Abstract
Introduction
Albuminuria is a marker of endothelial dysfunction and has been associated with adverse cardiovascular outcomes. The reasons for this association are unclear, but may be due to the relationship between endothelial dysfunction and intrinsic myocardial dysfunction.
Methods and Results
In the HyperGEN study, a population- and family-based study of hypertension, we examined the relationship between urine albumin-to-creatinine ratio (UACR) and cardiac mechanics (N=1894, all of whom had normal left ventricular ejection fraction and wall motion). We performed speckle-tracking echocardiographic analysis to quantify global longitudinal, circumferential, and radial strain (GLS, GCS, and GRS, respectively), and early diastolic (e′) tissue velocities. We used E/e′ ratio as a marker of increased LV filling pressures. We used multivariable-adjusted linear mixed effect models to determine independent associations between UACR and cardiac mechanics. The mean age was 50±14 years, 59% were female, and 46% were African-American. Comorbidities were increasingly prevalent among higher UACR quartiles. Albuminuria was associated with GLS, GCS, GRS, e′ velocity, and E/e′ ratio on unadjusted analyses. After adjustment for covariates, UACR was independently associated with lower absolute GLS (multivariable-adjusted mean GLS [95% CI] for UACR Quartile 1 = 15.3 [15.0–15.5]% vs. UACR Q4 = 14.6 [14.3–14.9]%, P for trend <0.001) and increased E/e′ ratio (Q1 = 25.3 [23.5–27.1] vs. Q4 = 29.0 [27.0–31.0], P= 0.003). The association between UACR and GLS was present even in participants with UACR < 30 mg/g (P<0.001 after multivariable adjustment).
Conclusions
Albuminuria, even at low levels, is associated with adverse cardiac mechanics and higher E/e′ ratio.
Keywords: albuminuria, cardiac mechanics, strain, echocardiography
INTRODUCTION
Albuminuria is associated with cardiovascular morbidity and mortality in diabetics, hypertensives, and the general population.1–5 In patients with heart failure (HF), there is increased prevalence of albuminuria, and higher urine albumin-to-creatinine ratio (UACR) is associated with greater overall cardiovascular mortality and more frequent hospitalization for HF.6, 7 In patients without HF, elevated UACR similarly predicts future hospitalization for HF.8 Increased UACR is also associated with left ventricular hypertrophy (LVH), a potent risk factor for progression to HF.9, 10 However, the relationship between albuminuria and subclinical myocardial dysfunction, prior to development of ventricular remodeling or overt HF, is not well understood, and has only been directly evaluated in small, selected samples.11, 12
Assessment of subclinical myocardial dysfunction has advanced considerably with the advent of tissue Doppler imaging (TDI) and speckle-tracking echocardiography, which allow for the measurement of tissue velocities and myocardial strain, respectively. These indices of cardiac mechanics are sensitive indicators of myocyte injury and malfunction, and can provide novel insight into potential risk factors, such as albuminuria (i.e., endothelial dysfunction), and their role in the pathogenesis of myocardial disease and adverse cardiovascular events.
We hypothesized that low-grade albuminuria, quantified by UACR, is associated with abnormal cardiac mechanics in individuals with a wide variety of cardiovascular risk factors, and that this association is present prior to development of overt echocardiographic structural abnormalities or symptomatic HF. We therefore performed speckle-tracking analysis for the ascertainment of cardiac mechanics in the Hypertension Genetic Epidemiology Network (HyperGEN) Study, a large population- and family-based study of hypertension.
METHODS
Study Population
HyperGEN, part of the National Institutes of Health Family Blood Pressure Program, is a cross-sectional study consisting of five U.S. sites, with four participating in an ancillary echocardiographic study (Salt Lake City, Utah; Forsyth County, North Carolina; Minneapolis, Minnesota; and Birmingham, Alabama). The goal of HyperGEN was to identify and characterize the genetic basis of familial hypertension; complete details of the HyperGEN study design have been reported previously.13 Study eligibility required a diagnosis of hypertension prior to the age of 60 years and at least one affected sibling willing to participate in the study. Normotensive, age-matched controls were drawn from the same base populations as the hypertensive participants. Hypertension was defined by an average systolic blood pressure ≥ 140 mmHg or an average diastolic blood pressure ≥ 90 mmHg (on at least 2 separate clinic visits) or by self-reported treatment for hypertension. Individuals with a history of type 1 diabetes mellitus (DM) or end-stage renal disease were excluded due to the high risk of secondary forms of hypertension. None of the HyperGEN participants had symptomatic HF. For the present study, participants with LVEF < 50% or abnormal wall motion score were excluded. All HyperGEN study participants gave written informed consent, and the HyperGEN study was approved by each study site’s local institutional review board.
Measurement of Urinary Albumin to Creatinine Ratio and Estimated Glomerular Filtration Rate
Overnight, 12-hour urine samples were collected from each patient.13 Urinary albumin was measured by immunoterbidimetry using the DiaSorin antibody. Urinary creatinine was measured using a colorimetric dye-binding technique based on its reaction with picric acid.14 UACR values are calculated as mg albumin/g creatinine. Glomerular filtration rate (GFR) was estimated by the abbreviated Modification of Diet in Renal Disease (MDRD) equation.
Clinical and Conventional Echocardiographic Characteristics
Demographic, clinical, and laboratory data were collected. Height, weight, and blood pressure were measured by trained personnel using a standardized protocol. Type 2 DM was defined by fasting glucose ≥ 126 mg/dl, use of hypoglycemic medication, or self-reported history. Coronary artery disease (CAD) was defined by self-reported history of myocardial infarction, coronary artery bypass grafting surgery, or percutaneous coronary intervention.
Echocardiography (including 2D, M-mode, and Doppler imaging) was acquired on all study participants using standardized acquisition protocols and stored in analog format (high grade, medical quality videocassette tapes) at the time of study visit.15, 16 Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (ASE).17, 18 LV ejection fraction (EF) was calculated using the biplane method of discs. LV mass was calculated using the linear method recommended by the ASE and indexed to body surface area. LVH was defined as LV mass index > 95 g/m2 in women or > 115 g/m2 in men.18 Diastolic function was quantified using early diastolic (E) and late/atrial diastolic (A) transmitral velocities, E/A ratio, isovolumic relaxation time, and E deceleration time.
Digitization of Echocardiograms and Interpretation of Image Quality
Archived echocardiograms in analog format were converted to digital format using the TIMS 2000 DICOM System (Foresight Imaging, Chelmsford, MA). Cine loops of 2–4 cardiac cycles from the parasternal short axis (papillary muscle level) and apical 4-chamber views were digitized at a frame rate of 30 to 40 frames per second and stored offline in DICOM format. Each echocardiographic view was assessed for image quality by an experienced operator, blinded to all other clinical and echocardiographic data, using a 4-point scale based on the degree of endocardial border visualized (1 = 0–25%; 2 = 25%–50%; 3 = 50%–75%; 4 = 75%–100%), similar to scales used previously.19, 20
Two-Dimensional Speckle-Tracking Analysis
Digitized cine loops were analyzed using 2D wall motion tracking software (2D Cardiac Performance Analysis [CPA], TomTec v4.5, Unterschleisshein, Germany). After isolating the highest quality cardiac cycle, the endocardial and epicardial borders were traced at end-systole in each view. Computerized speckle-tracking analysis was performed and endocardial and epicardial border tracings were manually adjusted to optimize tracking. Indices of LV mechanics included peak global longitudinal strain (GLS), peak global radial strain (GRS), peak global circumferential strain (GCS), and basal lateral wall early diastolic (e′) tissue velocities. E/e′ ratio was used as a marker LV filling pressures. For ease of display, all strain values were converted to absolute values (i.e., longitudinal and circumferential strain values were converted from negative to positive values). Lower absolute strain values, lower e′ tissue velocities, and higher E/e′ ratio were used to indicate worse cardiac function. A validation of the digitization and speckle-tracking techniques employed here has been published elsewhere.21
Statistical Analysis
Study participants were divided into UACR quartiles for descriptive purposes. Since the range of UACR values differed by sex, we used sex-specific UACR quartiles, an approach used previously.2 To do so, quartile cutoffs were determined independently for men and women, and then combined to give each quartile an equal gender composition. For all regression models, UACR (predictor variable) was log-transformed to normalize its distribution (raw UACR was right-skewed) and to increase the linearity of the association between UACR and speckle-tracking parameters (outcome variables).
We described clinical characteristics, laboratory data, and conventional echocardiographic parameters by UACR quartile. Continuous data were presented as mean ± standard deviation. Categorical variables were presented as a count and percentage. To test for trends in clinical characteristics, laboratory data, and conventional echocardiographic parameters across quartiles, we examined the association between each parameter and UACR using linear mixed effects models, adjusted only for the random effect of relatedness among HyperGEN participants. In these models, we treated UACR as a continuous (log-transformed) variable. Cardiac mechanics and filling pressures were similarly described by UACR quartile and analyzed using UACR as a continuous variable.
We used multivariable-adjusted linear mixed effects models (to account for relatedness among HyperGEN participants) to determine whether UACR was independently associated with worse cardiac mechanics and elevated E/e′ ratio. Subgroup analyses were performed in participants (1) without LVH; (2) without diabetes; and (3) without hypertension. We also analyzed the subgroup of participants who did not have clinical micro- or macroalbuminuria (i.e., UACR < 30 mg/g). For descriptive purposes, we reported the estimated mean value of each index of cardiac mechanics (or filling pressure) by quartile using the multivariable model. The estimated means in Quartiles 2, 3, and 4 were also compared to Quartile 1 to assess for significant differences.
For the multivariable analyses, covariates included speckle-tracking technician, image quality, study site (which accounts for differences in sonographers and echocardiography machines), and additional covariates that were selected using a combination of clinical relevance and association with albuminuria in previous studies. These additional covariates included age, sex, body mass index (BMI), diagnosis of diabetes, history of CAD, systolic blood pressure, use of antihypertensive medication, history of smoking, GFR, LV mass index, and EF. We also created and tested additional mixed effects linear models that adjusted for: (1) race/ethnicity instead of study site (race/ethnicity and study site were highly collinear because 2 sites recruited only white participants and 1 site only recruited African American participants); and (2) angiotensin converting enzyme-inhibitor (ACE-I)/angiotensin receptor blocker (ARB) and/or non-dihydropiridine calcium channel blocker use instead of any anti-hypertensive medication use, as these drugs are known to specifically reduce albuminuria beyond their blood pressure-lowering effects.22 For GLS, we further adjusted for arterial stiffness (pulse pressure/stroke volume ratio) and diastolic function (e′ velocity). To present β-coefficients and 95% confidence intervals (CIs) in a clinically relevant manner, β-coefficients and 95% CIs for natural log transformed UACR are multiplied by ln(2). This translates to the change in the outcome variable for each 100% increase (or doubling) in UACR.
We evaluated intra- and interobserver reliability in a randomly selected sample of 95 unrelated study participants. These echocardiograms were analyzed by 2 independent readers, blinded to each other’s measurements and all other data. Intraobserver measurements were performed by a single reader 1 month after initial measurement. We evaluated the reproducibility of speckle-tracking measurements by calculating intraclass correlation coefficient and mean bias (Supplementary Table S1). Statistical analyses were performed using Stata 12.1 software (StataCorp, College Station, TX).
RESULTS
Characteristics of the Study Participants
From an initial sample size of 2129 HyperGEN participants, 235 were excluded because of LVEF < 50% or presence of a wall motion abnormality, leaving a sample size of N=1894 for the present study. Median UACR was 4.1 (25th–75th percentile 2.4–8.8) mg/g in women and 3.3 (25th–75th percentile 2.1–7.3) mg/g in men. Microalbuminuria (UACR 30–300 mg/g) was present in 140 (7%) participants, and macroalbuminuria (UACR > 300 mg/g) was present in 50 (3%) participants. Table 1 shows baseline clinical and echocardiographic characteristics by quartile. The cohort had a mean age of 50 years. Females were more prevalent than males, making up 59% of the cohort. Nearly half of the participants were African-American, and they were more likely to have elevated UACR. Participants with elevated UACR were more likely to be older and have higher rates of cardiovascular risk factors, including hypertension, diabetes, obesity (greater BMI), and history of CAD.
Table 1.
Clinical and Echocardiographic characteristics by UACR quartile
| Clinical characteristic | Q1 N=473 |
Q2 N=474 |
Q3 N=472 |
Q4 N=475 |
|
|---|---|---|---|---|---|
|
| |||||
| Female UACR range, mg/g | <2.4 | 2.4–4.1 | 4.1–8.8 | >8.8 | |
| Male UACR range, mg/g | <2.1 | 2.1–3.3 | 3.3–7.3 | >7.3 | P-value |
| Age, years | 50.4±13.3 | 48±13.8 | 50.7±13.1 | 53±13.5 | 0.001 |
| Females, n(%) | 279(59) | 281(59) | 279(59) | 281(59) | 0.06 |
| African American, n(%) | 169(36) | 192(41) | 219(46) | 285(60) | <0.001 |
| Systolic blood pressure, mmHg | 120±17 | 121±17 | 128±20 | 134±24 | <0.001 |
| Diastolic blood pressure, mmHg | 70±10 | 70±9.6 | 73±10.9 | 74±11.3 | <0.001 |
| Coronary artery disease, n(%) | 24(5) | 17(4) | 29(6) | 50(11) | <0.001 |
| Hypertension, n(%) | 212(45) | 207(44) | 300(64) | 351(74) | <0.001 |
| Diabetes, n(%) | 40(9) | 50(11) | 70(15) | 147(31) | <0.001 |
| Smoking history, n(%) | 204(43) | 178(38) | 205(44) | 220(47) | 0.15 |
| BMI, kg/m2 | 29.8±6.6 | 30.4±6.6 | 30.4±6.8 | 32.1±7.5 | <0.001 |
| GFR, ml/min/1.73m2 | 80±18 | 87±18 | 88±19 | 86±24 | 0.04 |
| Urine sodium, mmol/L | 97±54 | 119±61 | 120±59 | 106±53 | 0.13 |
| Urine potassium, mmol/L | 28±16 | 35±18 | 35±20 | 32±20 | 0.08 |
| Anti-hypertensive medication, n(%) | 190(40) | 186(39) | 251(53) | 307(65) | <0.001 |
| Beta blocker | 62(13) | 48(10) | 61(13) | 67(14) | 0.80 |
| Calcium channel blocker | 69(15) | 67(14) | 110(23) | 164(35) | <0.001 |
| Non-dihydropyridine | 31(7) | 28(6) | 48(10) | 66(14) | <0.001 |
| Dihydropyridine | 38(8) | 40(8) | 62(13) | 98(21) | <0.001 |
| Angiotensin II receptor blocker | 10(2) | 8(2) | 17(4) | 12(3) | 0.78 |
| Angiotensin converting enzyme inhibitor | 71(15) | 83(18) | 92(20) | 129(27) | <0.001 |
| Thiazide diuretic | 49(10) | 48(10) | 75(16) | 69(15) | 0.08 |
| Sympatholytic | 30(6) | 16(3) | 45(10) | 52(11) | 0.08 |
| Statin | 36(8) | 29(6) | 32(7) | 43(9) | 0.70 |
|
| |||||
| Echocardiographic characteristic | |||||
|
| |||||
| LV end systolic volume, ml | 47.1±14.4 | 46.9±15.6 | 47.3±14.8 | 48.5±15.5 | 0.38 |
| LV end diastolic volume, ml | 125.1±25.2 | 125.8±28.2 | 126.2±26.7 | 129.1±27.9 | 0.17 |
| Ejection fraction, % | 62.8±5.7 | 63.3±6.1 | 63.1±5.8 | 63±5.7 | 0.68 |
| LV mass index, g/m2 | 79.2±15.7 | 80.3±17.9 | 84.2±18.3 | 89.7±26.1 | <0.001 |
| LV hypertrophy, n(%) | 50(11) | 58(12) | 81(17) | 121(25) | <0.001 |
| LV mass/volume ratio | 1.24±0.21 | 1.26±0.22 | 1.31±0.23 | 1.38±0.33 | <0.001 |
| Left atrial dimension, cm | 3.4±0.5 | 3.4±0.5 | 3.5±0.5 | 3.5±0.5 | 0.01 |
| Stroke volume, ml | 75±14.4 | 76.9±17.5 | 77±15.4 | 78.4±16.1 | 0.09 |
| Cardiac index, L/min/m2 | 2.5±0.5 | 2.6±0.6 | 2.7±0.6 | 2.7±0.6 | <0.001 |
| Pulse pressure/stroke volume, mmHg/ml | 0.70±0.20 | 0.70±0.21 | 0.73±0.24 | 0.75±0.24 | <0.001 |
| E velocity, cm/s | 63.7±16.6 | 68.7±18.5 | 67.8±20.8 | 66.7±19.8 | 0.22 |
| A velocity, cm/s | 60.3±18 | 62.5±18.9 | 67.7±19.4 | 71.6±21 | <0.001 |
| E/A ratio | 1.3±0.5 | 1.3±0.5 | 1.2±0.5 | 1.1±0.5 | <0.001 |
| Mitral valve deceleration time, ms | 201.2±54.7 | 201.2±54.7 | 199.3±55.2 | 213.5±66.2 | 0.02 |
| Isovolumic relaxation time, ms | 77.7±16.1 | 78.0±16.9 | 81.8±19 | 81.9±18.7 | 0.003 |
UACR = urinary albumin to creatinine ratio; BMI = body mass index; GFR = glomerular filtration rate; LV=left ventricular. Continuous data presented as mean±SD.
On echocardiographic analysis, higher levels of UACR were associated with increased LV mass, and increased prevalence of LVH across quartiles. Diastolic function was worse (including lower E/A ratio and increased isovolumic relaxation time) with increasing levels of UACR. Arterial stiffness increased with increasing levels of UACR, as indicated by higher pulse pressure/stroke volume ratio across UACR quartiles.
Association of albuminuria with worse cardiac mechanics
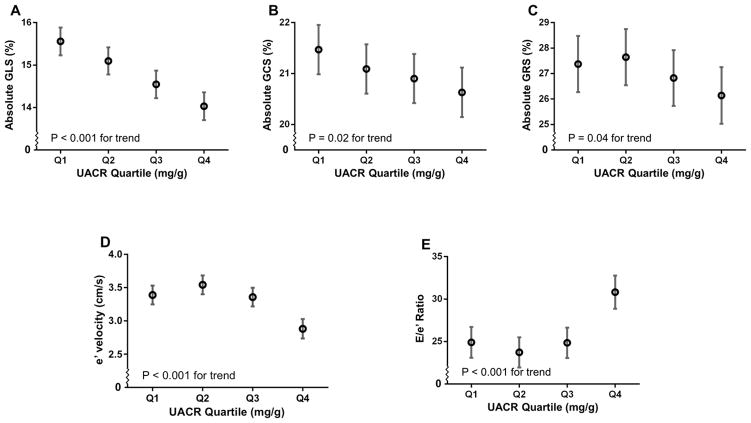
Figure 1 displays the unadjusted relationship between UACR quartile and four measures of cardiac mechanics (GLS, GCS, GRS, and e′ velocity), as well as E/e′ ratio, a marker of LV filling pressures. Absolute values of GLS, GRS, GCS, and e′ velocity decreased (indicating worse systolic and diastolic mechanics) with increasing UACR.
Figure 1.
Cardiac Indices by UACR Quartile. Estimated means and 95% confidence intervals for (A) global longitudinal strain; (B) global circumferential strain; (C) global radial strain; (D) early diastolic (e′) tissue velocity; and E/e′ by UACR quartile, adjusted only for relatedness among participants. P for trend indicates significance of association between log-transformed UACR and cardiac indices in linear mixed effects models (which accounts for relatedness among participants). Tissue velocities by speckle tracking are lower than values observed using tissue Doppler. Thus e′ tissue velocity is lower and E/e′ is higher than observed with tissue Doppler imaging.
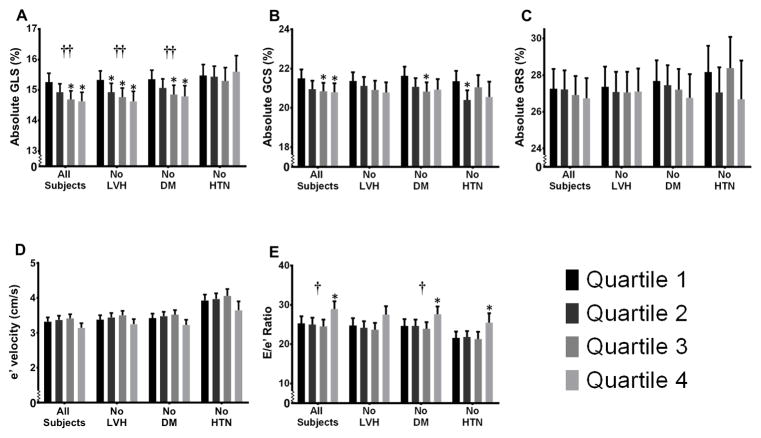
Figure 2 demonstrates the relationship between UACR and indices of cardiac mechanics after adjustment for familial relatedness and several covariates, including factors that affect the measurement of speckle-tracking parameters (speckle-tracking analyst, study site [which accounts for differences in sonographers], and image quality), demographic factors, comorbidities, and indicators of cardiac remodeling (LV mass index) and global systolic function (EF). The association between UACR and GLS persisted after multivariable adjustment (Figure 2A); however, the associations between UACR and GCS, GRS, and e′ velocity were attenuated after multivariable adjustment (Figures 2B–D). Of the speckle-tracking parameters, UACR levels were most closely associated with longitudinal strain on multivariable analysis. Covariates with the greatest effect on the β-coefficient for the association of UACR and GLS were BMI, diabetes mellitus, systolic blood pressure, speckle tracking image quality, and institution (Supplementary Tables S2 and S3).
Figure 2.
Cardiac Indices by UACR Quartile After Multivariable Adjustment. *P-value < 0.05 when compared to the referent quartile (Quartile 1). †P-value < 0.02 for continuous trend. ††P-value < 0.001 for continuous trend. The estimated means (with upper 95% confidence limits) for (A) global longitudinal strain; (B) global circumferential strain; (C) global radial strain; (D) early diastolic (e′) tissue velocity; and (E) E/e′ ratio are shown by UACR quartile for all subjects and 3 subgroups after adjustment for speckle-tracking analyst, study site, image quality, age, sex, body mass index, estimated glomerular filtration rate, diabetes mellitus, coronary artery disease, systolic blood pressure, use of antihypertensive medication, history of smoking, left ventricular mass index, and ejection fraction. LVH = left ventricular hypertrophy; DM = diabetes mellitus; HTN = hypertension. Tissue velocities by speckle tracking are lower than values observed using tissue Doppler. Thus e′ tissue velocity is lower and E/e′ is higher than observed with tissue Doppler.
In subgroup analyses, the association between UACR and longitudinal strain persisted on multivariable analysis among those without LVH and those without diabetes (Figure 2A). In normotensives, median UACR was 3.0 mg/g (interquartile range 2.0–5.0 mg/g). Microalbuminuria was present in 39/836 (5%) and macroalbuminuria in 15/836 (2%) of normotensives. After multivariable adjustment, the association between UACR and longitudinal strain was not present in normotensives. However, between Quartile 1 and Quartile 3, there was a stepwise decrease in GLS as seen in the other subgroups and the entire HyperGEN cohort. Subgroup analyses for GCS, GRS and e′ velocity showed non-significant trends toward worse mechanics.
Additional models which (1) adjusted for race/ethnicity instead of study site; (2) adjusted for ACE-I/ARB and/or non-dihydropyridine calcium channel blockers instead of any anti-hypertensive medication use; (3) included additional adjustment for e′ velocity as a marker of diastolic function; and (4) included additional adjustment for pulse pressure/stroke volume ratio as a marker of arterial stiffness did not change the association between UACR and GLS (Table 2).
Table 2.
Additional Models for the Association of Albuminuria with Global Longitudinal Strain and E/e′ Ratio
| Cardiac parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | β-coefficient (95% CI) | p-value | |
| GLS (All Subjects) | −0.16 (−0.24, −0.08) | <0.001 | −0.17 (−0.25, −0.09) | <0.001 | −0.16 (−0.23, −0.08) | <0.001 | −0.13 (−0.21, −0.05) | 0.001 | −0.16 (−0.24, −0.08) | <0.001 |
| GLS (No LVH) | −0.19 (−0.28, −0.11) | <0.001 | −0.21 (−0.29, −0.12) | <0.001 | −0.19 (−0.28, −0.11) | <0.001 | −0.18 (−0.26, −0.09) | <0.001 | −0.2 (−0.28, −0.11) | <0.001 |
| GLS (No DM) | −0.17 (−0.26, −0.08) | <0.001 | −0.18 (−0.27, −0.09) | <0.001 | −0.17 (−0.26, −0.08) | <0.001 | −0.13 (−0.22, −0.04) | 0.004 | −0.17 (−0.26, −0.08) | <0.001 |
| GLS (UACR <30mg/g) | −0.21 (−0.32, −0.10) | <0.001 | −0.23 (−0.35, −0.12) | <0.001 | −0.21 (−0.32, −0.09) | <0.001 | −0.17 (−0.28, −0.05) | 0.004 | −0.22 (−0.34, −0.11) | <0.001 |
| E/e′ Ratio (All subjects) | 0.78 (0.27, 1.29) | 0.003 | 0.81 (0.30, 1.32) | 0.002 | 0.77 (0.26, 1.27) | 0.003 | 0.56 (0.17, 0.95) | 0.005 | 0.61 (0.08, 1.15) | 0.025 |
| E/e′ Ratio (No DM) | 0.70 (0.17, 1.22) | 0.009 | 0.72 (0.19, 1.25) | 0.007 | 0.69 (0.17, 1.22) | 0.009 | 0.46 (0.06, 0.85) | 0.02 | 0.59 (0.03, 1.14) | 0.04 |
β-coefficient shown represents change in cardiac index per doubling of UACR; UACR = urinary albumin-to-creatinine ratio; GLS = global longitudinal strain; LVH = left ventricular hypertrophy; DM = diabetes mellitus; CI = confidence interval
Model 1: adjusted for speckle-tracking analyst, study site, image quality, age, sex, body mass index, estimated glomerular filtration rate, diabetes mellitus, coronary artery disease, systolic blood pressure, use of any antihypertensive medication, history of smoking, left ventricular mass index, and ejection fraction
Model 2: Model 1 except adjusted for ethnicity instead of study site
Model 3: Model 1 except adjusted for angiotensin converting enzyme inhibitor/angiotensin II receptor blocker and non-dihydropyridine calcium channel blocker use instead of any anti-hypertensive
Model 4: Model 1 + additional adjustment e′ velocity as a marker of diastolic dysfunction
Model 5: Model 1 + additional adjustment for pulse pressure/stroke volume as a marker of arterial stiffness
Association of albuminuria with increased left ventricular filling pressures
Higher levels of UACR were also significantly associated with higher E/e′ ratio, a marker of LV filling pressures. On univariate analysis (Figure 1E), E/e′ ratio was elevated in the highest UACR quartile (in which most participants still had low levels of albuminuria). Thus, even slight increases in UACR were associated with higher E/e′ ratio. The association between UACR and E/e′ ratio persisted after adjustment for potential confounders in the total cohort and in those without DM (Figure 2E). Adjustment for GFR, which was a covariate in the multivariable model, did not attenuate the association between UACR and E/e′ratio. Although the association between UACR and E/e′ did not meet statistical significance in those without LVH and in normotensives, the direction of the association was the same in these subgroups. Additional regression models did not alter the association between UACR and E/e′ ratio (Table 2).
Association of UACR and Cardiac Indices in Participants with UACR < 30 mg/g
Table 3 demonstrates the association between UACR and cardiac indices in participants with UACR < 30 mg/g (N=1722). On multivariable analysis, UACR remained associated with both longitudinal and circumferential strain, indicating that even in patients with low, subclinical levels of albuminuria, there is an association between UACR and cardiac mechanics.
Table 3.
Association between UACR and Cardiac Indices for Participants with UACR < 30 mg/g on Multivariable-Adjusted Linear Mixed Effects Models*
| Change in index per doubling in UACR and 95% CI (N=1722) | P-value | |
|---|---|---|
| Global longitudinal strain, % | −0.21 [−0.32, −0.10] | <0.001 |
| Global circumferential strain, % | −0.25 [−0.43, −0.08] | 0.005 |
| Global radial strain, % | −0.33 [−0.76, 0.08] | 0.12 |
| e′ velocity, cm/s | −0.04 [−0.09, 0.01] | 0.09 |
| E/e′ ratio | 0.47 [−0.21, 1.16] | 0.18 |
Adjusted for speckle-tracking analyst, study site, image quality, age, sex, body mass index, estimated glomerular filtration rate, diabetes mellitus, coronary artery disease, systolic blood pressure, use of antihypertensive medication, history of smoking, left ventricular mass index, and ejection fraction. UACR = urinary albumin-to-creatinine ratio. CI = confidence interval
DISCUSSION
In a speckle-tracking study of 1894 participants in the HyperGEN study, all of whom had LVEF > 50% and normal wall motion, we found that higher levels of UACR, even at levels below 30 mg/g, were independently associated with adverse cardiac mechanics. The relationship between UACR and cardiac mechanics remained after adjustment for several risk factors, including diabetes, blood pressure, CAD, and echocardiographic parameters including LV mass index and EF. To our knowledge, our study is the largest and most comprehensive investigation of the association between UACR and cardiac mechanics, and may help explain why increased UACR is associated with worse cardiovascular outcomes, including HF.
The association between UACR and adverse cardiac mechanics was most prominent for longitudinal strain, a sensitive marker for the health of the subendocardium, which is most susceptible to injury from cardiovascular risk factors.23 While differences in GLS between albuminuria quartiles were modest, the inverse relationship between UACR and longitudinal strain persisted after multivariable adjustment in the entire cohort and in multiple subgroups, suggesting a biological link between the two. Therefore, even small levels of albuminuria may signify generalized endothelial damage, which appears to relate most closely with endocardial and subendocardial myocardial dysfunction.
Our results extend and verify the results of small, single-center studies that have previously shown associations between albuminuria and TDI-based myocardial strain. A small study of 47 subjects with hypertension plus diabetes and 20 hypertensive controls showed an association between peak myocardial strain and 24-hr urine albumin.12 Similarly, another TDI-based study of 57 hypertensives and 48 healthy matched controls showed an association of septal strain and strain rate with the presence of microalbuminuria, but did not analyze the relationship across a range albuminuria levels.11
A previous analysis of data from 2 clinical trials of patients with diastolic dysfunction found an association between low-grade albuminuria, but not GFR, and reduced e′ tissue velocity in hypertensives.24 We found a similar association between albuminuria and reduced e′ velocity (Figure 1). However, this association was no longer significant after multivariable adjustment, which may be explained by differences in study design. In addition, the prior study did not adjust for LV mass index, CAD status, or DM as we did. Both studies, however, did find a consistent association between albuminuria and E/e′ ratio.
Our large sample allows for assessment of multiple subgroups. Namely, a significant association between UACR and longitudinal strain was present in the entire cohort, those without diabetes, and those without LVH. In the subgroup of normotensives, a similar association was present but it did not meet statistical significance, a finding that may be explained by the sample size in Quartile 4 (N=124 normotensives who had UACR levels that placed them in the highest UACR quartile [Quartile 4]). Importantly, only a small proportion of participants in our study had clinically defined micro- or macroalbuminuria (7% UACR= 30–300 mg/g and 3% UACR > 300 mg/g, respectively). An analysis of subjects with normal UACR (< 30 mg/g) did not alter the relationship between UACR and GLS. Taken together, these results support a continuous relationship between albuminuria and subclinical myocardial dysfunction, and suggest that there is a link between endothelial dysfunction and abnormal myocardial mechanics.
Albuminuria is thought to be the result of endothelial damage in the glomerulus.25, 26 In diabetes and hypertension, endothelial dysfunction is a global phenomenon. The cause of albuminuria in persons without diabetes or hypertension is less clear, though increased UACR has been linked to progression to hypertension in these individuals.27 Proteinuria occurs in dogs with renal venous congestion, and reduced renal blood flow has been associated with albuminuria in humans.28–30 Thus, it has been suggested that in HF, adverse hemodynamics may play a role in albuminuria in the absence of hypertension or diabetes.6 While HyperGEN participants did not have clinically evident HF, it is possible that subtle hemodynamic abnormalities play a role in the relationship observed in our study. Renal blood flow was not measured in HyperGEN, though models controlling for cardiac index (estimated on echocardiography) did not alter our results (data not shown). In addition, adjusting for GFR in our multivariable models did not attenuate the association between UACR and longitudinal strain.
An unobserved cause of both albuminuria and myocardial dysfunction, such as inflammation, may also account for the results.31 However, inflammatory markers such as CRP or IL-6 were not measured in HyperGEN. Alternatively, endothelial dysfunction leading simultaneously to albuminuria and subclinical CAD could explain the association.32, 33 However, assessment of subclinical CAD (i.e., coronary artery calcium) was not performed in HyperGEN. While the mechanism is unclear, our data suggest that albuminuria, even at low levels, is associated with adverse cardiac mechanics after adjustment for several risk factors and conventional echocardiographic parameters. These findings highlight a group of asymptomatic individuals who may be at increased risk for overt myocardial dysfunction and progression to HF.
Strengths and Limitations
Strengths of our study include its population-based design, comprehensive echocardiographic phenotyping, inclusion of a large number of African Americans, and inclusion of individuals without overt micro- or macroalbuminuria. In addition, our study is one of the largest speckle-tracking echocardiography studies to date.
Our study has some limitations. The UACR measurement was based on a single 12-hour urine collection, which may not accurately reflect the true level of albuminuria in some individuals because there is intrinsic variability in urinary albumin excretion. However, prior studies have shown that the correlation between a single-void UACR measurement and 24-hour urinary albumin excretion is high.34 Due to the cross-sectional design of our study we are also unable to evaluate the long-term outcomes of participants with higher levels of UACR and worse myocardial mechanics; thus, how many of these individuals ultimately developed symptomatic HF is unknown. In addition, digitization of analog echocardiograms with subsequent speckle-tracking analysis may have introduced noise into the data; however, ours is one of the largest studies of speckle-tracking echocardiography and cardiac mechanics to date, and any noise in the data would have attenuated the association between UACR and cardiac mechanics. The majority of images were of adequate quality, and image quality was used as a covariate in our multivariable analyses.
In our study, we utilized elevated E/e′ ratio as a marker of elevated LV filling pressures. While the E/e′ ratio may be better correlated with invasive LV filling pressures in those with reduced LVEF, it is still the best non-invasive estimate of LV filling pressures in the setting of LVEF > 50%.35 Furthermore, the E/e′ ratio has been independently associated with adverse outcomes in individuals with hypertension and no known cardiac disease.36
Finally, given the cross-sectional nature of our study, we cannot determine a cause-and-effect relationship between increased levels of UACR and worse cardiac mechanics. The association between UACR and cardiac mechanics may be due to several factors, including: (1) end-organ damage from hypertension, resulting in both albuminuria and reduced strain; (2) inflammation causing endothelial dysfunction which results in both albuminuria and abnormal cardiac mechanics;37 (3) decreased renal perfusion due to reduced myocardial performance; and/or (4) subclinical renal dysfunction resulting in abnormal cardiac mechanics. Controlled studies will be essential in determining the mechanisms underlying the association between albuminuria and abnormal cardiac mechanics.
Conclusions
Albuminuria, even at low levels, is associated with worse cardiac mechanics and higher E/e′ ratio. The association between albuminuria and worse mechanics and higher E/e′ ratio is present even in individuals without diabetes or LVH. These findings suggest a pathophysiological link between endothelial dysfunction and early, subclinical myocardial dysfunction. Future studies of therapies aimed at improving endothelial dysfunction may be helpful in determining whether decreasing UACR results in preservation of myocardial function.
Supplementary Material
Acknowledgments
Funding Sources: The HyperGEN cardiac mechanics ancillary study was funded by the National Institutes of Health (R01 HL 107577 to S.J.S.). The HyperGEN parent study was funded by cooperative agreements (U10) with the National Heart, Lung, and Blood Institute: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 2.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 3.Deckert T, Yokoyama H, Mathiesen E, Ronn B, Jensen T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen JS. Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. BMJ. 1996;312:871–874. doi: 10.1136/bmj.312.7035.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miettinen H, Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke. 1996;27:2033–2039. doi: 10.1161/01.str.27.11.2033. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Lin J, Solomon CG, Jablonski KA, Rice MM, Steffes M, Domanski M, Hsia J, Gersh BJ, Arnold JM, Rouleau J, Braunwald E, Pfeffer MA. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116:2687–2693. [Google Scholar]
- 6.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 7.Jackson CE, MacDonald MR, Petrie MC, Solomon SD, Pitt B, Latini R, Maggioni AP, Smith BA, Prescott MF, Lewsey J, McMurray JJ. Associations of albuminuria in patients with chronic heart failure: findings in the ALiskiren Observation of heart Failure Treatment study. Eur J Heart Fail. 2011;13:746–754. doi: 10.1093/eurjhf/hfr031. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, Investigators HS. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 9.Djousse L, Kochar J, Hunt SC, North KE, Gu CC, Tang W, Arnett DK, Devereux RB. Relation of albuminuria to left ventricular mass (from the HyperGEN Study) Am J Cardiol. 2008;101:212–216. doi: 10.1016/j.amjcard.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 10.Wachtell K, Olsen MH, Dahlof B, Devereux RB, Kjeldsen SE, Nieminen MS, Okin PM, Papademetriou V, Mogensen CE, Borch-Johnsen K, Ibsen H. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. J Hypertens. 2002;20:405–412. doi: 10.1097/00004872-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Bilge AK, Atilgan D, Onur I, Pamukcu B, Ozcan M, Adalet K. Relationship between left ventricular hypertrophy, hypertensive retinopathy, microalbuminuria and echocardiographic modalities in newly diagnosed hypertensive patients. Int J Card Imaging. 2010;26:405–412. doi: 10.1007/s10554-010-9589-0. [DOI] [PubMed] [Google Scholar]
- 12.Shim CY, Park S, Choi E-Y, Kang S-M, Cha B-S, Ha J-W, Rim S-J, Lee H-C, Chung N. Is albuminuria an indicator of myocardial dysfunction in diabetic patients without overt heart disease? A study with Doppler strain and strain rate imaging. Metabolism. 2008;57:448–452. doi: 10.1016/j.metabol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Beck SR, Rich SS, Heiss G, Lewis CE, Turner S, Province MA, Schwander KL, Arnett DK, Mellen BG. A genome-wide scan for urinary albumin excretion in hypertensive families. Hypertension. 2003;42:291–296. doi: 10.1161/01.HYP.0000087890.33245.41. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Roman MJ, de Simone G, O’Grady MJ, Paranicas M, Yeh JL, Fabsitz RR, Howard BV. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997;96:1416–1423. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 17.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Galema TW, Geleijnse ML, Yap SC, van Domburg RT, Biagini E, Vletter WB, Ten Cate FJ. Assessment of left ventricular ejection fraction after myocardial infarction using contrast echocardiography. European J Echocardiogr. 2008;9:250–254. doi: 10.1016/j.euje.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Peteiro J, Pinon P, Perez R, Monserrat L, Perez D, Castro-Beiras A. Comparison of 2- and 3-dimensional exercise echocardiography for the detection of coronary artery disease. J Am Soc Echocardiogr. 2007;20:959–967. doi: 10.1016/j.echo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar FA, Selvaraj S, Martinez EE, Beussink L, Kim K-Y, Ping J, Rasmussen-Torvik L, Sha J, Irvin R, Arnett DK, Shah SJ. Archeological Echocardiography: Digitization and Speckle-Tracking Analysis of Archival Echocardiograms in the HyperGEN Study. J Am Soc Echocardiogr. 2012;25:B10. doi: 10.1111/echo.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki DD, Ma JZ, Louis TA, Kasiske BL. Long-term effects of antihypertensive agents on proteinuria and renal function. Arch Intern Med. 1995;155:1073–1080. [PubMed] [Google Scholar]
- 23.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453–355. [DOI] [PubMed] [Google Scholar]
- 24.Shah AM, Lam CS, Cheng S, Verma A, Desai AS, Rocha RA, Hilkert R, Izzo J, Oparil S, Pitt B, Thomas JD, Zile MR, Aurigemma GP, Solomon SD. The relationship between renal impairment and left ventricular structure, function, and ventricular-arterial interaction in hypertension. J Hypertens. 2011;29:1829–1836. doi: 10.1097/HJH.0b013e32834a4d38. [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 26.Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond) 1995;88:629–633. doi: 10.1042/cs0880629. [DOI] [PubMed] [Google Scholar]
- 27.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 28.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 29.Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009;98:121–129. doi: 10.1007/s00392-008-0732-z. [DOI] [PubMed] [Google Scholar]
- 30.Wegria R, Capeci NE, Blumenthal MR, Kornfeld P, Hays DR, Elias RA, Hilton JG. The pathogenesis of proteinuria in the acutely congested kidney. J Clin Invest. 1955;34:737–743. doi: 10.1172/JCI103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakker SJ, Gansevoort RT, Stuveling EM, Gans RO, de Zeeuw D. Microalbuminuria and C-reactive protein: similar messengers of cardiovascular risk? Curr Hypertens Rep. 2005;7:379–384. doi: 10.1007/s11906-005-0075-3. [DOI] [PubMed] [Google Scholar]
- 32.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, Budoff MJ, Blumenthal RS, Nasir K. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer H, Jacobs DR, Jr, Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 34.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10:414–418. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]
- 35.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 36.Sharp AS, Tapp RJ, Thom SA, Francis DP, Hughes AD, Stanton AV, Zambanini A, O’Brien E, Chaturvedi N, Lyons S, Byrd S, Poulter NR, Sever PS, Mayet J, Investigators A. Tissue Doppler E/E′ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31:747–752. doi: 10.1093/eurheartj/ehp498. [DOI] [PubMed] [Google Scholar]
- 37.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.