Mucinous adenocarcinomas are uncommon and signet-ring cell carcinomas are rare histological subtypes of colorectal cancer, which differ from classical adenocarcinomas in clinicopathology and prognosis. In particular, signet-ring cell carcinomas could be taken into account for individual risk estimation because of their clearly different behavior.
Keywords: cancer, colorectal, mucinous, prognosis, signet ring
Abstract
Objectives:
To define the prognostic value of different histological subtypes of colorectal cancer.
Background:
Most colorectal cancers are classical adenocarcinomas (AC). Less frequent subtypes include mucinous adenocarcinomas (MAC) and signet-ring cell carcinomas (SC). In contrast to established prognostic factors such as TNM and grading, the histological subtype has no therapeutical consequences so far, although it may reflect different biological behavior.
Methods:
Between 1982 and 2012, a total of 3479 consecutive patients underwent surgery for primary colorectal cancer (AC, MAC, or SC). Clinical, histopathological, and survival data were analyzed.
Results:
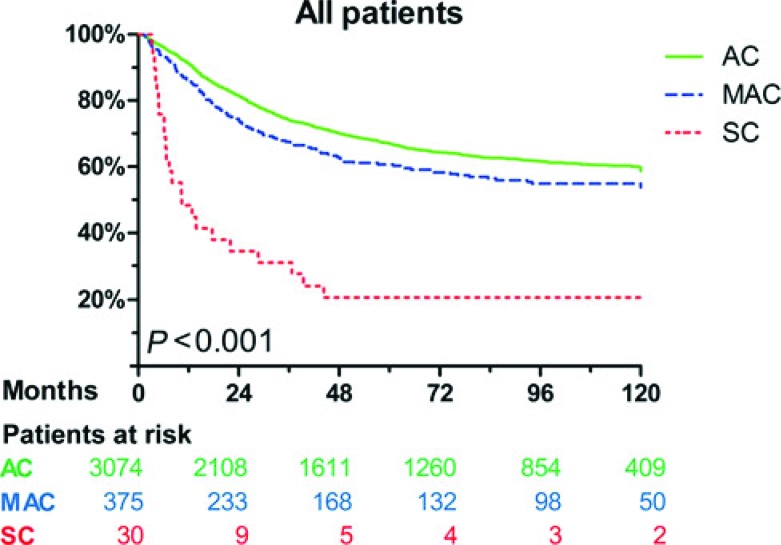
Of all 3479 patients, histological subtype was AC in 3074 cases (88%), MAC in 375 cases (11%), and SC in 30 cases (0.9%). MAC (51%, P < 0.001) and SC (50%, P = 0.029) occurred more frequently in right-sided tumors than AC (28%). Compared with AC, tumor stages and histological grading were higher in MAC and SC (P < 0.001 for each). Rates of angioinvasion were lower in MAC than in AC (5% vs 9%, P = 0.011). Rates of lymphatic invasion were higher in SC than in AC (67% vs 25%, P < 0.001). Five-year cause-specific survival was 67 ± 1% for AC, 61 ± 3% for MAC, and 21 ± 8% for SC (P < 0.001 for difference between the groups). In multivariable analysis, survival did not differ significantly between AC and MAC after correction for tumor stage. However, SC remained an independent prognostic factor associated with worse survival (hazard ratio = 2.5, 95% confidence interval = 1.6–3.8, P < 0.001).
Conclusions:
MAC and SC are histological subtypes of colorectal cancer with different characteristics than classical AC. Both are diagnosed in more advanced tumor stages, but the dismal prognosis of SC seems to be caused by its intrinsic tumor biology.
As early as 1979, the World Health Organization introduced the classification of colorectal cancers according to their histology. Histological subtypes were defined as classical adenocarcinomas (AC), which account for the large majority of cases, and mucinous adenocarcinomas (MAC), signet-ring cell carcinomas (SC), and other even less frequent forms (small cell carcinoma, squamous cell carcinoma, adenosquamous carcinoma, medullary carcinoma, and undifferentiated carcinoma).1,2 This classification exists in addition to the commonly used TNM staging and grading system and remains valid today.2 The histological subtype presumably plays a role in tumor biology and prognosis.3–6 However, in contrast to staging and grading of colorectal cancer, the histological subtype is currently not incorporated in clinical classification systems, although it potentially represents entities with different biological behavior, aggressiveness, and prognosis.4–7
In general, about 10% of all colorectal cancers are MAC, and about 1% are SC.3,8–10 Because of their relatively rare occurrence, in particular, the evaluation of the clinical impact of SC is difficult. However, compared with AC, both MAC and SC have been shown to be associated with young age, advanced tumor stage, accumulation in female patients, and distinct molecular patterns, such as microsatellite instability and activating mutations of the BRAF gene.3,4,8,11 Although ambiguous, recent data and meta-analyses suggest that the histological subtype MAC may be associated with worse outcome compared with AC.3,4,9,12–14 Poor prognosis of SC is more evident, mainly due to high rates of synchronous and metachronous distant organ metastasis associated with this histological subtype.3,8,11
The purpose of this study was to characterize patients with colorectal MAC and SC through evaluation of a large institution-based cohort.
PATIENTS AND METHODS
Patients
Since 1982, all patients undergoing surgery for colorectal cancer at the Department of Surgery, Klinikum rechts der Isar, Technische Universität München, Munich, Germany, are scheduled for periodic follow-up at our interdisciplinary ambulatory tumor center or outside of our hospital according to the recommendations of the German Cancer Society. The recommendations include—over a period of at least 5 years—regular physical examination, blood analysis, determination of carcinoembryonic antigen level, abdomen ultrasonography/computed tomography, chest radiography, and colonoscopy. All patient data are prospectively entered in a database, including preoperative tumor staging, preoperative multimodal treatment, details of the surgical procedure, occurrence of complications, postoperative histopathology, application of adjuvant or palliative treatment, and follow-up (date of last visit, date and site of tumor recurrence, date of tumor-related or unrelated death, cause-specific and recurrence-free survival).15 Information from patients followed outside of our institution is obtained by periodic phone calls to the responsible general practitioners or gastroenterologists.
For this analysis, consecutive complete data sets of patients with resection of colorectal AC, MAC, or SC were extracted. Patients with other histological subtypes and patients without oncological resection of their primary tumor were not included. The latest date of inclusion and follow-up was October 1, 2012. Histological, clinical, and survival data of patients with classical AC were compared with those of patients with MAC and SC. All patients were staged according to the seventh edition of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) tumor staging system.
Pathological Examination
After tumor resection, each surgical specimen was fixed in 10% formalin, opened and thoroughly examined macroscopically. Multiple representative slices of 3 to 5 mm were obtained from the tumor, the junction with adjacent uninvolved tissue, proximal and distal resection margins, uninvolved colon, and macroscopically abnormalities if any, such as polyps or other morphological changes. The mesenteric fat was thoroughly examined for lymph nodes. The obtained tissue blocks were processed for paraffin embedding and subsequent staining by hematoxylin and eosin.
The presence of AC, MAC, or SC was documented. For demonstrating mucin, Periodic acid-Schiff staining was performed. Classical gland-forming adenocarcinomas with variable size and configuration of the glandular structures were classified as AC (ICD-O[International Classification of Diseases for Oncology]: 8140/3).2 MAC were defined as tumors with more than 50% of the lesion being composed of mucin, typically characterized by pools of extracellular mucin that contain malignant epithelium as acinar structures, strips of cells, or single cells (ICD-O: 8480/3).2 SC were defined as tumors with more than 50% of the lesion being composed of tumor cells with prominent intracytoplasmic mucin, typically characterized by large mucin vacuoles that fill the cytoplasm and displace the nucleus (ICD-O: 8490/3).2
According to the World Health Organization, all tumors were graded as well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), or undifferentiated (G4).2 Grading was determined by the content and appearance of glandular structures, without a priori differentiation between AC, MAC, and SC.2,16 Lymphatic invasion and angioinvasion was reported on the basis of hematoxylin and eosin staining.
Statistical Analysis
Statistical evaluation was performed using IBM SPSS statistics Version 19 (SPSS Inc; IBM Corporation Software Group, Somers, NY). The distribution of nominal- or ordinal-scaled variables was compared using Pearson χ2 test. Cardinal variables were tested for normal distribution by the Kolmogorov-Smirnov test. Explorative comparison of independent groups was performed by the t test for normal distribution and the Mann-Whitney U test (2 groups) or the Kruskal-Wallis test (more than 2 groups) for nonnormal distribution. All statistical tests were performed 2-sided, and P values less than 0.05 were considered to be statistically significant. No correction of P values was applied to adjust for multiple test issue. However, results of all statistical tests being conducted were thoroughly reported so that an informal adjustment of P values can be performed while reviewing the data.17 Multivariable analysis of binary outcome data was assessed by logistic regression. Time-dependent survival probabilities were estimated with the Kaplan-Meier method, and the log-rank (Mantel-Cox) test was used to compare independent subgroups. Cause-specific survival was used as the primary outcome parameter. Cause-specific survival is equivalent to disease-specific survival regarding the initial malignant disease and considers only tumor-related deaths as events.18 It reflects the intrinsic biology of the initial colorectal cancer more precisely than, for example, cancer-specific survival, which could be considered as death because of any kind of cancer. Cox proportional hazard models were used to investigate the effect on survival of multivariable relationships among covariates. Cause-specific survival times and estimated hazard ratios (HRs) were calculated and reported in 95% confidence intervals (95% CIs).
RESULTS
Patient Cohort
Between January 1982 and October 2012, a total of 3881 patients underwent surgery for colorectal cancer at our institution. Of those patients, 402 were excluded because of nonresection procedures (defunctioning stoma/bypass) or histological subtypes other than AC, MAC, and SC. Thus, 3479 patients remained for analysis. Of these 3479 patients, 3074 had AC (88%), 375 had MAC (11%), and 30 had SC (0.9%). The median age was 65 years (range: 15–96 years). There were more men (n = 2016) than women (n = 1463) in the cohort. Intestinal tumor obstruction was present at the time of admission in 148 patients (4.3%). The tumor was located within the right hemicolon in 1052 patients (30%), within the left hemicolon in 991 patients (28%), and in the rectum in 1348 patients (39%). In 88 patients (2.5%), more than 1 colorectal cancer was found simultaneously. The prevalence of MAC and SC was not significantly altered after neoadjuvant treatment (P = 0.200). Of all 1348 patients with rectal cancer, 434 underwent neoadjuvant treatment. These 434 patients had AC in 90% (n = 389), MAC in 9% (n = 40), and SC in 1.2% (n = 5). On the contrary, 914 patients with rectal cancer did not undergo neoadjuvant treatment. Those had AC in 92% (n = 845), MAC in 7% (n = 63), and SC in 0.7% (n = 6).
All patients underwent oncological resection of their primary tumor, which required multivisceral surgical procedures in 673 patients (19%). The median number of resected lymph nodes per patient was 16. UICC tumor stages were stage I in 805 patients (23%), stage II in 966 patients (28%), stage III in 942 patients (27%), and stage IV in 766 patients (22%). For all 2713 patients without metastasis (stages I–III), R0 resection status was achieved in 96% (n = 2612). For the 766 patients who presented with distant organ metastasis or peritoneal carcinomatosis (stage IV), an R0 resection was achieved in 19% (n = 148). Further characteristics of the patient cohort are shown in Table 1.
TABLE 1. Clinicopathological Characteristics of the Patient Cohort.
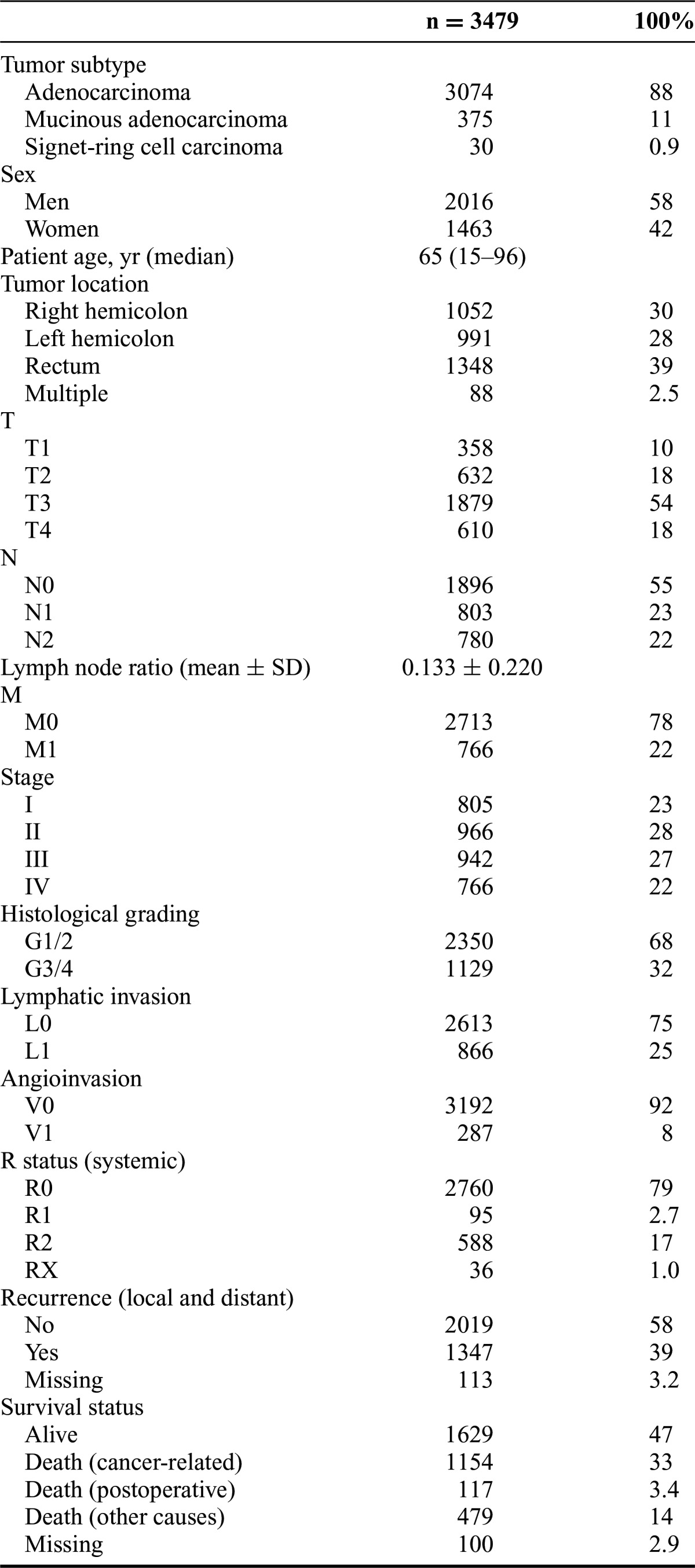
The median follow-up time was 98 months (range: 1–311 months). During follow-up, 1347 patients (39%) had developed at least 1 recurrence (175 local recurrences, 1029 distant recurrences, 143 both local and distant recurrences). Data on recurrence were not available for 113 patients (3.2%). Postoperatively, 117 patients (3.4%) died within 30 days of operation. At the time of follow-up, 1629 patients (47%) were alive, 1154 patients (33%) had died from cancer-related causes, and 479 patients (14%) had died because of other causes. Survival data were not available for 100 patients (2.9%).
Cancer Subtypes and Clinicopathology
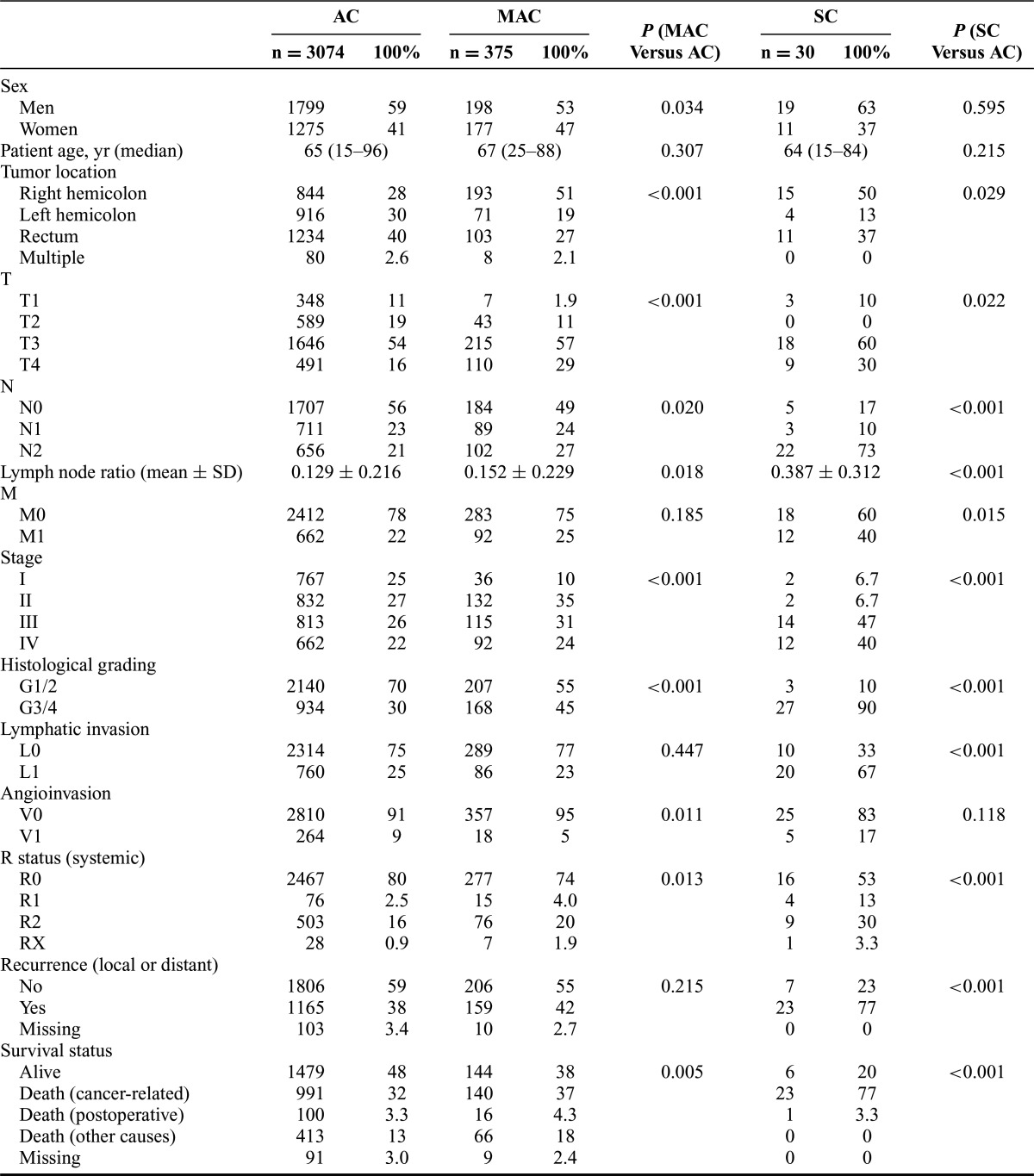
Age did not differ significantly between patients with AC, MAC, and SC. There was a higher proportion of women in the MAC group (47%, P = 0.034) than in the AC group (41%). Multivisceral surgery was performed more frequently for patients with MAC than for patients with AC (29% vs 18%, P < 0.001). Compared with AC (28%), MAC (51%, P < 0.001) and SC (50%, P = 0.029) were located more often within the right hemicolon. Furthermore, MAC and SC were diagnosed at more advanced stages than AC (P < 0.001 for each). UICC tumor stages III or IV were seen in 48% of patients with AC, in 55% of patients with MAC, and in 87% of patients with SC, respectively. Poor differentiation (G3/4) was found in 30% of patients with AC, in 45% of patients with MAC (P < 0.001, compared with AC), and in 90% of patients with SC (P < 0.001, compared with AC). Angioinvasion occurred more frequently in patients with AC than in patients with MAC (9% vs 5%, P = 0.011). Lymphatic vessel invasion occurred more frequently in patients with SC than in patients with AC (67% vs 25%, P < 0.001). For further details, see Table 2. Data regarding microsatellite instability were available for a subgroup of 251 patients, which reflected the relative frequencies of the histological subtypes in the whole collective. AC were microsatellite instable in 23% (50 of 221 patients), whereas MAC and SC were microsatellite instable in 50% (MAC: 14 of 28 patients, SC: 1 of 2 patients; P = 0.006).
TABLE 2. Comparison of the Clinicopathological Characteristics of Patients With Adenocarcinoma, Mucinous Adenocarcinoma, and Signet-Ring Cell Carcinoma.
Survival
Patients with MAC were more likely to develop local recurrence than those with AC (13% vs 8.6%, P = 0.014), whereas patients with SC were more likely to develop distant recurrence than those with AC (63% vs 33%, P = 0.002). Compared with the AC group (32%), more cancer-related deaths occurred in the MAC group (37%, P = 0.005) and SC group (77%, P < 0.001; Table 2). Cause-specific 5-year survival was 67 ± 1% for patients with AC, 61 ± 3% for patients with MAC, and 21 ± 8% for patients with SC (P < 0.001). Median cause-specific survival was not reached for AC and MAC. For SC, median cause-specific survival was 10 months (95% CI, 2–19 months). Compared with AC, cause-specific survival was significantly reduced for MAC (P = 0.011) and SC (P < 0.001). Furthermore, cause-specific survival was worse for SC compared with MAC (P < 0.001).
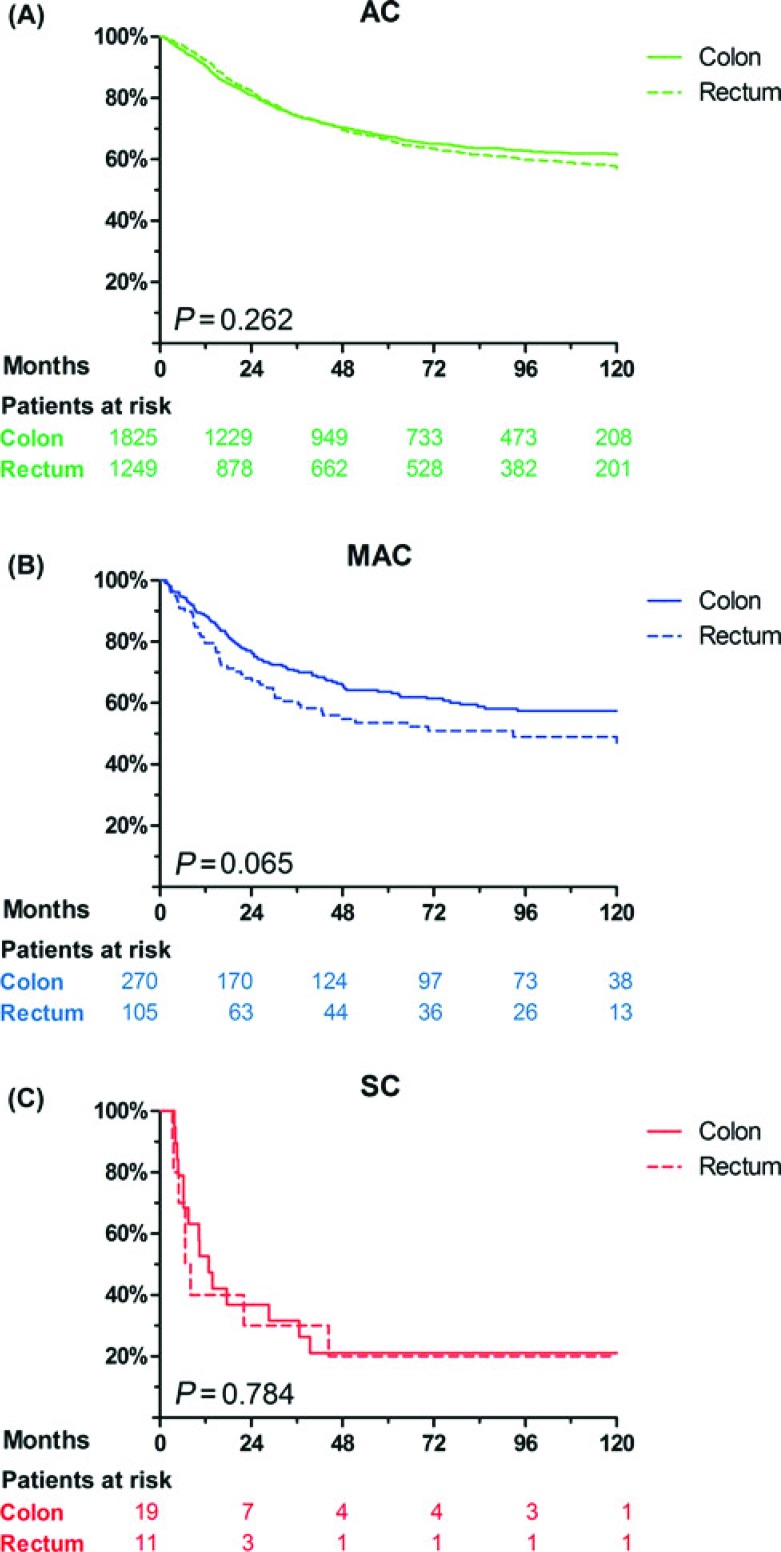
Figure 1 displays the Kaplan-Meier survival curves for the different histological subtypes. Within the groups AC, MAC, and SC, there were no significant differences in survival between patients with colon cancer and rectal cancer, although patients with MAC of the rectum showed a trend toward shorter survival than patients with MAC of the colon (P = 0.065, Fig. 2). The cause-specific 5-year survival was 64 ± 3% for MAC of the colon (n = 270), 56 ± 8% for MAC of the rectum, which had received neoadjuvant treatment (n = 40), and 52 ± 7% for MAC of the rectum without neoadjuvant treatment (n = 63). Five-year survival did not differ significantly among these groups (P = 0.108).
FIGURE 1.
Cause-specific survival for patients of all stages, corresponding to adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma.
FIGURE 2.
Cause-specific survival for patients with (A) adenocarcinoma, (B) mucinous adenocarcinoma, and (C) signet-ring cell carcinoma, corresponding to colon cancer and rectal cancer.
Cancer Subtypes and Prognosis
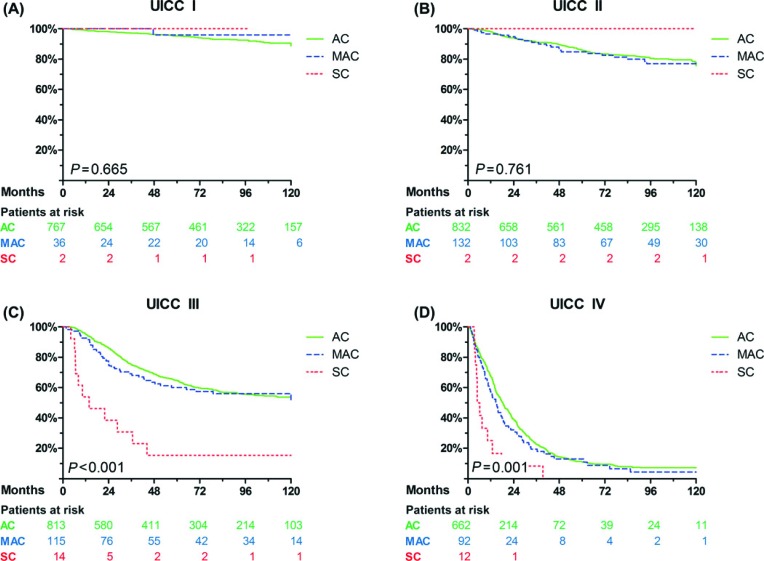
In addition to the histological subtype of cancer (AC, MAC, or SC), factors associated with poor cause-specific survival on univariable analysis were multivisceral surgery (P < 0.001), tumor, node, metastasis and UICC stage (P < 0.001, respectively), poor differentiation (G3/4, P < 0.001), lymphatic invasion (P < 0.001), angioinvasion (P < 0.001), resection status other than R0 (P < 0.001), and recurrence of disease (P < 0.001). On multivariable analysis, most of these parameters remained independent prognostic factors, with the exception of grading, lymphatic invasion, and angioinvasion (Table 3). MAC and SC were associated significantly more often with higher tumor stages than AC. After correction for tumor stage, the difference in survival between MAC and AC was no longer significant (HR = 1.0, 95% CI = 0.83–1.2; P = 0.991; Table 3). However, SC remained an independent predictor of poor prognosis, with an HR of 2.5 (95% CI = 1.6–3.8; P < 0.001). The stage-corrected survival characteristics confirmed these findings (Table 4 and Fig. 3).
TABLE 3. Independent Prognostic Factors in Multivariable Analysis.
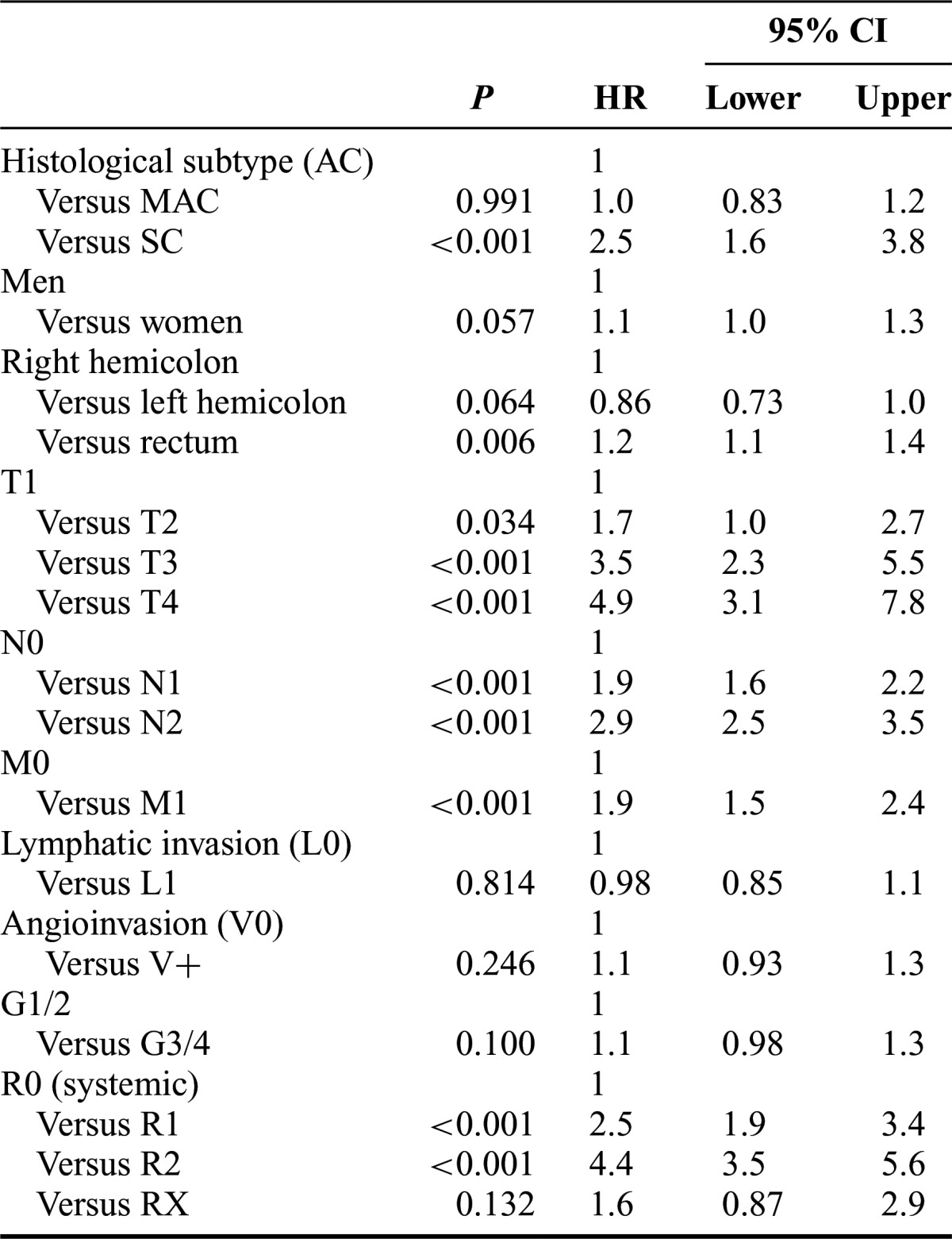
TABLE 4. Cause-Specific 5-Year Survival for Patients With Cancer With UICC Stages I, II, III, and IV, Corresponding to Adenocarcinoma, Mucinous Adenocarcinoma, and Signet-Ring Cell Carcinoma.
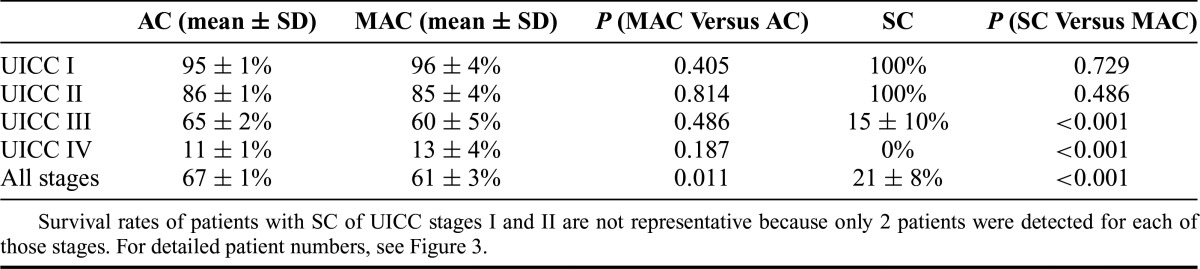
FIGURE 3.
Cause-specific survival for patients of (A) stage I, (B) stage II, (C) stage III, and (D) stage IV, corresponding to adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma. Levels of significance are listed in Table 4.
DISCUSSION
In this study, MAC and SC were shown to be uncommon, respectively, rare histological colorectal cancer subtypes with differences in clinical and histopathological findings. Compared with patients with AC, patients with MAC had more advanced tumor stages and reduced survival. SC were rare; however, these tumors implicated a clearly different behavior and reduced survival even after correction for tumor stage.
To our knowledge, we report on the largest European cohort investigating patients with AC, MAC, and SC and confirm previous nationwide epidemiologic data from the United States and Asia.9,10,19 The number of patients we studied is considerably smaller than in population-based reports; nevertheless, our thoroughly documented clinicopathological and long-term follow-up data are a strength of the cohort we describe.
The prevalence of 11% for MAC and 0.9% for SC in our cohort is in line with population-based data sets.9,10,19 Patients suffering from these tumor subtypes may be candidates for individually tailored treatment regimens, such as intensified systemic therapy and more frequent follow-up. Nevertheless, no prospective study has yet been performed to investigate different management of these entities, and specific therapeutic regimens are not recommended in current guidelines.7
In accordance with the majority of published series including cohorts from the United States and Asia, MAC and SC were largely located within the right hemicolon,4,9,10,20,21 had higher stages of the primary tumor4,5,13,20 and lymph node involvement,5,13,14,20,21 and more advanced UICC stages4,10,13,21 and poorer differentiation.4,9,10,14 Differences in these variables were more pronounced between SC and AC than between MAC and AC. AC of the right hemicolon were not associated with higher tumor stages than those of the left hemicolon. Thus, a putative delayed diagnosis for right-sided colon cancer in general does not explain the advanced tumor stages of MAC and SC. The more aggressive tumor biology of MAC and particularly SC is more likely.
Against our expectations, patients with MAC had low rates of angioinvasion but more local recurrences, whereas patients with SC had high rates of lymphatic invasion and more metachronous distant metastases. However, these results should be interpreted carefully because angioinvasion and lymphatic invasion were reported on the basis of routinely performed hematoxylin and eosin staining. A reduced sensitivity compared with detailed immunohistochemical staining methods cannot be excluded. More complete tumor resections (R0) were performed for MAC than for SC. However, the higher number of local recurrences for MAC could be due to higher rates of multivisceral resections in this group. The 3 large population-based studies from the United States9,10 and Asia19 did not investigate angioinvasion or lymphatic invasion, and there is no study that confirms or contradicts our findings. Thus, generalizability may be restricted because of the relatively small number of patients in our cohort. Overall, only a few studies have reported on angioinvasion and lymphatic invasion of AC, MAC, and SC so far.4,6,20,21 Lee et al21 and Sung et al6 found higher rates of angioinvasion in SC than in MAC; however, they did not evaluate AC. The same observation was made in our cohort when comparing SC with MAC (data not shown).
There was a significant shorter cause-specific survival for MAC than that for AC and for SC than that for AC and MAC. However, multivariable analysis including TNM stage confirmed SC to be an independent prognostic factor but not MAC. Survival curves confirmed no significant differences between MAC and AC when analyzed by tumor stage (Table 4 and Fig. 3). These findings are reflected in the current literature.4,5,8–10,14,20,21 However, small reports6,13 and 2 meta-analyses3,12 identified MAC as an independent prognostic factor for poor outcome. Study heterogeneity of the prognostic impact of MAC was high between the studies included in the meta-analyses, and the pooled HR did not exceed 1.05 (95% CI = 1.02–1.08, P < 0.001)3 and 1.06 (95% CI = 1.04–1.08, P < 0.001),12 respectively. Therefore, a significant prognostic effect of MAC is possible but might not be clinically relevant. In our multivariable analysis, the HR for MAC compared with AC was 1.001 (95% CI = 0.83–1.21, P = 0.991).
Neoadjuvant treatment is known to produce mucin pools.22 However, the prevalence of MAC or SC was not significantly increased after neoadjuvant treatment. Furthermore, survival of patients with MAC who underwent neoadjuvant chemoradiation did not significantly differ from those without preoperative treatment. Resistance to chemoradiation for MAC has been suggested recently.3 However, neither data from our study nor data from the current literature provide compelling evidence.3 For patients with MAC, late diagnosis at more advanced tumor stages and the observed high risk of local recurrence may be clinically more important than a slight difference in survival.
Regarding tumor biology, AC, MAC, and SC do not seem to represent consecutive steps of de-differentiation during the development of mucus-producing colorectal carcinoma but rather constitute different genetic pathways.5,11 Our study revealed distinct histopathological features and recurrence patterns for MAC and SC (Table 2), which were discussed previously. These results are supported by described genetic profiles specific for MAC and SC, including microsatellite instability and mutations of the genes BRAF, p53, and p16.4,5 In addition, downregulation of the adhesion molecules E-cadherin and β-catenin may lead to reduced cell adhesion in areas of high mucus content and promote scattering of tumor cells, further leading to advanced tumor stages and poorer prognosis.5,6 In our cohort, high rates of local recurrences including localized peritoneal carcinomatosis for patients with MAC and distant recurrences for patients with SC might reflect the aggressive phenotype of MAC and SC. In particular, local invasiveness may be increased in areas of high extracellular mucus content, as it is found in MAC.
SC have been described as being positive for intestinal trefoil factor and MUC2, 2 peptides that are usually produced only by goblet cells.5 Thus, SC could arise from different cells of origin than AC.5,23 Although they can be localized in the colorectum, SC may be genetically more related to signet-ring cell cancers of other organs (eg, gastric cancer) than to AC or MAC of the colorectum.5,11 The absence of E-cadherin/β-catenin and amplification of Bcl-2 are features typically shared with signet-ring cell cancer of the stomach but not with classical colorectal adenocarcinomas.5
In the past, all MAC and SC were classified as “poorly differentiated/high grade.”16 However, since 2010, the World Health Organization classification2 noted that grade of epithelial differentiation indeed formally determines the pathological grading (G1–G4, also described for the patients reported here). Microsatellite instability is an important molecular marker that occurs more frequently in MAC and SC than in classical AC and allows for risk stratification.2,7,16 Because of the association with right-sided tumors, the increased rate of microsatellite instability in MAC and SC may be caused by its embryologic origin. Although the distal part of the colon derives from the hindgut, the proximal part derives from the midgut and exhibits specific cellular genetic characteristics.24 Microsatellite instable tumors are associated with better prognosis and are currently classified as “low-grade.” If MAC or SC are microsatellite stable, they are associated with more aggressive biological behavior and are classified as “high-grade.”2,16,25 In current practice, microsatellite testing should be performed for all patients with MAC and SC.16 Because of lack of tissue samples from early patients of this cohort, we do not report on microsatellite data in detail here. In accordance with other reports,6 higher rates of microsatellite instability were detected in MAC and SC than in AC in a subgroup analysis (P = 0.006). Patients with microsatellite instable tumors had a better survival25; however, these findings were not statistically significant, most likely due to the small size of the individual groups (data not shown).
CONCLUSIONS
MAC and SC are uncommon, respectively, rare subtypes of colorectal cancer with a worse prognosis than classical AC. They are considered as independent tumor entities with different biological behavior. Our data suggest that patients with MAC and SC could profit from closer follow-up or even intensified adjuvant therapy because of their high rates of local and distant recurrence. The biological behavior of SC differs in specific, and these patients require special awareness, despite the relatively rare prevalence.
Footnotes
Reprints: Helmut Friess, MD, Hötzlring 22, 81737 Munich, Germany. E-mail: helmut@friess.cc.
Disclosure: Supported by a grant from the KKF (Kommission für klinische Forschung, Fakultät für Medizin, Technische Universität München) to U.N. and K.-P.J. The authors declare no conflicts of interest.
DISCUSSANTS
E. Rullier (Bordeaux, France):
I want to congratulate you for this very impressive cohort of more than 3000 patients extracted from a prospective database of colorectal cancers operated on at a single institution.
My first question concerns the period of evaluation that is very long, 30 years, and you know that during this period the TNM classification has been changed many times. So, how did you manage the pathologic classification of your database? Did you reclassify the tumors; did you review all the cases asking a single pathologist to do that?
The second question is about the resection that is very impressive, 96% of complete microscopic resection. I suspect that multivisceral resection is the explanation for that but I also suspect that maybe you have some incomplete or missing data, because the concept of R0 resection came after the beginning of your study. So, my question is about the definition of R0 resection. Could you specify what was the definition, was it only longitudinal or also circumferential resection margin?
Again, a question about the pathology methodology; you know that assessment of irradiated specimens, especially rectal resections, is very difficult because irradiation produces mucin pools. Did you have any difficulty in distinguishing between natural and irradiated mucin pools because you determined that 10% of your specimens were mucinous tumors?
During your presentation, you did not present the local recurrence data, but I read your article and you used local recurrence to demonstrate the potential difference in terms of biology and prognoses in your subgroup of mucinous tumors. In your article, you conclude that local recurrence is higher in the group of mucinous tumors. I am not sure I agree with you on that because you demonstrate clearly that the groups are not the same. You showed that there is higher stage in the mucinous group than in the conventional one.
So finally, when you analyzed survival, the worse prognoses of mucinous tumors disappeared after multivariate analysis. Did you use multivariate analysis also for local recurrence, which is not described in your article and not presented here? I suspect that the main conclusion of the article would be that signet-ring cell but not mucinous tumors may have a different biology and prognoses as compared with conventional colorectal cancer.
Response From U. Nitsche (Munich, Germany):
Thank you for these encouraging comments and questions. Regarding your first question, the long review period, you are correct. During these last 30 years, there were 5 different TNM classification systems, but, indeed, we did reclassify all the patients regarding the latest, seventh TNM classification system, which was possible by reviewing the pathological reports and our data.
You also asked whether all the specimens were reviewed by a single pathologist; this was not the case. What we did was to review all the pathological reports and we included or defined only those tumors as mucinous or signet-ring carcinomas in which we clearly had the definition according to the pathological report or at least the statement of the amount of mucin pools. But you raise a good point because nearly every tumor is described as having some mucus component, but that does not qualify every tumor as a mucinous carcinoma.
You mentioned the multivisceral resection and the high R0 rate. Indeed, we had a 96% R0 rate for stages 1 to 3 and this was possible by a 19% rate of multivisceral resection. However, for the stage 4 disease, we obtained R0 in 19%.
With regard to the earlier pathological reports, I'm not quite sure when resection was first included in the TNM classification but, of course, whenever it was stated, we included it for our analysis. In the earlier cases when R0 may have not been stated, at least our pathologist routinely reported for every tumor if there were microscopic or macroscopic tumor cells at the resection margin, which obviously is one of the most important findings for the surgeon. This was done for all specimens as well for the longitudinal and also for the circumferential resection margin.
Your next question was a very interesting one, the rate of mucinous adenocarcinomas or of cancers with mucinous differentiation, which obviously according to the literature is higher after neoadjuvant radiation. Unfortunately, we were not able to investigate whether there was a higher rate of mucinous adenocarcinomas after radiation as we did not have the preoperative findings, but we did investigate if there was a higher rate of mucinous adenocarcinomas for patients with rectal cancer if they were radiated in comparison to if they did not undergo preoperative radiation. And this was the case, it was 7% compared with 9%, which was not significant.
I think we also agree with your final comment. The mucinous adenocarcinomas were diagnosed at higher tumor stages and this may have contributed to the higher rates of local recurrence. We did a multivariable analysis on these data and found similar results to the other multivariable analysis; the mucinous adenocarcinoma was not an independent predictor of local recurrence. We concluded that mucinous adenocarcinomas do represent some specific characteristics that clearly differ from the classical adenocarcinomas. However, the signet-ring cell carcinomas seem really to represent another different histological or biological type.
DISCUSSANTS
N. Senninger (Munster, Germany):
These findings are shown in a large series and you should be congratulated because we all know how difficult it is to bring back the files of previous surgical generations. The second point is not completely new because in pathology, in the 1970s we have been told about the difficult biological properties of tumors.
My question is, we saw the advent of neoadjuvant regimens, new drugs, and so forth in the last 30 years, and how could you make Kaplan-Meier curves with patients who had been treated so differently inside the last 30 years? If you did not do that, I would strongly recommend that you compare the decades, first decade, second, and third, because there the patients would be rather homogenous.
Response From U. Nitsche (Munich, Germany):
I think one of the strengths of our series, even if not all the findings were new, is that we had very complete documentation of lymphatic and vascular invasion; however, you are correct, we did not compare the Kaplan-Meier curves regarding decades. Perhaps that is a good idea. However, to our mind the treatment may have shifted during recent years. This should be the same effect for all cancers in regard to the histological subtypes. Therefore, we do not assume that a bias may be raised because of this factor.
DISCUSSANTS
J. Izbicki (Hamburg, Germany):
I enjoyed your presentation very much. Following on Professor Senninger's comment, it is not a new finding that a signet-ring cell carcinoma had a different biological behavior, worse prognosis than a normal adenocarcinoma. So, my at least a little bit provocative question is what is exactly new in your presentation?
The second comment is with regard to the pathological workup. Did I understand this correctly that you just adhered to the pathological written reports and reviewed them or did a specific pathologist rereview the slides of the specimen?
Response From U. Nitsche (Munich, Germany):
We adhered to the pathological reports and we did not review every single slide of all the more than 3000 patients. And to your first question, indeed, there have been many reports that signet-ring cell cancers are different in terms of behavior. However, our study still remains a large analysis that compares the 3 most frequent cancer types; mucinous, classical adenocarcinoma, and signet-ring cell carcinomas. We report on comprehensive markers such as lymphatic invasion, lymph node invasion, local recurrence rate, or overall survival that has not been done in this extensive method so far.
DISCUSSANTS
P. Gertsch (Zurich, Switzerland):
This is a very nice study and I congratulate you. Those treating peritoneal carcinomatosis often see the dismal prognoses of appendix tumors with signet-ring histology. In your presentation, you mentioned that most signet-ring cell carcinomas were on the right side of the colon. Have you been able to differentiate the appendix from the right colon tumor?
Response From U. Nitsche (Munich, Germany):
We included appendix tumors with the right-sided cancers but you make a good point, the appendix tumors can be different and include neuroendocrine tumors. If that was the case, they were excluded from this analysis as we investigated only the classical, the mucinous, and the signet-ring cell carcinomas.
REFERENCES
- 1.Morson BC, Sobin LH. Histological Typing of Intestinal Tumours (International Histological Classification of Tumours, No. 15). 1st ed Geneva, Switzerland: World Health Organization; 1976 [Google Scholar]
- 2.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th ed Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer; 2010 [Google Scholar]
- 3.Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–388 [DOI] [PubMed] [Google Scholar]
- 4.Chew MH, Yeo SA, Ng ZP, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25:1221–1229 [DOI] [PubMed] [Google Scholar]
- 5.Borger ME, Gosens MJ, Jeuken JW, et al. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol. 2007;212:278–286 [DOI] [PubMed] [Google Scholar]
- 6.Sung CO, Seo JW, Kim KM, et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol. 2008;21:1533–1541 [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047 [DOI] [PubMed] [Google Scholar]
- 8.Gopalan V, Smith RA, Ho YH, et al. Signet-ring cell carcinoma of colorectum–current perspectives and molecular biology. Int J Colorectal Dis. 2011;26:127–133 [DOI] [PubMed] [Google Scholar]
- 9.Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161–1168 [DOI] [PubMed] [Google Scholar]
- 11.Kelemen LE, Kobel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol. 2011;12:1071–1080 [DOI] [PubMed] [Google Scholar]
- 12.Chen JX, Tang XD, Xiang DB, et al. TNM stages and prognostic features of colorectal mucinous adenocarcinomas: a meta analysis. Asian Pac J Cancer Prev. 2012;13:3427–3430 [DOI] [PubMed] [Google Scholar]
- 13.Song W, Wu SJ, He YL, et al. Clinicopathologic features and survival of patients with colorectal mucinous, signet-ring cell or non-mucinous adenocarcinoma: experience at an institution in southern China. Chin Med J (Engl). 2009;122:1486–1491 [PubMed] [Google Scholar]
- 14.Chen JS, Hsieh PS, Chiang JM, et al. Clinical outcome of signet ring cell carcinoma and mucinous adenocarcinoma of the colon. Chang Gung Med J. 2010;33:51–57 [PubMed] [Google Scholar]
- 15.Rosenberg R, Friederichs J, Schuster T, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–978 [DOI] [PubMed] [Google Scholar]
- 16.Aust DE. [WHO classification 2010 for the lower gastrointestinal tract: what is new?]. Pathologe. 2011;32(suppl 2):326–331 [DOI] [PubMed] [Google Scholar]
- 17.Saville DJ. Multiple comparison procedures—the practical solution. Am Statistician. 1990;44:174–180 [Google Scholar]
- 18.Nitsche U, Maak M, Schuster T, et al. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg. 2011;254:793–800; discussion 800–801. [DOI] [PubMed] [Google Scholar]
- 19.Du W, Mah JT, Lee J, et al. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78–85 [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Taniguchi H, Fujita S, et al. Clinicopathological characteristics and prognostic factors of advanced colorectal mucinous adenocarcinoma. Histopathology. 2012;61:162–169 [DOI] [PubMed] [Google Scholar]
- 21.Lee WS, Chun HK, Lee WY, et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg. 2007;194:294–298 [DOI] [PubMed] [Google Scholar]
- 22.Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2011;35:127–134 [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol. 1999;14:1057–1061 [DOI] [PubMed] [Google Scholar]
- 24.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408 [DOI] [PubMed] [Google Scholar]
- 25.Nitsche U, Rosenberg R, Balmert A, et al. Integrative marker analysis allows risk assessment for metastasis in stage II colon cancer. Ann Surg. 2012;256:763–771 [DOI] [PubMed] [Google Scholar]