Abstract
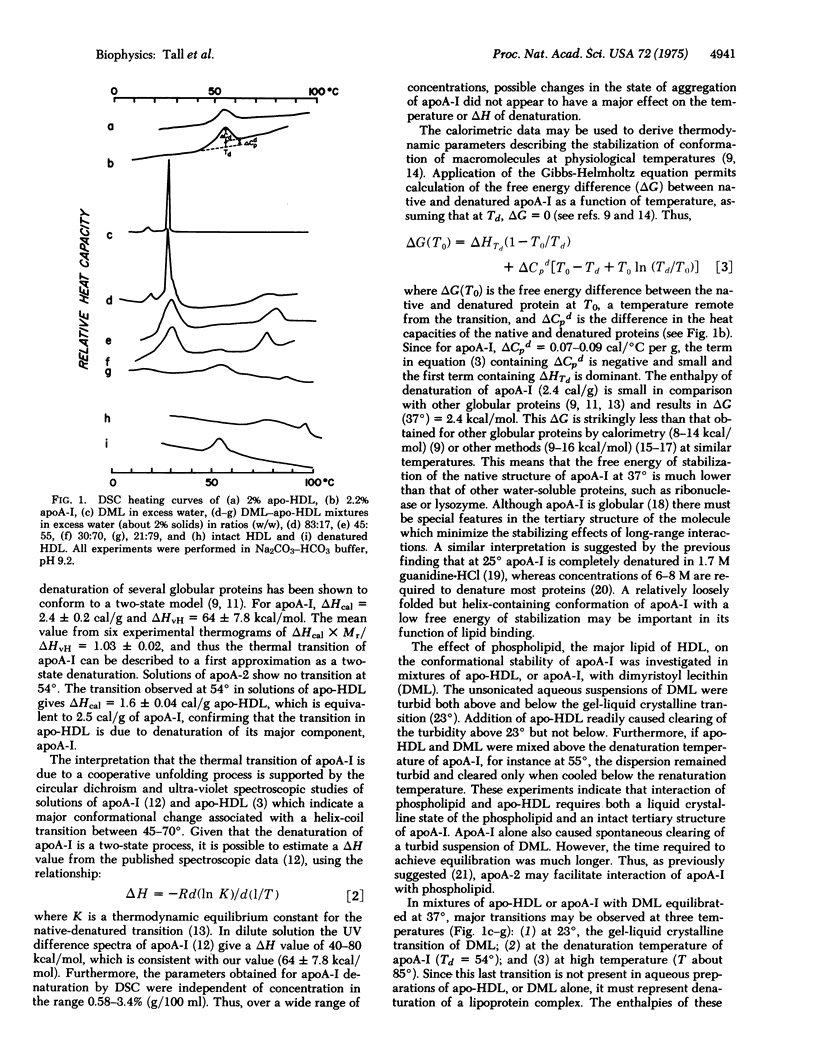
Temperature-dependent conformational changes of the principal apoprotein of human plasma high density lipoprotein (HDL), apoA-I, have been studied in the isolated apoprotein, in complexes of apoprotein with phospholipid, and in intact HDL. Differential scanning calorimetry shows that in solution apoA-I undergoes a reversible, two-state thermal denaturation (midpoint temperature 54 degrees). The enthalpy (2.4 cal/g)(10.0 J/g) and specific heat change (0.08 cal/degrees C per g)(0.33 J/degrees C per g) associated with the denaturation were used to calculate the free energy difference (deltaG) between native and unfolded apoA-I at 37 degrees. DeltaG (2.4 kcal/mol)(10.0 kJ/mol) is less than that of other globular proteins (typically 8-14 kcal/mol)(33-59 kJ/mol), indicating that at 37 degrees native apoA-I has a loosely folded conformation. Turbidity studies show that apoA-I is able to solubilize phospholipid in its native but not in its denatured form. Mixtures of apo-HDL (the total apoprotein of HDL) or apoA-I with dimyristoyl lecithin show a thermal transition at about 85 degrees not present in the lecithin or the apoprotein alone, which indicates that the native conformation of the apoprotein is stabilized by phospholipid. Scanning calorimetry of intact HDL shows a high-temperature endotherm associated with disruption of the HDL particle, suggesting that in HDL the conformation of apoA-I is also stabilized by interaction with lipid. The loosely folded conformation of native, uncomplexed apoA-I may be especially adapted to the binding of lipid, since this process may involve both hydrophobic sites on the surface of the protein and concealed apolar amino acid residues that are exposed by a cooperative, low energy unfolding process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann G., Brewer H. B., Jr A molecular model of high density lipoproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1534–1538. doi: 10.1073/pnas.71.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Brewer H. B., Jr A molecular model of high density lipoproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1534–1538. doi: 10.1073/pnas.71.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune K. C., Tanford C. Thermodynamics of the denaturation of lysozyme by guanidine hydrochloride. II. Dependence on denaturant concentration at 25 degrees. Biochemistry. 1969 Nov;8(11):4586–4590. doi: 10.1021/bi00839a053. [DOI] [PubMed] [Google Scholar]
- Baker H. N., Delahunty T., Gotto A. M., Jr, Jackson R. L. The primary structure of high density apolipoprotein-glutamine-I. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3631–3634. doi: 10.1073/pnas.71.9.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. N., Gotto A. M., Jr, Jackson R. L. The primary structure of human plasma high density apolipoprotein glutamine I (ApoA-I). II. The amino acid sequence and alignment of cyanogen bromide fragments IV, III, and I. J Biol Chem. 1975 Apr 10;250(7):2725–2738. [PubMed] [Google Scholar]
- Biltonen R., Lumry R. Studies of the chymotrypsinogen family of proteins. VII. Thermodynamic analysis of transition I of alpha-chymotrypsin. J Am Chem Soc. 1969 Jul 16;91(15):4256–4264. doi: 10.1021/ja01043a039. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Henry R., Leslie R. B., Robertson R. N. NMR studies of pig low- and high-density serum lipoproteins. Molecular motions and morphology. Biochim Biophys Acta. 1975 Feb 20;380(2):320–327. doi: 10.1016/0005-2760(75)90018-1. [DOI] [PubMed] [Google Scholar]
- Gwynne J., Brewer B., Jr, Edelhoch H. The molecular properties of ApoA-I from human high density lipoprotein. J Biol Chem. 1974 Apr 25;249(8):2411–2416. [PubMed] [Google Scholar]
- Gwynne J., Brewer H. B., Jr, Edelhoch H. The molecular behavior of apoA-I in human high density lipoproteins. J Biol Chem. 1975 Mar 25;250(6):2269–2274. [PubMed] [Google Scholar]
- Jackson W. M., Brandts J. F. Thermodynamics of protein denaturation. A calorimetric study of the reversible denaturation of chymotrypsinogen and conclusions regarding the accuracy of the two-state approximation. Biochemistry. 1970 May 26;9(11):2294–2301. doi: 10.1021/bi00813a011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laggner P., Müller K., Kratky O., Kostner G., Holasek A. Studies on the structure of lipoprotein A of human high density lipoprotein HDL3: the spherically averaged electron density distribution. FEBS Lett. 1973 Jun 15;33(1):77–80. doi: 10.1016/0014-5793(73)80163-2. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Hirz R., Shrager R. I., Gotto A. M. The influence of lipid on the conformation of human plasma high density apolipoproteins. J Biol Chem. 1972 Apr 25;247(8):2598–2606. [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Simon R. H. The interaction of polypeptide components of human high density lipoprotein with sodium dodecyl sulfate. J Biol Chem. 1974 Jun 25;249(12):3937–3940. [PubMed] [Google Scholar]
- Salahuddin A., Tanford C. Thermodynamics of the denaturation of ribonuclease by guanidine hydrochloride. Biochemistry. 1970 Mar 17;9(6):1342–1347. doi: 10.1021/bi00808a007. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Wisdom C. Serum lipoproteins structure and function. Annu Rev Biochem. 1972;41:703–730. doi: 10.1146/annurev.bi.41.070172.003415. [DOI] [PubMed] [Google Scholar]
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Shiao D. D., Sturtevant J. M. Heats of thermally induced helix-coil transitions of DNA in aqueous solution. Biopolymers. 1973;12(8):1829–1836. doi: 10.1002/bip.1973.360120810. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Atkinson D., Scanu A. M. Small-angle x-ray scattering of human serum high-density lipoproteins. J Supramol Struct. 1972;1(2):98–104. doi: 10.1002/jss.400010203. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Zierenberg O., Tunggal B., Schreiber E. 13C nuclear magnetic resonance spectroscopic evidence for hydrophobic lipid-protein interactions in human high density lipoproteins. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3696–3700. doi: 10.1073/pnas.71.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Träuble H., Middelhoff G., Brown V. W. Interaction of a serum apo-lipoprotein with ordered and fluid lipid bilayers. Correlation between lipid and protein structure. FEBS Lett. 1974 Dec 15;49(2):269–275. doi: 10.1016/0014-5793(74)80528-4. [DOI] [PubMed] [Google Scholar]


