Abstract
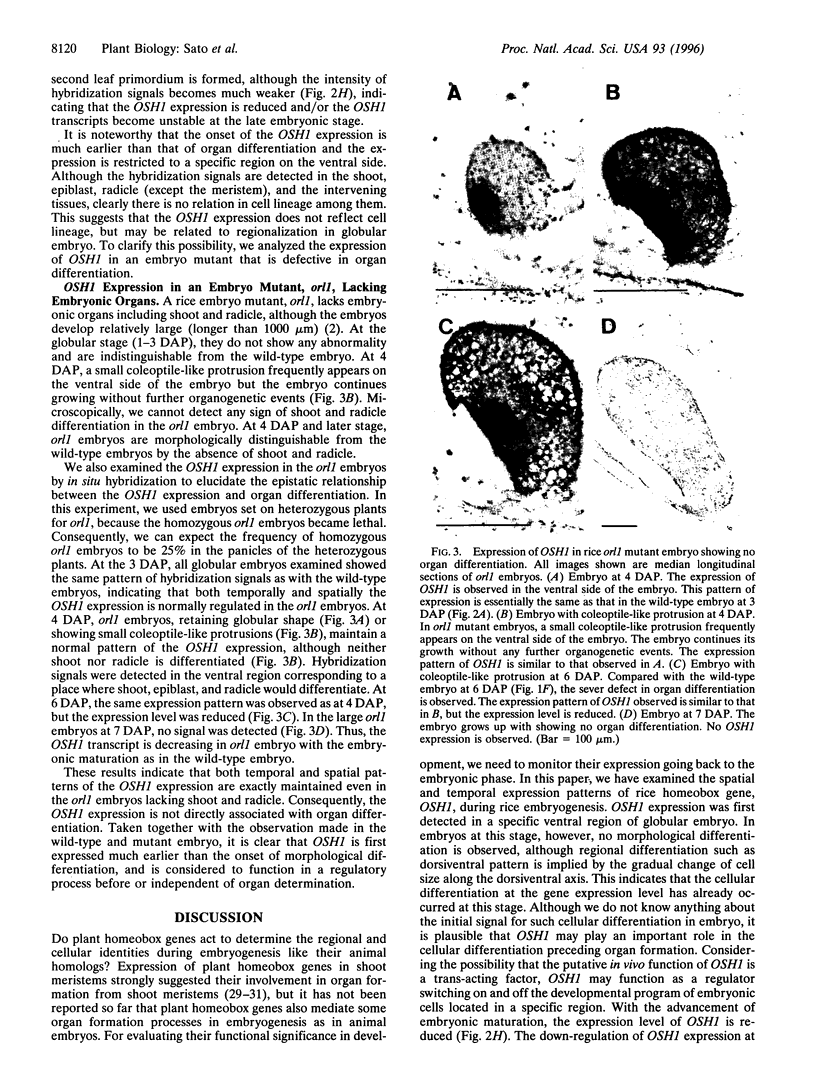
Homeobox genes encode a large family of homeodomain proteins that play a key role in the pattern formation of animal embryos. By analogy, homeobox genes in plants are thought to mediate important processes in their embryogenesis, but there is very little evidence to support this notion. Here we described the temporal and spatial expression patterns of a rice homeobox gene, OSH1, during rice embryogenesis. In situ hybridization analysis revealed that in the wild-type embryo, OSH1 was first expressed at the globular stage, much earlier than organogenesis started, in a ventral region where shoot apical meristem and epiblast would later develop. This localized expression of OSH1 indicates that the cellular differentiation has already occurred at this stage. At later stages after organogenesis had initiated, OSH1 expression was observed in shoot apical meristem [except in the L1 (tunica) layer], epiblast, radicle, and their intervening tissues in descending strength of expression level with embryonic maturation. We also performed in situ hybridization analysis with a rice organless embryo mutant, orl1, that develops no embryonic organs. In the orl1 embryo, the expression pattern of OSH1 was the same as that in the wild-type embryo in spite of the lack of embryonic organs. This shows that OSH1 is not directly associated with organ differentiation, but may be related to a regulatory process before or independent of the organ determination. The results described here strongly suggest that, like animal homeobox genes, OSH1 plays an important role in regionalization of cell identity during early embryogenesis.
Full text
PDF
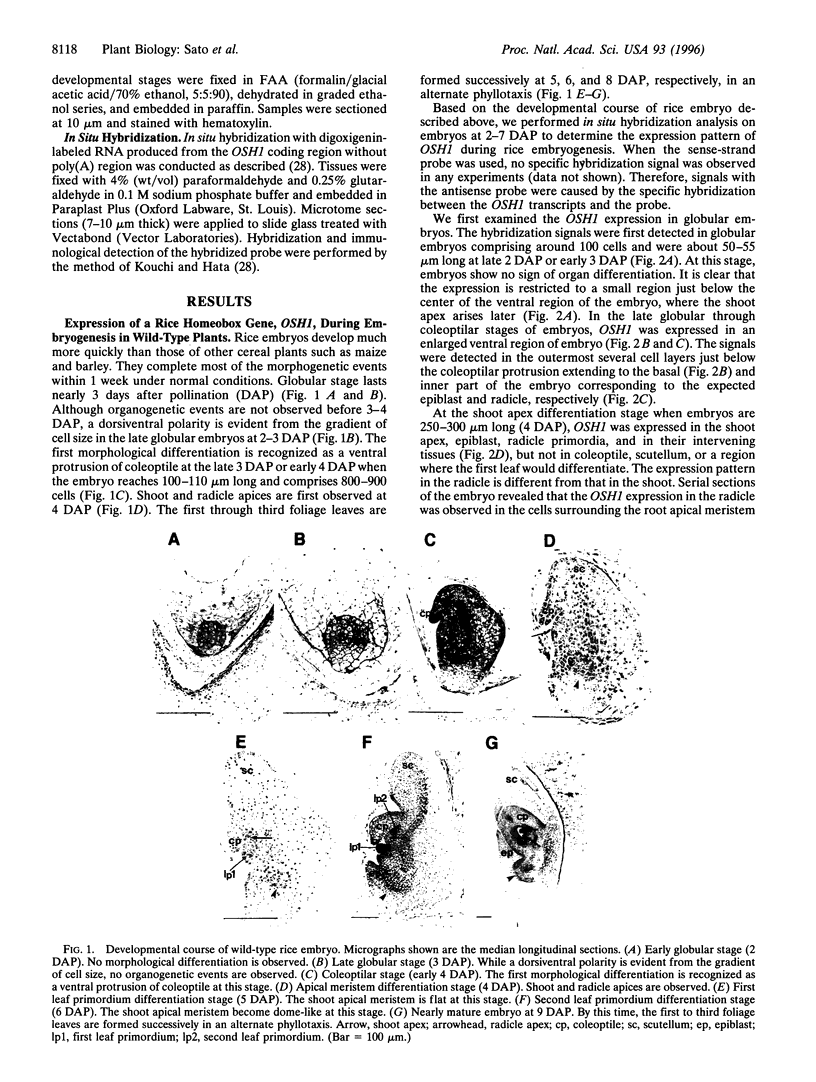
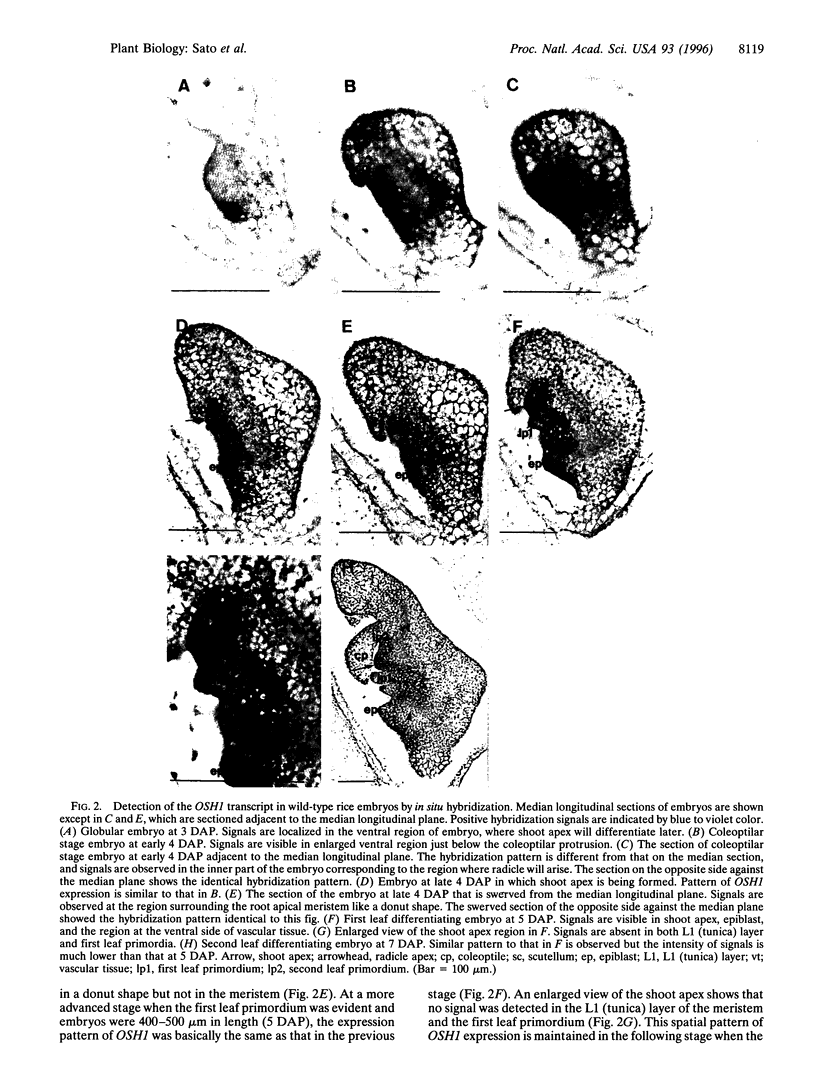


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellmann R., Werr W. Zmhox1a, the product of a novel maize homeobox gene, interacts with the Shrunken 26 bp feedback control element. EMBO J. 1992 Sep;11(9):3367–3374. doi: 10.1002/j.1460-2075.1992.tb05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. K., Sheridan W. F. Isolation and Characterization of 51 embryo-specific Mutations of Maize. Plant Cell. 1991 Sep;3(9):935–951. doi: 10.1105/tpc.3.9.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errampalli D., Patton D., Castle L., Mickelson L., Hansen K., Schnall J., Feldmann K., Meinke D. Embryonic Lethals and T-DNA Insertional Mutagenesis in Arabidopsis. Plant Cell. 1991 Feb;3(2):149–157. doi: 10.1105/tpc.3.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Barker S. J., Perez-Grau L. Regulation of gene expression during plant embryogenesis. Cell. 1989 Jan 27;56(2):149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., de Paiva G., Yadegari R. Plant embryogenesis: zygote to seed. Science. 1994 Oct 28;266(5185):605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Jofuku K. D., Schipper R. D., Goldberg R. B. A frameshift mutation prevents Kunitz trypsin inhibitor mRNA accumulation in soybean embryos. Plant Cell. 1989 Apr;1(4):427–435. doi: 10.1105/tpc.1.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano-Murakami Y., Yanai T., Tagiri A., Matsuoka M. A rice homeotic gene, OSH1, causes unusual phenotypes in transgenic tobacco. FEBS Lett. 1993 Nov 22;334(3):365–368. doi: 10.1016/0014-5793(93)80713-5. [DOI] [PubMed] [Google Scholar]
- Kouchi H., Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet. 1993 Apr;238(1-2):106–119. doi: 10.1007/BF00279537. [DOI] [PubMed] [Google Scholar]
- Lincoln C., Long J., Yamaguchi J., Serikawa K., Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994 Dec;6(12):1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. A., Moan E. I., Medford J. I., Barton M. K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996 Jan 4;379(6560):66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- Ma H., McMullen M. D., Finer J. J. Identification of a homeobox-containing gene with enhanced expression during soybean (Glycine max L.) somatic embryo development. Plant Mol Biol. 1994 Feb;24(3):465–473. doi: 10.1007/BF00024114. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Ichikawa H., Saito A., Tada Y., Fujimura T., Kano-Murakami Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 1993 Sep;5(9):1039–1048. doi: 10.1105/tpc.5.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J., Söderman E., Svenson M., Borkird C., Engström P. A new homeobox-leucine zipper gene from Arabidopsis thaliana. Plant Mol Biol. 1992 Mar;18(5):1019–1022. doi: 10.1007/BF00019223. [DOI] [PubMed] [Google Scholar]
- Ruberti I., Sessa G., Lucchetti S., Morelli G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991 Jul;10(7):1787–1791. doi: 10.1002/j.1460-2075.1991.tb07703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Davis R. W. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Lloyd A. M., Davis R. W. The HAT4 gene of Arabidopsis encodes a developmental regulator. Genes Dev. 1993 Mar;7(3):367–379. doi: 10.1101/gad.7.3.367. [DOI] [PubMed] [Google Scholar]
- Sinha N. R., Williams R. E., Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993 May;7(5):787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Smith L. G., Greene B., Veit B., Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992 Sep;116(1):21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- Tamaoki M., Tsugawa H., Minami E., Kayano T., Yamamoto N., Kano-Murakami Y., Matsuoka M. Alternative RNA products from a rice homeobox gene. Plant J. 1995 Jun;7(6):927–938. doi: 10.1046/j.1365-313x.1995.07060927.x. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991 Mar 21;350(6315):241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]