Abstract
Clonal mouse neuroblastoma cells without tyrosine 3-monooxygenase [EC 1.14.16.2; tyrosine hydroxylase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating)] activity were fused with normal cells from embryonic mouse sympathetic ganglia. One of the 37 hybrid cell lines obtained possesses high tyrosine 3-monooxygenase activity and synthesizes dopamine. These cells also have excitable membranes and generate action potentials in response to electrical stimuli. Thus hybrid cells, generated by fusion of neuroblastoma cells with normal cells from the nervous system, can acquire neural properties not found with the parental neuroblastoma cells.
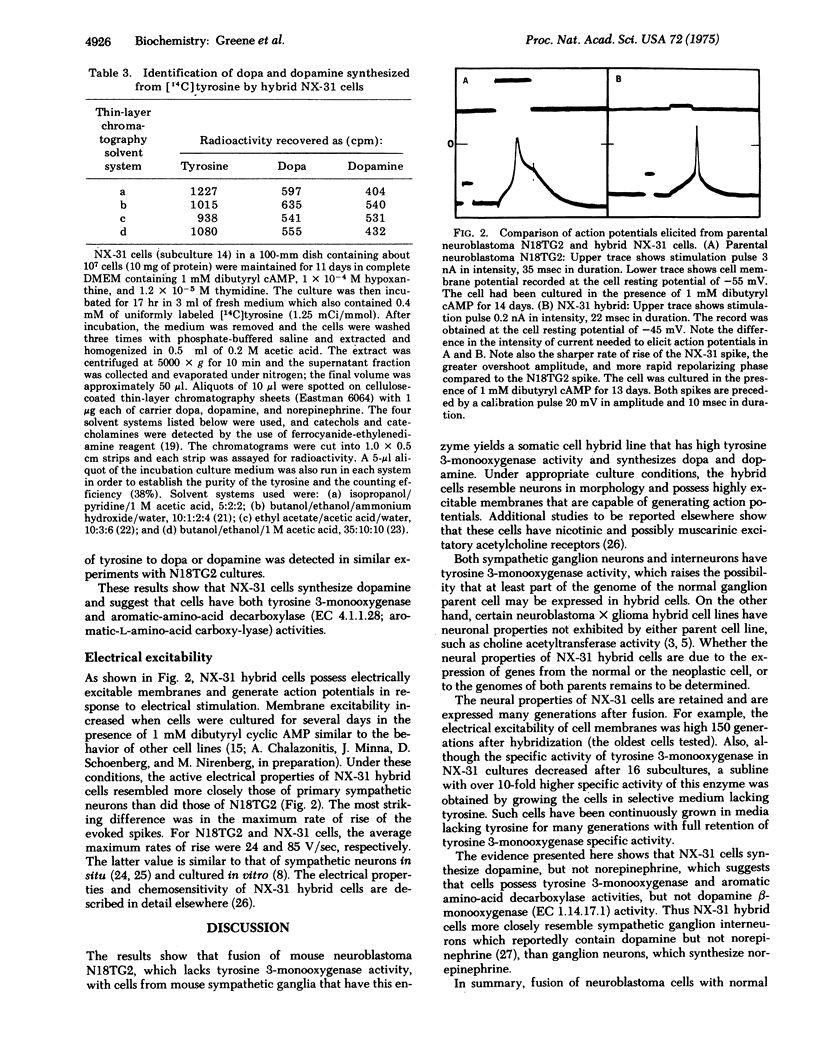
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Hamprecht B., Kemper W. High activity of choline acetyltransferase induced in neuroblastoma x glia hybrid cells. Exp Cell Res. 1974 Apr;85(2):399–408. doi: 10.1016/0014-4827(74)90142-6. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Cegrell L., Falck B., Ritzén M., Rosengren E. Dopamine-containing cells in sympathetic ganglia. Acta Physiol Scand. 1970 Mar;78(3):334–338. doi: 10.1111/j.1748-1716.1970.tb04668.x. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O., Nirenberg M. W. Selection for neuroblastoma cells that synthesize certain transmitters. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2530–2533. doi: 10.1073/pnas.71.6.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A., Greene L. A. Enhancement in excitability properties of mouse neuroblastoma cells cultured in the presence of dibutyryl cyclic AMP. Brain Res. 1974 Jun 7;72(2):340–345. doi: 10.1016/0006-8993(74)90878-6. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A., Greene L. A., Nirenberg M. Electrophysiological chracteristics of chick embryo sympathetic neurons in dissociated cell culture. Brain Res. 1974 Mar 22;68(2):235–252. doi: 10.1016/0006-8993(74)90393-x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Ruddle F. H. Genetic control of two electrophoretic variants of glucosephosphate isomerase in the mouse (Mus musculus). Biochem Genet. 1969 Apr;3(2):151–162. doi: 10.1007/BF00520350. [DOI] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Minna J., Nelson P., Peacock J., Glazer D., Nirenberg M. Genes for neuronal properties expressed in neuroblastoma x L cell hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):234–239. doi: 10.1073/pnas.68.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., McMorris F. A., Nelson P. G. Electrical excitability and chemosensitivity of mouse neuroblastoma X mouse or human fibroblast hybrids. Exp Eye Res. 1973 Apr;79(1):199–212. [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Roser R., Tocci P. M. Detection of catecholamines and related compounds with diazonium reagents by thin-layer chromatography. J Chromatogr. 1972 Oct 5;72(1):207–211. doi: 10.1016/0021-9673(72)80026-8. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER F. H., GILLIS C. N. CATECHOLAMINE BIOSYNTHESIS IN VIVO: AN APPLICATION OF THIN-LAYER CHROMATOGRAPHY. Biochem Pharmacol. 1965 Apr;14:623–626. doi: 10.1016/0006-2952(65)90235-2. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Shuster L. A simplified radiochemical assay for choline acetyltransferase. J Neurochem. 1967 Oct;14(10):977–985. doi: 10.1111/j.1471-4159.1967.tb09509.x. [DOI] [PubMed] [Google Scholar]
- Vahidi A., Sankar D. V. The application of paper and partition thin-layer chromatography to the separation of catecholamines and their metabolites. J Chromatogr. 1969 Aug 5;43(1):135–140. doi: 10.1016/s0021-9673(00)99177-5. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Schrier B. K., Farber J. L., Thompson E. J., Rosenberg R. N., Blume A. J., Nirenberg M. W. Markers for gene expression in cultured cells from the nervous system. J Biol Chem. 1972 May 25;247(10):3159–3169. [PubMed] [Google Scholar]



