed. We hypothesized that, by systematically testing interaction terms involving phenotypic traits, we would identify modifying effects on nevus formation involving skin sun sensitivity, hair color, and skin color, among non-Hispanic white children. Freckling and eye color were initially included in this hypothesis, but insufficient numbers of study participants representing variations of freckling and eye color within categories of these three main phenotypic traits precluded their systematic evaluation.
METHODS
Study Design and Sample
The study population analyzed was part of a nested randomized controlled trial within a prospective cohort study. Longitudinal data on phenotypic characteristics, sun exposure and nevus counts were obtained from a cohort of children in Colorado, US, starting at age 6 and continuing through age 9.22 The participants were children born between January-September, 1998, recruited from private pediatric practices and a large health maintenance organization in the Denver metropolitan area. 19, 22 Children were excluded if they had a debilitating condition or if their parents could not speak English. The majority of the children (86.5%) lived in Colorado since birth. This study was reviewed and approved by the Colorado Multiple Institutional Review Board.
For the present analysis, we included only those children who had a skin examination in 2007 (age 9), the most current and complete data set available for analysis (n=850). We excluded redheads (n=38) because they are known to have fewer nevi than those with other hair colors, suggesting that the development of nevi in red-haired children is fundamentally different, 15,27 possibly due to polymorphisms in the melanocortin 1 receptor (MC1R) gene. 28 Children with a parent-reported race/ethnicity designation of Hispanic, African American/Black, Asian/Pacific Islander, Native American and unknown (n= 158) were excluded because these populations have been shown to have fewer nevus counts overall22 and have a considerably lower melanoma risk.29
Skin Examination
Skin examinations in 2007 were conducted by a team of 7 dermatologists, pediatricians, pediatric nurses and nurse practitioners during the summer months (June-early September) to allow for observation of summer tanning. Skin examinations provided full body melanocytic nevus counts (excluding the genitals and scalp). Nevi were differentiated from freckles and café-au-lait macules by whether or not they were raised (since only nevi are raised) and if flat, by the fact that early junctional nevi are dark brown, have regular edges, and do not occur in patches as do freckles. 13 Warts were distinguished from nevi by their verrucous nature. 13 Congenital nevi were excluded from total nevus counts. Plastic stencils were used to measure nevus size. Nevi were recorded as being either raised or flat, and coded by size: < 2 mm, ≥ 2 mm to < 5mm, or ≥ 5 mm. Placement of nevi was recorded on a body map;13 this information was used to classify nevi as residing on “intermittently” or “chronically” sun-exposed body sites. Prior to the beginning of the 2007 data collection period, nevus counting protocols were reviewed as part of skin examination retraining sessions conducted by the lead study dermatologist (JGM). Over the duration of the data collection period, 53 children were evaluated separately by two different examiners to allow determination of interrater reliability and to allow any discrepancies in procedures to be corrected. All examiners participated in the duplicate examinations. Using a 2-way ANOVA mixed effects model, the interrater reliability coefficient was calculated to be 0.88.
Phenotype of the subjects was assessed in part by visual examination. Eye color was recorded (blue, green, hazel, light brown, or dark brown), and for analysis was dichotomized based on similar mean nevus counts as “light” (blue, green, hazel) or “dark” (light or dark brown). Hair color was assessed using hair dye samples and dichotomized as “light” (blonde, light brown) or “dark” (medium or dark brown, black) based on precedence in the literature. 30 Freckling was assessed by means of a diagram with pictures of various freckling patterns on the face, back/shoulders and anterior arms. 13 Examiners recorded the picture that best represented the freckling pattern of each child. For analysis, freckling was dichotomized as “any” versus “none”. Skin coloration was measured using a Chroma Meter CR-400. The Chroma Meter CR-400 measures across the visible light spectrum using the Hunter Lab parameters. The L scale represents the color spectrum from total black to total white. Increasing values on the L scale (more white) are indicative of lighter skin color. 31–32 To obtain an inner arm measurement representing base skin color, a ruler was placed with the zero mark in the center of the child’s axilla. Five readings were taken at a point 7.5 centimeters below the axilla. To qualify as light skinned, the average L reading was greater than or equal to 60 L units. Thus for analysis, base skin color was dichotomized as “light” (≥ 60 L) and “dark” (<60L). This cut-point was determined by consensus of a team of dermatologists and investigators. 26
Surveys
Risk factors for nevus development, including sun exposure history, sunburns and a sun sensitivity rating similar to the Fitzpatrick score33, were reported by parents each summer from 2004–2007 when recall of sun exposures was expected to be optimal. Trained interviewers conducted the interviews by telephone with the parent or guardian who was the child’s primary caregiver. The survey response rates for 2004–2007 among the subset of study participants eligible for this analysis were 96, 98, 99, and 98 percent respectively.
Usual daily summer sun exposure was assessed using the composite of two variables from each interview year from 2004–2007: the number of days per week in the summer the child typically spent more than 15 minutes outside between 11:00 am and 3:00 pm, and the usual number of hours spent outside during these occasions. These variables were multiplied to create a single variable for each year representing the total number of hours outside per week between 11:00 am and 3:00 pm. The four resulting variables were averaged and dichotomized as 0 to 14 h/wk (“half-time or less”) versus 15–28 h/wk (“most-all the time”). The number of sunny waterside vacations taken between birth through age 8 was collected through questions asked each year about vacations to sunny locations. “Waterside” locations were defined as destinations known to be associated with recreational activities such as swimming, surfing, waterskiing, and boating. 34 Vacations to these locations were only included if they occurred during a season in which outdoor water activities would be expected to occur. 34 We analyzed number of sunny waterside vacations through age 8 instead of age 9 because in a previous analysis, we found a lag of at least one year in the relationship between vacation sun exposure and nevus development. 34 The number of waterside vacations was used as a continuous variable in multivariate analysis. A sun protection index was created for each year by taking the mean of four variables assessing the frequency of using sunscreen, shade, clothes covering most of the body, and hats (5- all of the time, 4- most of the time, 3- about half the time, 2- not very often, 1- never) while outside between 11:00 am and 3:00 pm for more than 15 minutes. Index scores for the four years were averaged and a score of 3.5 or above was considered ‘high sun protection’, while a score below 3.5 was considered ‘low sun protection’. Sun sensitivity was assessed in 2007 by the following question: “If your child were outside in strong sunshine at the beginning of the summer for one hour with no protection, which statement describes what would happen?” The sun sensitivity skin ratings were read to the respondent as possible answer choices categorizing the degree of sunburn the following day relative to the degree of tan one week later: painful/none (type 1), painful/light (type 2), slight/little (type 3), none/good (type 4). Sunburns were assessed annually by a question asking whether the child experienced any sunburn in the past year that caused reddening of the skin lasting until the next day regardless of severity. The resulting information was used to create a dichotomous variable: “any burn” in the previous four years versus “no burn.”
Data Analysis
Based on our hypothesis that phenotypic traits may interact in relation to nevus development, we began our analysis with five phenotype main effect variables [presence of freckling (any/none), hair color (light/dark), eye color (light/dark), base skin color (light/dark) and sun sensitivity scores (types 1–4)]. A five-way table was created that included all variables to assess adequate cell size for each phenotypic combination. Presence of freckling and eye color had several cells with low numbers, prohibiting their inclusion in further analyses. For example, for children with no freckles, light hair, dark skin, and sun sensitivity type 1, the cell counts for both eye colors (light and dark) were less than 5. Hair color, skin color and sun sensitivity were retained and we confirmed that all remaining cell counts had sufficient numbers (≥ 5) to proceed with the analysis.
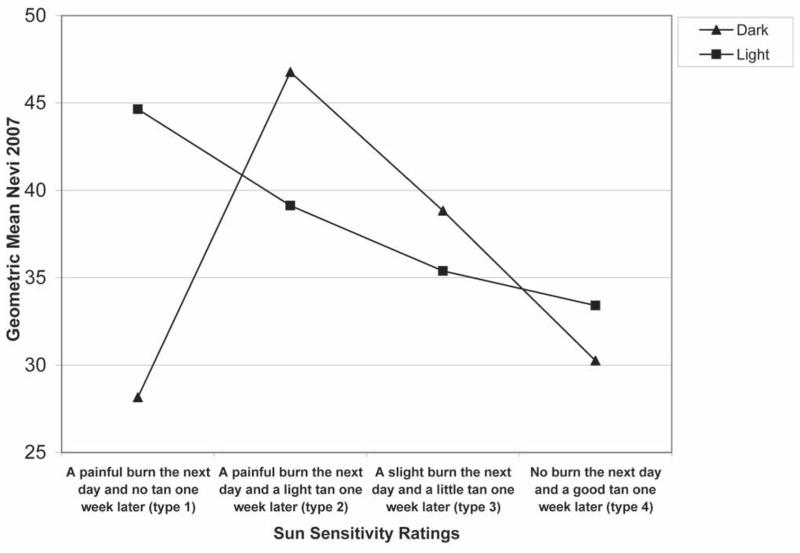
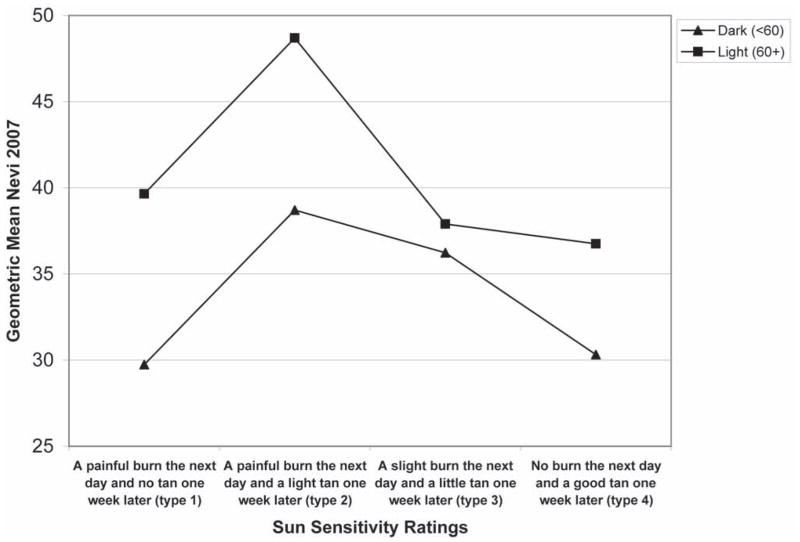
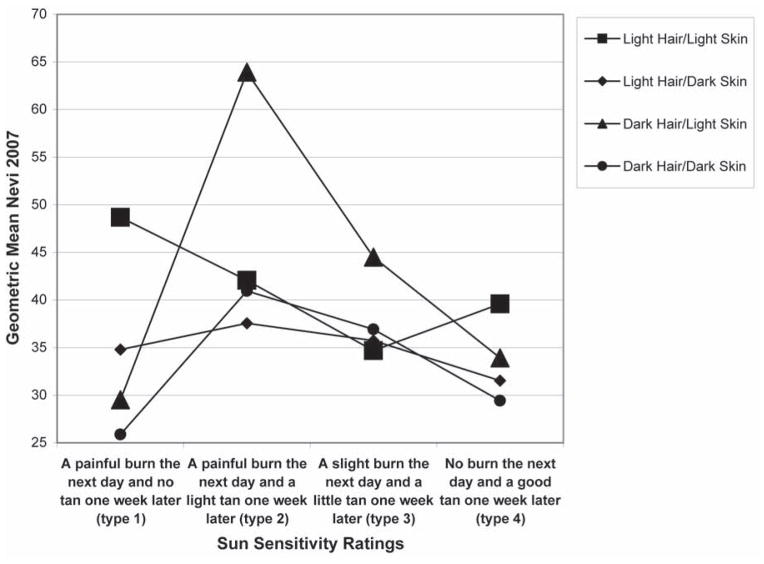
The second step in the analysis was to plot nevus counts by hair color, base skin color and sun sensitivity rating in order to visualize the potential interactions between these variables. In Fig. 1, the mean number of nevi is plotted by sun sensitivity rating, stratified by hair color. Fig. 2 represents the mean number of nevi by sun sensitivity rating, stratified by base skin color. Figure 3 is the combination of Figs. 1 and 2, which is the number of nevi by sensitivity score, stratified by base skin color and hair color. The plots suggest that within sun sensitivity type 2 the relationships between hair color, base skin color and mean number of nevi is modified. Therefore, we dichotomized sun sensitivity rating as type 2 versus all others (types 1, 3, 4).
Figure 1.
Mean number of Nevi by Sun Sensitivity by Hair Color
Note: Y-axis begins at 25
Figure 2.
Mean number of Nevi by Sun Sensitivity by Base Skin Color
Note: Y-axis begins at 25
Figure 3.
Mean number of Nevi by Sun Sensitivity by Hair Color by Base Skin Color
Note: Y-axis begins at 25
Interaction terms were created by coding the phenotypic traits of interest as 0 (reference: dark hair; dark skin; skin sensitivity types 1, 3, 4) versus 1 (light hair; light skin; sun sensitivity- type 2) and multiplying the values to create up to 3-way interaction terms (e.g., light hair X light skin X type 2).
The number of 2007 log-transformed total body nevus counts (all sizes, excluding scalp and genitals) was the main dependent variable. The choice to use log-transformed nevus counts in our multivariate analysis (Table II) and report geometric means of nevus counts (Table I) resulted from the observation of a skewed distribution of the 2007 nevus counts and thereby recognizing the need to stabilize the data variance.35
Table II.
Relationship between nevi, phenotype, sun exposure and interaction terms in White non-Hispanic Colorado children age 9, N=654, multivariate analysis.
| Predictor | B | s.e | antilog (B)a | P value |
|---|---|---|---|---|
| Gender | ||||
| Female | −0.12 | 0.05 | .88 | 0.01 |
| Male | ref | |||
| Sun Sensitivity | ||||
| A painful burn/light tan (type 2) | 0.29 | 0.09 | 1.34 | <0.001 |
| All others (types 1,3,4) | ref | |||
| Hair Color | ||||
| Light (blonde, light brown) | 0.01 | 0.05 | 1.01 | 0.85 |
| Dark (medium-dark brown, black) | ref | |||
| Eye color | ||||
| Light (blue, green, hazel) | 0.14 | 0.05 | 1.15 | 0.01 |
| Dark (brown) | ref | |||
| Presence of Freckling | ||||
| Any | 0.25 | 0.05 | 1.28 | <0.001 |
| None | ref | |||
| Base Skin Color (L-scale)b | ||||
| Light (60+) | 0.07 | 0.05 | 1.07 | 0.17 |
| Dark (<60) | ref | |||
| Sun Protection Indexc | ||||
| High score (3.5+) | −0.07 | 0.07 | 0.94 | 0.34 |
| Low score (<3.5) | ref | |||
| Sunburn in 2004–2007 | ||||
| Any burn | 0.12 | 0.07 | 1.13 | 0.07 |
| No burn | ref | |||
| Usual Daily Sun Exposured | ||||
| Most-all the time | 0.09 | 0.07 | 1.10 | 0.16 |
| Half time or less | ref | |||
| Waterside Vacation Sun Exposuree (# waterside vacations birth thru age 8) | 0.03 | 0.01 | 1.03 | 0.01 |
| Interaction term | ||||
| Light hair X skin type 2 | −0.23 | 0.11 | 0.79 | 0.03 |
Factor by which nevus counts change for every one unit increase in predictor.
Measures skin reflectance, higher values indicate lighter skin color.
Mean sun protection index from 2004–2007.
Mean sun exposure from 2004–2007.
Used as continuous variable in multiple regression.
Note:
The 3 way interaction term of light hair X light skin color X type 2 was tested and was not significant.
The 2 way interaction terms: light hair X light skin and type 2 X light skin were tested and were not significant.
Table I.
Whole body melanocytic nevus counts (in geometric means and 95% confidence intervals) by phenotype and demographic characteristics in White non-Hispanic Colorado children age 9, N=654.
| Characteristic | Nevus count | |||
|---|---|---|---|---|
| n | % | GM (95% CI) | P-valuea | |
| Gender | ||||
| Male | 304 | 46% | 39.4 (36.9–42.1) | <0.001 |
| Female | 350 | 54% | 34.3 (32.1–36.6) | |
| Sun Sensitivityb | ||||
| Painful burn/no tan (type 1) | 56 | 9% | 36.3 (29.8–44.3) | 0.002 |
| Painful burn/light tan (type 2) | 167 | 26% | 41.7 (38.2–45.4) | |
| Slight burn/little tan (type 3) | 295 | 46% | 36.7 (34.3–39.3) | |
| No burn/good tan (type 4) | 121 | 19% | 31.7 (28.4–35.2) | |
| Hair Color | ||||
| Light (blonde, light brown) | 378 | 58% | 36.5 (34.4–38.7) | 0.93 |
| Dark (medium-dark brown, black) | 276 | 42% | 36.7 (33.9–39.7) | |
| Eye Color | ||||
| Light (blue, green, hazel) | 482 | 74% | 38.4 (36.4–40.5) | <0.001 |
| Dark (brown) | 172 | 26% | 32.0 (29.1–35.2) | |
| Presence of Frecklingc | ||||
| Any | 476 | 73% | 39.7 (37.6–41.9) | <0.001 |
| None | 174 | 27% | 29.2 (26.7–31.9) | |
| Base Skin Color (L-scale)d | ||||
| Light (60+) | 219 | 34% | 40.9 (37.6–44.4) | <0.001 |
| Dark (<60) | 433 | 66% | 34.6 (32.8–36.6) | |
| Sun Protection Indexe,f | ||||
| High Score (3.5+) | 88 | 13% | 35.7 (31.1–40.9) | 0.70 |
| Low Score (<3.5) | 565 | 87% | 36.7 (34.9–38.5) | |
| Sunburn in 2004–2007 | ||||
| Any burn | 561 | 86% | 37.6 (35.7–39.5) | 0.007 |
| No burn | 93 | 14% | 31.2 (27.3–35.7) | |
| Usual Daily Sun Exposureg | ||||
| Most-all the time | 90 | 14% | 40.5 (36.4–45.0) | 0.09 |
| Half time or less | 564 | 86% | 36.0 (34.2–37.9) | |
| Waterside Vacation Sun Exposure (birth through age 8) | ||||
| One or more vacation | 504 | 77% | 37.7 (35.8–39.8) | 0.02 |
| None | 150 | 23% | 32.9 (29.8–36.4) | |
| Household Incomeh | ||||
| <$75,000 | 245 | 39% | 36.7 (34.1–39.5) | 0.52 |
| $75,000–$99,999 | 178 | 28% | 35.2 (31.9–39.0) | |
| ≥$100,000 | 208 | 33% | 37.9 (34.9–41.1) | |
| Parent Education | ||||
| High school or less | 41 | 6% | 43.6 (36.5–52.1) | 0.24 |
| Some college | 115 | 18% | 34.8 (33.7–40.5) | |
| College graduate | 284 | 43% | 36.3 (33.6–39.1) | |
| Beyond college | 214 | 33% | 36.7 (34.0–40.0) | |
GM=geometric mean.
p-value based on ANOVA of log-transformed nevus counts.
15 missing cases; Bonferroni analysis shows that type 2 is significantly different from type 4, p = 0.001.
4 missing cases.
2 missing cases; Measures skin reflectance, higher values indicate lighter skin color.
1 missing case.
Mean sun protection index from 2004–2007.
Mean sun exposure from 2004–2007.
23 missing cases.
To systematically test for interaction effects among the three main phenotypic variables, the three main effects and 3-way and 2-way interaction terms were included in the initial regression model. 35 Then, in a step-down backwards approach, the 3-way interaction term was assessed first. If the 3-way interaction term was not revealed as statistically significant (p<0.05), then all 2-way interaction terms were tested and removed from the model if not significantly associated with nevus counts. To verify that the relationships between phenotype main effects or interaction terms and nevi were not due to confounding, variables including waterside vacation sun exposure, usual daily sun exposure, sun protection index, sunburn in 2004–2007 and gender were controlled for in the multiple regression models. For interpretation, the antilog was used to convert multiple linear regression coefficients to the multiplicative factor by which nevus counts are expected to change, on average, for every one unit increase in the predictor variable.
To further investigate the relationship between hair color, skin color, sun sensitivity and nevus counts, we repeated our analysis on 2007 log-transformed nevus counts less than 2 mm, greater than or equal to 2 mm, nevus counts on chronically exposed body sites (defined as face, anterior neck, lateral forearms, dorsa of the hands, and, for boys only, the posterior neck) and intermittently exposed body sites (defined as trunk, legs, lateral upper arms, medial forearms, and female posterior neck. 22
RESULTS
The cohort of children included in this study was relatively advantaged, as indicated by the education level of parents (76% of parents reported at least a college degree) and income (61% had annual incomes > $75,000) (see Table I). As shown in Table I, children with a sun sensitivity type 2 had the highest mean nevus counts when compared to the other sun sensitivity ratings (type 2: 41.7 vs. type 1: 36.3, type 3: 36.7 and type 4: 31.7, p=0.002). Light eye color, presence of freckling, light base skin color, sunburn in 2004–2007 and waterside vacation sun exposure were all significantly related to increased nevus counts (all p < 0.05). Boys also had more nevi, an average of five more compared to girls (p < 0.001). Hair color, sun protection index, usual daily sun exposure, household income, and parent education were not significantly related to nevus count.
Table II reports the final results of the multiple linear regression analysis examining interaction effects of the three phenotypic traits (hair color, skin color, sun sensitivity). The 3-way interaction (light hair x light skin x type 2) was not significant and was removed from the model. Subsequently, the following 2-way interactions were sequentially removed due to lack of significance: light hair x light skin and type 2 x light skin (both p>0.05). Only one 2-way interaction remained: light hair x type 2 (B= −0.23, p= 0.03). The antilog (B) transformation for this term (0.79) reveals that the simultaneous presence of both light hair and sun sensitivity type 2 is associated with 21% fewer nevi compared to all other combinations of these two factors. Other variables significant in the multivariate model include: waterside vacation sun exposure, gender, presence of freckling, and light eye color.
Replication of analysis for nevi by size and body location
Nevi measuring less than 2 mm represented 92% of the 2007 total nevus counts while nevi greater than or equal to 2 mm represented only 8%. Chronically exposed body sites contained 31% of the total nevus counts and intermittently exposed body sites contained 69%. The interaction term found to be a significant predictor of all nevi (light hair x type 2) was found to be significantly associated with counts of nevi less than 2 mm (B= −0.26, p= 0.02), but not for counts of nevi greater than or equal to 2 mm (B= −0.17, p= 0.55). The interaction term was also significantly associated with nevi in intermittently sun exposed body sites (B= −0.28, p=.02), but not with nevi in chronically exposed body sites (B= −0.18, p=.14).
DISCUSSION
Hair color modifies the relationship between sun sensitivity type 2 and nevus counts in white children in Colorado. For those with dark hair the mean number of nevi spiked at sun sensitivity type 2, and for those with light hair color we saw a decrease in the mean number of nevi from type 1 to type 2 and then a continued decrease in mean number of nevi in types 3 and 4 (Fig. 1). As shown, those with light hair and type 2 had considerably fewer nevi than those with dark hair and type 2. The spike at type 2 for those with dark hair was an unexpected finding and is contrary to the body of literature indicating that those with dark hair color have fewer nevi. 16,24,36–38 These relationships were independent of eye color, presence of freckling, gender, usual daily sun exposure, sunburn in 2004–2007, sun protection index and waterside vacation sun exposure.
Previous studies that investigated phenotype and its relationship to nevus development have reported on the phenotypic main effects. Two studies that reported interactions between phenotype and nevus development did not approach the interaction term analysis systematically. Oliveria et al18 observed a small subpopulation within their cohort with light skin, light hair, and a tendency to burn who had few nevi. However, the finding was reported only in the discussion of the paper and did not distinguish between the Fitzpatrick skin types. Although Bauer et al39 investigated all possible 2-way interactions, higher order interaction terms were not evaluated nor were the 2-way interaction terms tested systematically. The significant interaction they reported was that children who are a Fitzpatrick type 2 with fair hair had greater numbers of incident melanocytic nevi than all other skin types. The differences between our findings and those of Bauer et al39 are likely due to differences in the study populations and definition of the dependent variable. Our study investigated the relationship between phenotype and nevus counts in 9-year-old children in Colorado whereas they examined German children from ages 2–7 years over a 3-year period, looking at incident nevi over that time period. Differences in altitude and climate between Germany and Colorado are likely to result in a very different pattern of sun exposure, which may lead to differences in nevus acquisition. Further, there may be genetic differences between the two study populations.
It is widely accepted that those with red hair have fewer nevi compared to those with other hair colors. 15,27,28 Recent findings regarding MC1R polymorphisms suggest that the penetrance of the MC1R variants is additive, signifying that the more MC1R polymorphisms one has the lighter one’s skin color, independent of hair color. 28 It is biologically plausible that non-red haired individuals with MC1R variant genotypes may acquire fewer nevi similar to individuals with red hair. Therefore, it is possible that the lower nevus counts in our light-haired sun sensitivity type 2 children may be a result of MC1R variants.
It is also possible that children with light hair and sun sensitivity type 2 undergo nevogenesis at different rates than children with dark hair and sun sensitivity type 2. Thus, children with light hair and sun sensitivity type 2 may eventually acquire similar numbers of nevi to their dark-haired counterparts, but may do so over a longer time span. The lack of relationship between the usual daily sun exposure and sun protection index variables and nevus counts could reflect an inability of parents to accurately report sun exposure, or the lack of precision in the measures. It could also mean that genetic factors are more important than sun exposure in influencing nevus development. This is supported by two studies that found that genes account for about 67% of variation in nevus counts while environment accounts for 33%.4 It could also mean that it may take very little sun exposure to induce nevus development in those who are genetically susceptible.
Our analysis of the relationship between hair color, sun sensitivity and nevi by body location revealed that the finding of higher nevus counts among children with dark hair and sun sensitivity type 2 was present for nevi in intermittently sun exposed body sites, but not for nevi in chronically exposed body sites. The “divergent pathway” model presented by Whiteman et al42 may help to explain these findings. According to this model, in individuals who have a high propensity to develop nevi, melanocytes are initiated by sun exposure early in life and induced to proliferate and become neoplastic with little additional requirement for UV exposure. In individuals with a low propensity to develop nevi, chronic sun exposure is thought to be required for melanoma development. It is expected that in the first pathway, more melanomas will occur in “intermittently” exposed body sites (e.g., the trunk), while in the second pathway, more melanomas will occur in “chronically” exposed body sites (e.g., the head and neck). Research has generally supported the existence of these two pathways. 42–45 The children in our study with dark hair and sun sensitivity type 2 are most likely following the first pathway: they have a propensity to develop nevi on the intermittently exposed body sites with little sun exposure and would be expected to develop melanomas earlier in life and on intermittently exposed sites such as the trunk. Our blonde children with sun sensitivity type 2 have few moles and are therefore likely in the group that will develop melanoma through chronic sun exposure; their melanomas would be predicted to occur on the chronically exposed head and neck rather than the trunk, and later in life. This has clear implications for prevention. For the dark haired children with many nevi, once initiation of melanocytes has occurred early in life it may be difficult to prevent the development of melanoma and early detection may need to be especially vigilant. For light haired children with sensitive skin types but few nevi, primary prevention (avoidance of UV light) should be practiced throughout life, since we would expect them to develop melanoma through a pattern of chronic exposure. The lower nevus counts in these individuals may reflect presence of the nevus suppressing MC1R gene. Like red-heads, who develop fewer nevi and have a high risk for melanoma, nevi may not be a good marker for melanoma risk among light haired individuals with type 2 sun sensitivity. Studies that examine both nevus development and genetic factors should focus on this group to investigate whether MC1R polymorphisms, or other genetic factors such as those in a recent report,46 could be contributing to the suppression of nevus development. Our team is in the process of collecting DNA specimens and testing this hypothesis.
Our findings emphasize that it is important to understand the heterogeneity of the relationship between phenotype and nevus development. The relationship we found should be examined in other studies of nevi in children, as well as in case control studies of melanoma, to determine whether similar phenotypic interactions predict melanoma risk. This would provide the opportunity to discover how phenotype and nevus development in childhood relate to melanoma risk as an adult. In this regard, based on our findings in non-Hispanic white children, we predict that individuals with both dark hair and skin type 2 are among those with the highest risk for melanoma. Risk of melanoma in this group may have been previously under-appreciated. Developing a clearer understanding of how differences in phenotype relate to nevus development and melanoma could lead to individualized primary and secondary prevention recommendations that may reduce melanoma incidence and mortality.
Acknowledgments
Funding/Support: This study was supported in part by a grant to Dr. Crane from the National Cancer Institute (RO1-CA74592).
We are indebted to Dr. H. Alan Arbuckle, Dr. Joanna Burch, Brenda Mokrohisky, Cathi Sommer, and Laura Wilson for conducting skin examinations. Dr. Robert Dellavalle contributed to the study design.
Footnotes
The authors have no conflict of interest to declare
Statement on prior presentation: Contents of the manuscript have not been previously published and are not currently submitted elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jenny Aalborg, Email: jenny.aalborg@ucdenver.edu.
Joseph G. Morelli, Email: joseph.morelli@ucdenver.edu.
Tim E. Byers, Email: tim.byers@ucdenver.edu.
Stefan T. Mokrohisky, Email: stefan.mokrohisky@ucdenver.edu.
Lori A. Crane, Email: lori.crane@ucdenver.edu.
References
- 1.American Cancer Society. [Accessed April 1, 2009];Detailed Guide: Skin Cancer - Melanoma. Available at: http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_melanoma_50.asp?sitearea=
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al., editors. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.Osterlind A, Tucker MA, Hou-Jensen K, Stone BJ, Engholm G, Jensen OM. The Danish case-control study of cutaneous malignant melanoma, I. Importance of host factors. Int J Cancer. 1988;42:200–6. doi: 10.1002/ijc.2910420210. [DOI] [PubMed] [Google Scholar]
- 5.Holman CDJ, Armstrong BK. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst. 1984;72:257–66. [PubMed] [Google Scholar]
- 6.Bauer J, Garbe C. Acquired Melanocytic Nevi as Risk Factor for Melanoma Development. A Comprehensive Review of Epidemiological Data. Pigment Cell Res. 2003;16:297–306. doi: 10.1034/j.1600-0749.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 7.Skender-Kalnenas TM, English DR, Heenan PJ. Benign melanocytic lesions: Risk markers or precursors of cutaneous melanoma? J Am Acad Dermatol. 1995;33:1000–7. doi: 10.1016/0190-9622(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 8.Daland EM, Holmes JA. Malignant melanomas: a clinical study. N Engl J Med. 1939;230:651–60. [Google Scholar]
- 9.Cameron JR. Melanoma of skin: clinical account of a series of 209 malignant neoplasms of the skin. J R Coll Surg Edinb. 1968;13:233–54. [PubMed] [Google Scholar]
- 10.Bliss JM, Ford D, Swerdlow AJ, Armstrong BK, Cristofolini M, Elwood JM, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:367–76. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- 11.Berwick M, Wiggins C. The Current Epidemiology of Cutaneous Malignant Melanoma. Front Biosci. 2006;11:1244–54. doi: 10.2741/1877. [DOI] [PubMed] [Google Scholar]
- 12.Pope DJ, Sorahan T, Marsden JR, Ball PM, Grimley RP, Peck IM. Benign pigmented nevi in children. Prevalence and associated factors: The West Midlands, United Kingdom mole study. Arch Dermatol. 1992;128:1201–06. doi: 10.1001/archderm.128.9.1201. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher RP, McLean DI, Yang P, Coldman AJ, Silver HK, Spinelli JJ, et al. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Similarities to melanoma: the Vancouver mole study. Arch Dermatol. 1990a;126:770–6. [PubMed] [Google Scholar]
- 14.Gallagher RP, McLean DI, Yang CP, Coldman AJ, Silver HK, Spinelli JJ, et al. Anatomic distribution of acquired melanocytic nevi in white children. A comparison with melanoma: the Vancouver Mole Study. Arch Dermatol. 1990b;126:466–71. [PubMed] [Google Scholar]
- 15.Dulon M, Weichenthal M, Blettner M, Breitbart M, Hetzer M, Greinert R, et al. Sun exposure and number of nevi in 5- to 6-year-old European children. J Clin Epidemiol. 2002;55:1075–81. doi: 10.1016/s0895-4356(02)00484-5. [DOI] [PubMed] [Google Scholar]
- 16.Synnerstad I, Nilsson L, Fredrikson M, Rosdahl I. Frequency and distribution pattern of melanocytic naevi on Swedish 8–9 year-old children. Acta Derm Venereol. 2004;84:271–6. doi: 10.1080/00015550410025903. [DOI] [PubMed] [Google Scholar]
- 17.Milne E, Simpson JA, English DR. Appearance of melanocytic nevi on the backs of young Australian children: a 7-year longitudinal study. Melanoma Res. 2008;18:22–8. doi: 10.1097/CMR.0b013e3282f20192. [DOI] [PubMed] [Google Scholar]
- 18.Oliveria SA, Satagopan JM, Geller AC, Dusza SW, Weinstock MA, Berwick M, et al. Study of Nevi in Children (SONIC): baseline findings and predictors of nevus count. Am J Epidemiol. 2009;169:41–53. doi: 10.1093/aje/kwn289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd AT, Morelli J, Mokrohisky ST, Asdigian N, Byers TE, Crane LA. Melanocytic nevi and sun exposure in a cohort of Colorado children: anatomic distribution and site-specific sunburn. Cancer Epidemiol Biomarkers Prev. 2007;16:2136–43. doi: 10.1158/1055-9965.EPI-07-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Autier P, Severi G, Pedeux R, Cattaruzza MS, Boniol M, Grivegnee A, et al. Number and size of nevi are influenced by different sun exposure components: implications for the etiology of cutaneous melanoma (Belgium, Germany, France, Italy) Cancer Causes Control. 2003;14:453–9. doi: 10.1023/a:1024961100651. [DOI] [PubMed] [Google Scholar]
- 21.Darlington S, Siskind V, Green L, Green A. Longitudinal study of melanocytic nevi on adolescents. J Am Acad Dermatol. 2002;46:715–22. doi: 10.1067/mjd.2002.120931. [DOI] [PubMed] [Google Scholar]
- 22.Crane LA, Mokrohisky ST, Dellavalle RP, Asdigian N, Aalborg J, Byers TE, et al. Melanocytic nevus development in Colorado children born in 1998: A longitudinal study. Arch Dermatol. 2009;145(2):148–56. doi: 10.1001/archdermatol.2008.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cockburn M, Hamilton A, Mack T. The simultaneous assessment of constitutional, behavioral, and environmental factors in the development of large nevi. Cancer Epidemiol Biomarkers Prev. 2007;16:200–6. doi: 10.1158/1055-9965.EPI-06-0273. [DOI] [PubMed] [Google Scholar]
- 24.Green A, Siskind V, Hansen ME, Hanson L, Leech P. Melanocytic nevi in schoolchildren in Queensland. J Am Acad Dermatol. 1989;20:1054–60. doi: 10.1016/s0190-9622(89)70131-6. [DOI] [PubMed] [Google Scholar]
- 25.Luther H, Altmeyer P, Garbe C, Ellwanger U, Jahn S, Hoffmann K, et al. Increase of melanocytic nevus counts in children during 5 years of follow-up and analysis of associated factors. Arch Dermatol. 1996;132:1473–8. [PubMed] [Google Scholar]
- 26.Aalborg J, Morelli JG, Mokrohisky S, Asdigian N, Byers TE, Dellavalle RP, Crane LA. Tanning increases nevus development in light-skinned children without red hair. Arch Dermatol. 2009;145:989–96. doi: 10.1001/archdermatol.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dellavalle RP, Johnson KR, Hester EJ, Deas AM, Mokrohisky S, Morelli JG, Crane LA. Children with red hair have more freckles but fewer melanocytic nevi: results from a cohort study of 280 three-year-olds. Arch Dermatol. 2005;141(8):1042–3. doi: 10.1001/archderm.141.8.1042. [DOI] [PubMed] [Google Scholar]
- 28.Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, et al. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–61. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute (NCI) [Accessed May 11, 2009];1975–1991 = SEER 9; 1992–2005 = SEER 13. http://www.cdc.gov/cancer/skin/statistics/race.htm.
- 30.Whiteman DC, Brown RM, Purdie DM, Hughes MC. Melanocytic nevi in very young children: the role of phenotype, sun exposure, and sun protection. J Am Acad Dermatol. 2005;52:40–7. doi: 10.1016/j.jaad.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 31.Eckhardt L, Mayer JA, Creech L, Johnston MR, Lui KJ, Sallis JF, et al. Assessing children’s ultraviolet radiation exposure: the potential usefulness of a colorimeter. Am J Public Health. 1996;86:1802–4. doi: 10.2105/ajph.86.12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter®, the DermaSpectrometer® and the Mexameter®. Skin Res Technol. 2000;6(4):230–9. doi: 10.1034/j.1600-0846.2000.006004230.x. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 34.Pettijohn KJ, Asdigian NL, Aalborg J, Morelli JG, Mokrohisky ST, Dellavalle RP, Crane LA. Vacations to waterside locations result in nevus development in Colorado children. Cancer Epidemiol Biomarkers Prev. 2009;18(2):454–63. doi: 10.1158/1055-9965.EPI-08-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aiken L, West S. Testing and interpreting interactions. Newbury Park, CA: Sage Publications; 1991. Multiple Regressions. [Google Scholar]
- 36.Green A, Siskind V, Green L. The incidence of melanocytic nevi in adolescent children in Queensland, Australia. Melanoma Res. 1995;5:155–60. doi: 10.1097/00008390-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Fritschi L, McHenry P, Green A, Mackie R, Green L, Siskind V. Naevi in schoolchildren in Scotland and Australia. Br J Dermatol. 1994;130:599–603. doi: 10.1111/j.1365-2133.1994.tb13106.x. [DOI] [PubMed] [Google Scholar]
- 38.Carli P, Naldi L, Lovati S, La Vecchia C The Oncology Cooperative Group of the Italian Group for Epidemiological Research in Dermatology. The density of melanocytic nevi correlates with constitutional variables and history of sunburns: a prevalence study among Italian schoolchildren. Int J Cancer. 2002;101:375–79. doi: 10.1002/ijc.10629. [DOI] [PubMed] [Google Scholar]
- 39.Bauer J, Büttner P, Wiecker TS, Luther H, Garbe C. Risk factors of incident melanocytic nevi: a longitudinal study in a cohort of 1,232 young German children. Int J Cancer. 2005;115:121–6. doi: 10.1002/ijc.20812. [DOI] [PubMed] [Google Scholar]
- 40.Zhu G, Duffy DL, Eldridge A, Grace M, Mayne C, O’Gorman L, et al. A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum-likelihood combined linkage and association analysis in twins and their sibs. Am J Hum Genet. 1999;65:483–492. doi: 10.1086/302494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachsmuth RC, Turner F, Barrett JH, Gaut R, Randerson-Moor JA, Bishop DT, et al. The effect of sun exposure in determining nevus density in UK adolescent twins. J Invest Dermatol. 2005;124:56–62. doi: 10.1111/j.0022-202X.2004.23548.x. [DOI] [PubMed] [Google Scholar]
- 42.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003 Jun 4;95:806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 43.Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Bronstein BR, Mihm MC, Jr, Speizer FE. Moles and site-specific risk of nonfamilial cutaneous malignant melanoma in women. J Natl Cancer Inst. 1989 Jun 21;81:948–52. doi: 10.1093/jnci/81.12.948. [DOI] [PubMed] [Google Scholar]
- 44.Hacker E, Hayward NK, Dumenil T, James MR, Whiteman DC. The Association between MC1R Genotype and BRAF Mutation Status in Cutaneous Melanoma: Findings from an Australian Population. J Invest Dermatol. 2009 Jul 2; doi: 10.1038/jid.2009.182. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Olsen CM, Zens MS, Stukel TA, Sacerdote C, Chang YM, Armstrong BK, et al. Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int J Cancer. 2009 Feb 15;124(4):937–44. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125:909–17. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]