Abstract
Purpose
Pseudomonas aeruginosa (P. aeruginosa) microbial keratitis (MK) is a sight-threatening disease. Previous animal studies have identified an important contribution of the complement system to the clearance of P. aeruginosa infection of the cornea. Mannose-binding lectin (MBL), a pattern recognition receptor of the lectin pathway of complement, has been implicated in the host defense against P. aeruginosa. However, studies addressing the role of the lectin pathway in P. aeruginosa MK are lacking. Hence, we sought to determine the activity of the lectin pathway in human MK caused by P. aeruginosa.
Methods
Primary human corneal epithelial cells (HCECs) from cadaveric donors were exposed to two different P. aeruginosa strains. Gene expression of interleukin (IL)-6, IL-8, MBL, and other complement proteins was determined by reverse transcription-polymerase chain reaction (RT–PCR) and MBL synthesis by enzyme-linked immunosorbent assay and intracellular flow cytometry.
Results
MBL gene expression was not detected in unchallenged HCECs. Exposure of HCECs to P. aeruginosa resulted in rapid induction of the transcriptional expression of MBL, IL-6, and IL-8. In addition, expression of several complement proteins of the classical and lectin pathways, but not the alternative pathway, were upregulated after 5 h of challenge, including MBL-associated serine protease 1. However, MBL protein secretion was not detectable 18 h after challenge with P. aeruginosa.
Conclusions
MK due to P. aeruginosa triggers activation of MBL and the lectin pathway of complement. However, the physiologic relevance of this finding is unclear, as corresponding MBL oligomer production was not observed.
Introduction
Microbial keratitis (MK) is a common and severe ocular infection characterized by a corneal epithelial defect with underlying stromal inflammation caused by various microorganisms [1]. Despite intensive treatment with topical antibiotics [2], MK may lead to permanent scarring and loss of vision. Affected patients experience significant pain and distress, and often require multiple outpatient visits or even hospitalization [3]. Several risk factors for MK have been identified, the most common ones being contact lens use in the young and ocular surface diseases in the elderly; these render the corneal epithelium more vulnerable to microbial infection [4]. In the developed world, wearing contact lenses is probably the most important risk factor for MK, particularly in individuals with healthy eyes [5]. MK is caused by a variety of microorganisms, with Pseudomonas aeruginosa (P. aeruginosa) being the most commonly isolated pathogen in patients with contact lens–related MK [6]. P. aeruginosa is a gram-negative opportunistic human pathogen that has been implicated in severe infections in the community and hospital settings [7]. The treatment of P. aeruginosa infections is complicated by the fact that most strains are both intrinsically resistant to several antibiotics and are able to acquire resistance during therapy [8]. Hence, P. aeruginosa MK is often a severe infection causing significant damage to the cornea and is difficult to treat effectively [9,10]. The development of contact lens–related MK involves several stages, starting with the contamination of the contact lenses, for example with P. aeruginosa. Additional mechanical trauma of the corneal epithelium seems mandatory to initiate adherence and invasion of the cornea. Subsequently, bacterial virulence factors and excessive activation of the host innate immune system contribute to the damage or destruction of the cornea in severe MK [11]. The healthy cornea has been shown to possess functional complement activity [12]), and the complement cascade can be aggressively activated in the cornea during an inflammatory reaction [13]. In addition, in earlier studies, the complement system was found to be crucially involved in the clearance of P. aeruginosa infection of the cornea [14,15].
Mannose-binding lectin (MBL), a pattern recognition receptor of the lectin pathway of complement, is a circulating oligomeric protein that is produced primarily by the liver with minor transcription in the intestine and testis [16]. Its carbohydrate-recognition domain allows binding to several oligosaccharides on a wide range of microorganisms and on dying cells, leading to complement activation in an antibody- and complement C1q–independent manner via its MBL-associated serine proteases (MASPs). The concentration of circulating functional MBL multimers is profoundly influenced by polymorphisms in the coding and promoter regions of the MBL2 gene on chromosome 10, resulting in remarkable variations in serum MBL concentration in healthy individuals. Notably, almost a third of the population worldwide displays moderate to severe MBL deficiency [17]. Only two studies have evaluated the involvement of MBL in the defense against ocular infection [18,19]. MBL expression was found to be significantly upregulated in the corneas of mice with fungal keratitis [19]. Whether MBL is expressed constitutively in human corneal epithelial cells (HCECs) and in response to infection has not been tested.
In the case of P. aeruginosa, evidence is conflicting as to whether it is recognized and bound by MBL [20-23]. Importantly, MBL has been found to play a key role in containing and preventing a systemic spread of P. aeruginosa infection following a burn injury in a rodent model [24]. In addition, MBL deficiency has been associated with earlier acquisition of P. aeruginosa and an increased rate of death in adult patients with cystic fibrosis [25]. Given these data on the potential role of MBL in the immune response to P. aeruginosa, we investigated the activity of the lectin pathway of complement in an experimental model of human MK caused by P. aeruginosa.
Methods
The study was approved by the Royal Victorian Eye and Ear Hospital Human and Research Ethics Committee, and was conducted according to the principles of the revised Declaration of Helsinki. Written consent was obtained for all human samples.
Materials and reagents
Mouse monoclonal antibody against MBL (HYB 131–01) was purchased from BioPorto Diagnostics (Gentofte, Denmark). Lipopolysaccharide (LPS) from P. aeruginosa, insulin-transferrin-sodium selenite media supplement (ITS Liquid Media Supplement), human recombinant epithelial growth factor and mannan from Saccharomyces cerevisiae were purchased from Sigma (Sydney, Australia). Dulbecco’s modified Eagle’s medium, Ham’s F12, penicillin-streptomycin-amphotericin B solution (Antibiotic-Antimycotic), gentamicin, trypan blue, PureLink RNA kit, PureLink DNase I, SuperScript III First-Strand Synthesis System, and all TaqMan reagents came from Life Technologies (Mulgrave, Australia). Brilliant III Ultra-Fast SYBR Green QPCR Mastermix was purchased from Agilent Technologies (Mulgrave, Australia).
Primary human corneal epithelial cell culture
Corneal rims from cadaveric donors were obtained post corneal graft from the Lions Eye Donation Service Melbourne (Melbourne, Australia). Under a dissecting microscope, the limbal tissue was dissected out and divided into smaller explants (approximately 1 mm x 2 mm), which were seeded onto 12-well tissue culture plates containing a small amount of culture medium (equal amounts of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium supplemented with 10% fetal calf serum, 0.5 μg/ml human insulin, 0.5 μg/ml human transferrin, 0.5 ng/ml sodium selenite, 10 ng/ml human recombinant epithelial growth factor, and penicillin [100 U/ml], streptomycin [100 μg/ml] and amphotericin B [2.5 μg/ml], Life Technologies) [26]. Cultures were incubated at 37 °C under 5% CO2 and 95% humidity for 3 h to allow adequate attachment before being supplemented with 2 ml of medium, which was changed every 2–3 days after attachment of the explants. Confluent cultures of HCECs were used for experiments (after 14–16 days of culturing).
Mannose-binding lectin 2 genotyping of human corneal epithelial cells
DNA lysates of HCECs were prepared according to the manufacturer’s instructions (TaqMan Sample-to-SNP, Life Technologies). The MBL2 promoter and first exon polymorphisms were determined by allele-specific PCR using TaqMan fluorescent probes (Life Technologies). For assay details, see Table 1. MBL2 genotypes were classified as low (XA/YO, YO/YO), intermediate (XA/XA, YA/YO), or high (YA/YA, XA/YA), producing genotypes according to the published literature [27] with exon variant alleles collectively designated as O and the wild-type gene as A, and the promoter variant allele and the wild-type gene designated as X and Y, respectively.
Table 1. PCR primer sequences and details of TaqMan gene expression and genotyping assays.
| Gene | Primer sequence (5′-3′) | SNP Database/GenBank ID | Assay reference |
|---|---|---|---|
| MBL2 -X/Y |
rs7096206 |
C__27858274_10 |
|
| MBL2 -B (codon 54) |
rs1800450 |
C___2336609_20 |
|
| MBL2 -C (codon 57) |
rs1800451 |
C___2336608_20 |
|
| MBL2 -D (codon 52) |
rs5030737 |
C___2336610_10 |
|
| GAPDH |
F: ATGGAAATCCCATCACCATCTT |
NM_002046.4 |
|
| R: CGCCCCACTTGATTTTGG |
|||
| IL-6 |
F:CCACACAGACAGCCACTCAC |
NM_000600.3 |
|
| R: AGGTTGTTTTCTGCCAGTGC |
|||
| IL-8 |
F: CCAGGAAGAAACCACCGGA |
NM_000584.3 |
|
| R: GAAATCAGGAAGGCTGCCAAG |
|||
| MBL2 |
NM_000242.2 |
Hs00175093_m1 |
|
| GAPDH | NM_002046.4 | Hs99999905_m1 |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MBL, mannose-binding lectin; IL, interleukin.
Challenge/infection with Pseudomonas aeruginosa
P. aeruginosa (invasive strains PAO1 and ATCC 27,853) was grown overnight in Lysogeny broth at 37 °C until absorbance at 600 nm reached an optical density of 0.3–0.4 (mid-logarithmic phase). The bacterial culture was centrifuged at 6000 × g for 10 min and resuspended in culture medium. Confluent cell layers of HCECs were inoculated with viable P. aeruginosa at different bacteria-to-cell ratios or with LPS (100 ng/ml, Sigma). Culture plates were centrifuged at 150 g for 5 min to allow bacteria to come into contact with the cells, followed by further culturing of the cells in graded intervals up to 3 h. Subsequently, after removing the bacteria- or LPS-containing medium, the cells were washed and supplemented with fresh medium containing 100 μg/ml gentamicin to kill extracellular and adherent bacteria. After various culture times, HCECs were harvested for mRNA (mRNA) and DNA preparation, respectively. Cell cytotoxicity was examined by staining with 0.4% trypan blue following treatment with 0.05% (w/v) trypsin-EDTA (Life Technologies).
RNA isolation and reverse transcription
Total RNA was purified from HCECs and the human cell line HepG2 (kindly provided by Dr Angelika Sturm, Ph.D., University of Melbourne, Australia) using the PureLink RNA kit with on-column digestion of DNA by PureLink DNase I according to the manufacturer’s instructions (Life Technologies). Reverse transcription of 0.8 μg of total RNA was performed with the SuperScript III First-Strand Synthesis System using 50 ng/μl of random hexamer primers according to the manufacturer’s instructions (Life Technologies).
Quantitative reverse transcription polymerase chain reaction (RT–PCR) analysis
Primers and TaqMan Gene Expression Assays used to quantify the expression of MBL, interleukin (IL)-6, and IL-8 are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene for normalization. In addition, expression of various genes of the complement system was tested in selected samples using the TaqMan Array Human Complement Pathway Plate (cat. no. 4,414,120, Life Technologies). Amplification of the cDNA template (10–100 ng) was performed in a total volume of 10 μl with TaqMan Gene Expression Mastermix in combination with TaqMan Gene expression assays or arrays, and with Brilliant III Ultra-Fast SYBR Green QPCR Mastermix (Agilent Technologies) in combination with 300 nM forward and reverse primer (IL-6, IL-8, and GAPDH). Samples were assayed in triplicate. The relative mRNA levels of MBL and selected genes of the complement system, IL-6 and IL-8, were compared using the delta–delta cycle threshold (ΔΔCT) method [28] based on the normalization of each amplicon to the endogenous control. Results are reported as fold-difference relative to control RNA from unchallenged HCECs based on three independent experiments. cDNA from HepG2 liver-derived cells was used as positive control for MBL expression, whereas RNA, no template, and minus-reverse transcriptase samples were used as negative controls.
Quantification of mannose-binding lectin oligomer secretion
MBL levels were quantified using a mannan-binding assay as previously described [29], modified by a horseradish peroxidase signal amplification step at the end (ELAST enzyme linked immunosorbent assay [ELISA] Amplification System, Perkin Elmer Inc., Glen Waverley, Australia) [30]. The detection limit was 0.5 ng/ml. To prevent binding of secreted MBL to extracellular P. aeruginosa, HCECs were washed after 2–5 h of challenge with P. aeruginosa, and new medium containing 100 μg/ml gentamicin was added to kill extracellular bacteria. A washing step was repeated after 30 min followed by supplementation with new medium. Supernatants of HCECs after challenge with P. aeruginosa were harvested 6 and 18 h later, centrifuged to remove cell debris and used in 1:2 and 1:5 dilutions. Flow cytometry with intracellular staining for MBL was performed after 4 h of challenge with bacteria, and then samples were cultured overnight in fresh media containing the protein transport inhibitor brefeldin A (BD Cytofix/Cytoperm, BD Biosciences, North Ryde, Australia). Cells were then fixed and permeabilized to allow staining for intracellular MBL as described previously [31].
Statistical analysis
Data are expressed as mean and standard deviation (SD). Differences in the normalized cycle thresholds (ΔCT) and relative mRNA levels were analyzed using one-way ANOVA (ANOVA) followed by Bonferroni’s multiple comparison test. All testing was two-tailed and p values less than 0.05 were considered statistically significant. All analyses were performed using Prism for Windows, version 4 (GraphPad, La Jolla, CA).
Results
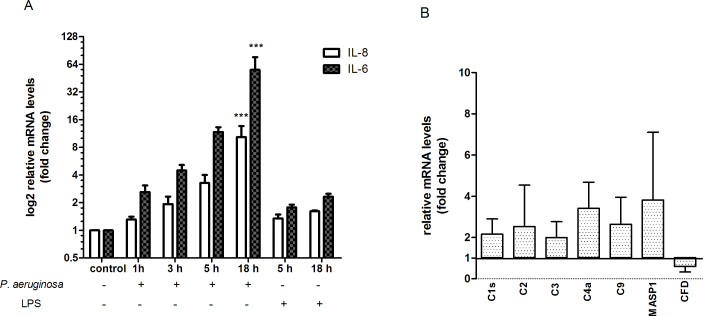
Exposure of confluent monolayers of HCECs to both invasive P. aeruginosa strains at a ratio of 50:1 (bacteria to cell) resulted in >90% HCEC viability after 3 h of incubation and up to 15 h of subsequent culture after adherent and extracellular bacteria had been killed. Of the primary HCECs from five different donors, three displayed a high-producing MBL2 genotype (YA/YA or XA/YA) with one an intermediate (YA/YO) and another a low-producing MBL2 genotype (YO/YO). GAPDH was included as an internal standard to ensure that the levels of cDNAs in the different samples were comparable. Expression of GAPDH was not influenced by challenge with P. aeruginosa, and mean CT values (18.46±0.86) over all samples; PCR runs showed good reproducibility (coefficient of variation, 4.17%). An increase in IL-6 and IL-8 expression (up to 50- and 10 fold, respectively) was detected 1 h after challenge with P. aeruginosa (strain PAO1) with a significant difference after 18 h compared to a much less dramatic response to P. aeruginosa LPS (Figure 1A). Similar changes were observed with the second P. aeruginosa strain (ATCC 27,853, data not shown). In contrast, MBL mRNA was only detected at very low levels after challenge with P. aeruginosa (CT of ≥34) compared to liver-derived HepG2 cells (positive control, CT of 21). MBL expression was not detectable in any unchallenged or LPS challenged primary HCEC. However, a small albeit significant continuous increase in expression up to 18 h after the initial challenge was observed (mean ΔCT [SD] 17.99 [0.65] at 1 h, 17.57 [0.21] at 3 h, 17.09 [0.79] at 5 h, 15.37 [1.26] at 18 h; p<0.05 for the comparison of 1 h versus 18 h; one-way ANOVA with Bonferroni’s multiple comparison test).
Figure 1.
Challenge of human corneal epithelial cells with Pseudomonas aeruginosa induced differential changes in relative levels of interleukin-6, interleukin-8 and complement proteins mRNA compared to lipopolysaccharide or unchallenged control. Human corneal epithelial cells (HCEC) were challenged with Pseudomonas aeruginosa or lipopolysaccharide (LPS) for up to 3 h and subsequently cultured for up to 15 h. Data are presented as mean and standard deviation based on three independent experiments. A: Relative levels of interleukin-6 (IL-6) and interleukin-8 (IL-8) mRNA (mRNA) in HCEC normalized to housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH, p<0.0001 (“***”) for the comparison of 18 h versus control and versus 1 h; one-way ANOVA with Bonferroni's Multiple Comparison Test). B: Relative levels of complement proteins mRNA in HCEC normalized to housekeeping genes GAPDH, beta glucuronidase and phosphoglycerate kinase 1.
The response of the complement system to P. aeruginosa infection was further characterized in three primary HCEC samples after 5 h of incubation. Gene expression was significantly upregulated in the majority of complement proteins compared to the control samples with the exception of complement Factor D (Figure 1B), which was downregulated. However, the difference was small in general (two- to fourfold increase). Interestingly, MASP-1 mRNA levels were significantly elevated in infected HCECs compared to control, and expression of MASP-2 mRNA was only detected after challenge with P. aeruginosa albeit at very low levels, similar to MBL (CT of 37).
Secretion of MBL oligomers was assessed in the supernatants of HCEC from three different donors at 6 and 18 h after challenge with P. aeruginosa. Analysis of mutations in the MBL2 gene revealed high-producing genotypes in HCEC from two cases and an intermediate producing genotype in one donor (XA/XA). MBL oligomers were not detected in the supernatants in any of the tested samples after challenge with P. aeruginosa (data not shown). Intracellular MBL oligomers were not detected by flow cytometry after challenge with P. aeruginosa in three donor corneas with intermediate and low producing genotypes (data not shown).
Discussion
MBL and the lectin pathway of complement have been implicated in the immune response to P. aeruginosa infection and in murine fungal keratitis. In this study, we demonstrated for the first time that the lectin pathway of complement is upregulated in vitro in HCECs after challenge with P. aeruginosa. In line with a recent rodent study demonstrating increased expression of MBL after challenge with aspergillus, we detected low but consistent expression of MBL mRNA in our in vitro model using HCECs from cadaveric donors. The MBL serine protease MASP-1 was also found to be significantly upregulated in response to challenge with P. aeruginosa, providing further evidence that the lectin pathway of complement is activated in P. aeruginosa MK.
Previous studies have shown that MBL is almost exclusively expressed in the human liver with only minor signals in the human intestine and testis [16]. However, recent studies have provided evidence of low-level expression of MBL mRNA in the tonsils [32] and thyroid [33]. Although the amount of MBL mRNA in our corneal samples was very low, we are confident that this represents true gene expression for the following reasons. First, MBL expression was consistently detected only in the challenged HCECs as compared to the challenged or unchallenged LPS controls. Second, we were able to demonstrate an increase in MBL expression with longer in vitro culture intervals. Third, we included a range of negative controls, which never showed any positive signal, and used a specific probe-based real-time PCR assay. In addition, our results are supported by evidence of an activated lectin pathway (MASP-1/-2) in the same samples and a previous murine study demonstrating increased corneal expression of MBL after challenge with aspergillus [19]. Previous studies [16] have failed to demonstrate MBL expression in human peripheral blood leukocytes or mononuclear cells. Hence, it seems unlikely that the low expression of MBL is related to the presence of dendritic cells or macrophages in our cadaveric corneal samples rather than to expression by the HCECs themselves.
The physiologic relevance of observed increase in corneal MBL transcription upon challenge with P. aeruginosa remains unclear, as we were unable to detect any corneal MBL oligomer production or secretion by flow cytometry and ELISA. This is not surprising given the observed low level of MBL expression in the same cells. We note that synthesis of MBL oligomers only reached a concentration of 30 pg/ml after more than 24 h of stimulation in a liver cell line [34]. Given the markedly stronger MBL expression by a factor of 1,000 or more in the liver cell line compared to stimulated HCECs, our ELISA and flow cytometry assays certainly have limitations in their sensitivity for the detection of small amounts of intracellular or secreted MBL oligomers in stimulated HCECs. The level of MBL production required to contribute to local antimicrobial activity has not been determined to date. Investigators who have shown low levels of MBL production in the respiratory tract of patients with pulmonary infection have suggested that when sample dilution factors are taken into account, the measured levels may be functional [35]. It may be that by analogy, the locally transcribed MBL2 product—although not measurable in our experimental setup—could also be effective in MK. Our results should be contrasted to those from an animal model of fungal keratitis where murine MBL-A protein expression in the whole corneas of MBL-A wild-type mice was demonstrated. Only a small increase in MBL RNA expression was detectable (1.4-fold) in this murine study [19].
Similar to MBL, significant MASP-2 expression is confined to the liver with no detectable expression in lymphoid or myeloid cells including monocytes [36], whereas MASP-1 seems to be expressed in a wider range of human tissues. In our study, MASP-1 was found to be upregulated in response to P. aeruginosa corneal infection, although the difference was rather small. MASP-2 expression was only detected at very low levels. Interestingly, a recent study has identified MASP-1 as crucially involved in the lectin pathway in humans by activating MASP-2, which is ultimately required for C4 cleavage [37]. As MBL expression was low in our model, other lectins like the ficolins could potentially be more important in activating the lectin pathway via MASP-1/-2. Corneal expression of these multimeric lectins has not been investigated.
Genes of the classical and alternative complement pathway have been shown to be selectively expressed in human corneal tissue, leading to continuous low-level activation even without any chemical or infectious challenge [12]. In murine models of P. aeruginosa infection, C3 has been identified as a crucial component in the host response as C3 depletion led to more severe disease with a decreased number of neutrophils recruited locally and consequently persistence of bacteria [14,15]. However, as C3 can be activated by all three complement pathways, it is important to investigate the contribution of each pathway. We found a more than twofold upregulation in genes of the classical and lectin but not the alternative pathway (as complement factors B and D did not increase) in response to P. aeruginosa infection. Previous studies have identified all three pathways as being capable of activating the complement system in response to P. aeruginosa [20]. However, results are conflicting in relation the importance of the lectin pathway in the host response to P. aeruginosa infection [24,38]. A recent study suggested a redundant role of the lectin pathway and a prominent role of the alternative pathway, at least in a MASP-2 deficient animal model of invasive pneumonia [20]. This does not exclude a significant effect of MBL in P. aeruginosa infection via lectin pathway–independent opsonophagocytosis or via activation of the lectin pathway in human MK, the latter being supported by our results demonstrating upregulation of MBL and its associated serine proteases.
Apart from proteins of the complement system, IL-6 and IL-8 expression was markedly upregulated in response to P. aeruginosa infection, consistent with a previous study using semiquantitative PCR [39] and underscoring the ability of HCECs to initiate an immune response to P. aeruginosa. In particular, IL-6 seems to be critical to the host response of the cornea after infection with P. aeruginosa [40].
The limited number of cadaveric corneal samples is the main limitation of our study. However, primary HCECs isolated from human donor corneas seem to be more appropriate when investigating differences in the lectin pathway activation compared to cell lines that only display one MBL genotype. Another important limitation is the fact that we were not able to confirm that the observed MBL expression leads to measurable protein secretion.
In conclusion, our studies suggest that MBL and the lectin pathway of complement are upregulated in a human model of P. aeruginosa MK. However, the physiologic relevance of this finding is unclear, as corresponding MBL oligomer production was not observed. Given that MBL deficiency is common in the general population, further studies are necessary to verify the involvement of MBL and the lectin pathway of complement in P. aeruginosa MK, which might lead to the development of targeted therapies that can stimulate innate immune defense and prevent some of the detrimental consequences of P. aeruginosa MK.
Acknowledgments
Our work was supported by a “Small Grant” with the “Royal Victorian Eye and Ear Hospital (East Melbourne, Australia)” as the funding source and with “11/1041H” as the grant number.
References
- 1.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129–41. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 2.Morlet N, Daniell M. Microbial keratitis: what's the preferred initial therapy? View 2: Empirical fluoroquinolone therapy is sufficient initial treatment. Br J Ophthalmol. 2003;87:1169–72. doi: 10.1136/bjo.87.9.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Clinical, epidemiological and cost aspects of contact lens related infectious keratitis in Belgium: results of a seven-year retrospective study. Bull Soc Belge Ophtalmol. 2005;(297):7–15. [PubMed] [Google Scholar]
- 4.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–24. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, Brian G, Holden BA. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–62. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Contact lens-related corneal ulcers requiring hospitalization: a 7-year retrospective study in Belgium. Acta Ophthalmol Scand. 2006;84:522–6. doi: 10.1111/j.1600-0420.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–19. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 8.Tam VH, Chang KT, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, Weston JS, Caeiro JP, Garey KW. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:1160–4. doi: 10.1128/AAC.01446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allan BD, Dart JK. Strategies for the management of microbial keratitis. Br J Ophthalmol. 1995;79:777–86. doi: 10.1136/bjo.79.8.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniell M. Overview: Initial antimicrobial therapy for microbial keratitis. Br J Ophthalmol. 2003;87:1172–4. doi: 10.1136/bjo.87.9.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. American Academy of Optometry. 2007;84:273–8. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 12.Diehn JJ, Diehn M, Marmor MF, Brown PO. Differential gene expression in anatomical compartments of the human eye. Genome Biol. 2005;6:R74. doi: 10.1186/gb-2005-6-9-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondino BJ, Sumner HL. Generation of complement-derived anaphylatoxins in normal human donor corneas. Invest Ophthalmol Vis Sci. 1990;31:1945–9. [PubMed] [Google Scholar]
- 14.Cleveland RP, Hazlett LD, Leon MA, Berk RS. Role of complement in murine corneal infection caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1983;24:237–42. [PubMed] [Google Scholar]
- 15.Hazlett LD, Berk RS. Effect of C3 depletion on experimental Pseudomonas aeruginosa ocular infection: histopathological analysis. Infect Immun. 1984;43:783–90. doi: 10.1128/iai.43.3.783-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1–3 genes. Mol Immunol. 2006;43:962–71. doi: 10.1016/j.molimm.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7:85–94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- 18.Chow SP, Dean MM, Depla JA, Daniell MD, Eisen DP. Mannose-binding lectin as part of the complement pathway: characterization in non-inflamed and inflamed human eyes. Clin Experiment Ophthalmol. 2011;39:871–7. doi: 10.1111/j.1442-9071.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu T, Gong H, Zhou Q, Sun S, Xie L. Gene profiling in murine corneas challenged with Aspergillus fumigatus. Mol Vis. 2007;13:1226–33. [PubMed] [Google Scholar]
- 20.Kenawy HI, Ali YM, Rajakumar K, Lynch NJ, Kadioglu A, Stover CM, Schwaeble WJ. Absence of the lectin activation pathway of complement does not increase susceptibility to Pseudomonas aeruginosa infections. Immunobiology. 2012;217:272–80. doi: 10.1016/j.imbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers S, Aerts PC, van Dijk H. Differential microorganism-induced mannose-binding lectin activation. FEMS Immunol Med Microbiol. 2003;36:33–9. doi: 10.1016/S0928-8244(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 22.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies J, Neth O, Alton E, Klein N, Turner M. Differential binding of mannose-binding lectin to respiratory pathogens in cystic fibrosis. Lancet. 2000;355:1885–6. doi: 10.1016/S0140-6736(00)02297-2. [DOI] [PubMed] [Google Scholar]
- 24.Møller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, Jensenius JC, Ezekowitz RA, Takahashi K. Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J Immunol. 2006;176:1769–75. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalmers JD, Fleming GB, Hill AT, Kilpatrick DC. Impact of mannose-binding lectin insufficiency on the course of cystic fibrosis: A review and meta-analysis. Glycobiology. 2011;21:271–82. doi: 10.1093/glycob/cwq161. [DOI] [PubMed] [Google Scholar]
- 26.Francis D, Abberton K, Thompson E, Daniell M. Myogel supports the ex-vivo amplification of corneal epithelial cells. Exp Eye Res. 2009;88:339–46. doi: 10.1016/j.exer.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Eisen DP, Dean MM, Boermeester MA, Fidler KJ, Gordon AC, Kronborg G, Kun JF, Lau YL, Payeras A, Valdimarsson H, Brett SJ, Ip WK, Mila J, Peters MJ, Saevarsdottir S, van Till JW, Hinds CJ, McBryde ES. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47:510–6. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56:630–41. doi: 10.1046/j.1365-3083.2002.01167.x. [DOI] [PubMed] [Google Scholar]
- 30.Kwok JY, Augst RM, Yu DY, Singh KK. Sensitive CSF ELISAs for the detection of MBL, MASP-2 and functional MBL/MASP-2. J Neurosci Methods. 2012;209:255–7. doi: 10.1016/j.jneumeth.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downing I, Koch C, Kilpatrick DC. Immature dendritic cells possess a sugar-sensitive receptor for human mannan-binding lectin. Immunology. 2003;109:360–4. doi: 10.1046/j.1365-2567.2003.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasso DL, Segat L, Zocconi E, Radillo O, Trevisiol C, Crovella S. MBL expression in patients with recurrent tonsillitis. Int J Pediatr Otorhinolaryngol. 2009;73:1550–3. doi: 10.1016/j.ijporl.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Sun G, Liu G, Shi Y, Han Y, Yu F, Xiang X, Li W, Xiao H, Liu X, Li S. Clinical Significance of Mannose-Binding Lectin Expression in Thyroid Carcinoma Tissues. Pathol Oncol Res. 2013;19:259–66. doi: 10.1007/s12253-012-9577-x. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen CM, Hansen TK, Steffensen R, Jensenius JC, Thiel S. Hormonal regulation of mannan-binding lectin synthesis in hepatocytes. Clin Exp Immunol. 2006;145:173–82. doi: 10.1111/j.1365-2249.2006.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidler KJ, Hilliard TN, Bush A, Johnson M, Geddes DM, Turner MW, Alton EW, Klein NJ, Davies JC. Mannose-binding lectin is present in the infected airway: a possible pulmonary defence mechanism. Thorax. 2009;64:150–5. doi: 10.1136/thx.2008.100073. [DOI] [PubMed] [Google Scholar]
- 36.Kuraya M, Matsushita M, Endo Y, Thiel S, Fujita T. Expression of H-ficolin/Hakata antigen, mannose-binding lectin-associated serine protease (MASP)-1 and MASP-3 by human glioma cell line T98G. Int Immunol. 2003;15:109–17. doi: 10.1093/intimm/dxg008. [DOI] [PubMed] [Google Scholar]
- 37.Degn SE, Jensen L, Hansen AG, Duman D, Tekin M, Jensenius JC, Thiel S. Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. J Immunol. 2012;189:3957–69. doi: 10.4049/jimmunol.1201736. [DOI] [PubMed] [Google Scholar]
- 38.Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72:2899–906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Wu XY, Yu FS. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr Eye Res. 2005;30:527–34. doi: 10.1080/02713680590968150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole N, Bao S, Stapleton F, Thakur A, Husband AJ, Beagley KW, Willcox MD. Pseudomonas aeruginosa keratitis in IL-6-deficient mice. Int Arch Allergy Immunol. 2003;130:165–72. doi: 10.1159/000069006. [DOI] [PubMed] [Google Scholar]