Abstract
Objective
Microtia is treated with rib cartilage sculpting and staged procedures; though aesthetically pleasing, these constructs lack native ear flexibility. Tissue-engineered (TE) elastic cartilage may bridge this gap; however, TE cartilage implants lead to hypertrophic changes with calcification and loss of flexibility. Retaining flexibility in TE cartilage must focus on increased elastin, maintained collagen II, decreased collagen X, with prevention of calcification. This study compares biochemical properties of human cartilage to TE cartilage from umbilical cord mesenchymal stem cells (UCMSCs). Our goal is to establish a baseline for clinically useful TE cartilage.
Methods
Discarded cartilage from conchal bowl, microtic ears, pre-auricular tags, rib, and TE cartilage were evaluated for collagen I, II, X, calcium, glycosaminoglycans, elastin, fibrillin I and III. Human UCMSCs were chondroinduced on 2-D surfaces and 3-D D, L-lactide-co-glycolic acid (PLGA) fibers.
Results
Cartilage samples demonstrated similar staining for collagens I, II, X, elastin, fibrillin I, and III, but differed from rib. TE pellets and PLGA-supported cartilage were similar to auricular samples in elastin and fibrillin I staining. TE samples exclusively stained for fibrillin III. Only microtic samples demonstrated calcium staining.
Conclusions
TE cartilage expressed similar levels of elastin, fibrillin I, collagens I and X when compared to native cartilage. Microtic cartilage demonstrated elevated calcium, suggesting this abnormal tissue may not be a viable cell source for TE cartilage. TE cartilage appears to recapitulate the embryonic development of fibrillin III, which is not expressed in adult tissue, possibly providing a strategy to control TE elastic cartilage phenotype.
Keywords: Mesenchymal Stem Cells, Chondrogenesis, Microtia, Nanofibers, Tissue Engineering, Fibrillin, Elastin
Introduction
Microtia is a deformity of the external auricle, which presents in .843 to 4.34 cases in 10,000 live births 1. Because microtia is a noticeable physical deformity, it can have a detrimental impact on a child’s psychosocial well-being and development. Children with microtia become self-aware of the malformation at the ages 5 to 6 and have been shown to be at a higher risk for interpersonal difficulties, depression, and aggression/hostility2. Treatments for microtia utilize endogenous costal cartilage grafts or synthetic implants. In costal cartilage implantation, techniques typically performed are ones popularized by Brent, Nagata, and Firmin3. These surgical techniques involve rib cartilage harvest and sculpting, with implantation, followed by staged surgeries to create the semblance of an external ear 4. Risks associated with harvest include pneumothorax, chest wall retrusion, and postoperative thoracic scoliosis; risks associated with the implant site include infection, extrusion, and loss of the graft5. While the reconstructed ear may be aesthetically pleasing, 6 costal cartilage (a hyaline cartilage) does not have the same deformability as native auricular tissue. Alternatives to autologous cartilage include synthetic implants, such as Medpor®, but have higher risks of infection and extrusion, while still lacking the deformability of native auricular tissue 7. Solutions to these limitations noted in both autologous and synthetic ear reconstruction may be found in tissue-engineered (TE) cartilage. The potential advantages of TE cartilage constructs include an unlimited supply of engineered cartilage, fewer surgeries, and the avoidance of attendant complications. However, to date, the major limitation to TE cartilage is that it tends to calcify, and become inflexible in a predictable fashion after implantation 8. From a clinical standpoint, TE elastic cartilage must maintain its elastic phenotype, have characteristics that allow ear flexibility, and yet, must be rigid enough to withstand the deforming forces of the healing soft tissue envelope.
In previously published work, our laboratory has utilized human umbilical cord mesenchymal stem cells (hUCMSCs) as a cell source for TE cartilage 9. Nanofiber-supported hUCMSC chondrogenesis promoted increased glycosaminoglycans (GAG) and improved collagen II to I ratio (differentiation index) compared to standard pellet formation, indicating an elastic cartilage phenotype 10. However, we also noted increased expression of collagen X, and decreased expression of elastin mRNA, both of which suggest the development of a hypertrophic cartilage phenotype. Because hypertrophic cartilage tends to be less flexible, and may indicate a tendency to calcify after implantation, we wanted to further evaluate our tissue-engineered cartilage in comparisons to normal auricular cartilage (conchal bowl), pre-auricular cartilage remnants, microtia samples, and hyaline cartilage from rib, which is the current gold standard for cartilage source during external ear reconstruction.
In order to create and maintain flexible TE elastic cartilage beyond our current capabilities, we need to maintain control over elastic fiber deposition, fibrillin production, eliminate calcium deposition, and diminish collagen X production. Elastic fibers are formed when tropoelastin binds to fibrillin I in the ECM and becomes cross-linked11. As such, maintaining fibrillin I content in tissue engineered cartilage provides the appropriate template for elastin deposition and cross-linking, thereby maintaining tissue engineered cartilage flexibility. In addition to fibrillin I, two other types of fibrillin have been identified. Fibrillin II is expressed with fibrillin I prenatally, and is only minutely expressed in mature tissues 12. Fibrillin III is expressed prenatally and no longer present following birth13. To our knowledge fibrillin expression in TE cartilage has not been characterized and may eventually provide insight into maintaining elastic cartilage phenotype following implantation.
Elastic cartilage flexibility also decreases as calcium deposition in the matrix increases14. Calcium cross-links to binding domains in fibrillin, making the fibrillin template inflexible; therefore, minimizing calcium matrix deposition is another strategy to maintain cartilage flexibility. A last set of strategies to prevent phenotype change from elastic cartilage to hypertrophic cartilage is to control the presence of certain collagens, namely, to maintain a differentiation index (collagen II/I ratio) above 1 (with greater collagen II); a ratio below 1 is an indication of fibrocartilage development, and to control the collagen X content of the cartilage. Increased collagen X is an initial indication that hypertrophic cartilage is developing.
Lastly, MSC source may play a role in maintaining an elastic cartilage phenotype. To date, abdominal adipocytes have proven to be the most reliable source for adult tissue-engineering15. In infants and children, minimal sources of fat exist for harvesting, making this a less than ideal cell source. Other discarded tissue sources–including cartilage itself-- may be useful to generate TE elastic cartilage, including pre-auricular cartilage-skin remnants, microtic cartilage harvested at the time of staged ear reconstruction, and rib cartilage. In order to determine whether a cell source is a legitimate alternative for TE cartilage, the biochemical properties of these sources must be determined.
The goal of this study is to accurately define the expression of various extracellular matrix (ECM) components in auricular cartilage, including collagen I, II, X, calcium; glycosaminoglycans (GAGS), elastin, fibrillin I and II. We wish to compare our tissue engineered pellets and nanofiber--supported TE cartilage to these tissues to determine a baseline of understanding how to create and maintain elastic cartilage phenotype in TE cartilage.
Materials/Methods
Discarded human cartilage samples were collected with IRB approval (IRB # 10-1580 and 10-1299) and included conchal bowl, microtia, pre-auricular skin and cartilage remnants, and rib cartilage. Samples were fixed in 10% formalin solution up to 48 hours and stored in 70% ethanol before biochemical characterization.
Tissue engineered cartilage was generated from hUCMSCs harvested using an explant technique. Passage 2 cells were chondroinduced as previously described 9. Briefly, 4 × 105 isolated hUCMSCs were placed in 0.3 mL of chondrogenic media in a 2 mL conical tube and grown for 21 days, after which the pellet was fixed in 10% formalin and frozen. PLGA nanofibers were electrospun as mats as previously described16. 4 × 105 hUCMSCs were seeded on the nanomats and subjected to chondrogenesis for 21 days, after which, scaffolds were fixed in 10% formalin solution up to 48 hours and stored in 70% ethanol before biochemical characterization.
Antibodies and Immunohistochemistry
Mouse monoclonal anti-Collagen I, II and X antibodies were purchased from Abcam (Cambridge, MA). Rabbit polyclonal anti-Fibrillin I was obtained from Sigma-Aldrich (St. Louis, MO); goat polyclonal anti-Fibrillin III was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Chondrocyte cultures and human samples were fixed in neutral-buffered formalin for 24 hours and stored in 70% ethanol for staining. Samples were paraffin embedded; 4 μm sections were cut with a microtome. IHC was performed in a Bond Autostainer (Leica Microsystems Inc. Norwell, MA). Slides were de-waxed in Bond Dewax solution (AR9222) and hydrated in Bond Wash solution (AR9590). Antigen retrieval for collagen I and X and fibrillin I antibodies was performed for 15 min at 37° C in Pepsin (Invitrogen; Camarillo, CA). Collagen II antigen retrieval was performed using the Bond Enzyme Pretreatment kit (AR9551); no antigen retrieval was necessary for fibrillin III. Detection of the collagen I, II, X and fibrillin I antibodies was performed using the Bond Polymer Refine Detection System (DS9800). For fibrillin III the secondary antibody and polymer from the kit were replaced by Goat IgG and the HRP polymer from Biocare (Concord, CA). Stained slides were dehydrated and prepared with a coverslip. Positive and negative controls were included for each antibody.
Histological Stains
Elastin was detected using the Verhoeff Elastic Stain kit (KTVEL); calcium was detected with the Alizarin Red Stain kit (STARE; American MasterTech; Lodi, CA).
Digital imaging and image analysis
Stained slides were digitally imaged at 20× magnification using the Aperio ScanScope XT (Aperio Technologies, Vista, CA). Digital images were stored and analyzed within the Aperio Spectrum Database. Expression levels of biomarkers were measured using the Aperio Color Deconvolution V9 algorithm with minor modifications. Optical densities and intensity thresholds were modified based on levels in positive and negative controls. The percent area with strong (+3), medium (+2) and weak (+1) positive signals, as well as total positive signal, were used to compare biomarker levels.
Results
Cartilage from conchal bowl, preauricular remnants, microtic, and TE samples (pellets grown on 2-D surfaces, and PLGA-supported cartilage) stained in similar distributions for collagen I and X; however, costal cartilage stained less intensely for both (Figure 1). Conchal bowl and preauricular cartilage remnants stained similarly for collagen II; microtic cartilage stained less positively; both costal and TE cartilage stained lightly for collagen II. Alizarin red stain was negative in all samples, with the exception of microtia samples, where calcium was noted in the extracellular matrix. All of the human cartilage samples demonstrated similar percentage of cells positive for collagen II (Table 1); the TE pellet (45%) and PLGA cartilage (63%) stained less intensely for collagen II. Collagen X was expressed similarly in native tissues (>95%) but were below 91% in both TE pellets and PLGA-supported cartilage.
Figure 1.
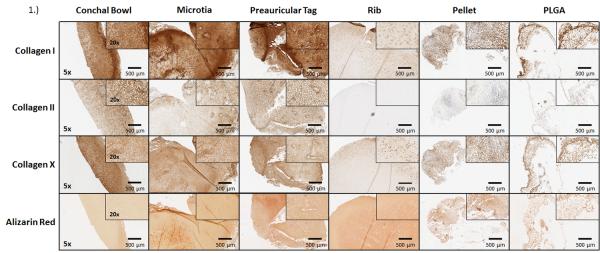
Histochemical analysis of cartilage. Images are representative of collagen I, II, X, and alizarin red staining for calcium of frozen adult conchal bowl, pediatric microtia, pediatric preauricular cartilage remnants, and adult costal cartilage. Similar distributions of collagens I, II, and X were seen in all samples, with the exception of rib cartilage (a hyaline cartilage). Calcium was present in only the microtia samples. The TE pellet and PLGA –supported cartilage demonstrated similar distribution of Collagen I, X, and calcium in the extracellular matrix as native auricular cartilage. Collagen II distribution was less than native auricular cartilage samples, but greater than rib.
Table 1.
Semi-quantitative image analysis of collagens I, II, X, and calcium deposition. Total positivity of stains was analyzed using Spectrum software. Positivity was designated as strong, medium, or weak, depending on the intensity/darkness of each pixel. Similar distributions were seen in the TE (both pellet and PLGA-supported cartilage) and auricular-derived cartilage for collagens I and X, with marginal presence in rib samples. The microtia samples had a significantly higher presence of calcium, unlike the other samples. Collagen II was most intense in auricular-derived cartilage, lower in the TE cartilage, and nearly absent in rib samples.
| Stain | Tissue | % Positive | %Strong+ | %Medium+ | % Weak+ |
|---|---|---|---|---|---|
| Collagen I | Conchal bowl | 99 | 66 | 29 | 4 |
| Microtia | 99 | 63 | 28 | 8 | |
| Preauricular Tag | 100 | 84 | 14 | 2 | |
| Rib | 93 | 0 | 21 | 72 | |
| Pellet | 91 | 32 | 39 | 20 | |
| PLGA | 92 | 26 | 42 | 24 | |
| Collagen II | Conchal bowl | 97 | 23 | 50 | 23 |
| Microtia | 90 | 9 | 45 | 36 | |
| Preauricular Tag | 96 | 26 | 49 | 22 | |
| Rib | 3 | 0 | 1 | 2 | |
| Pellet | 45 | 0 | 12 | 33 | |
| PLGA | 63 | 3 | 21 | 39 | |
| Collagen X | Conchal bowl | 100 | 53 | 41 | 6 |
| Microtia | 99 | 14 | 62 | 23 | |
| Preauricular Tag | 99 | 17 | 57 | 25 | |
| Rib | 95 | 6 | 19 | 69 | |
| Pellet | 91 | 21 | 40 | 31 | |
| PLGA | 90 | 17 | 38 | 34 | |
| Alizarin Red | Conchal bowl | 0 | 0 | 0 | 0 |
| Microtia | 4.14 | 0.55 | 1.18 | 2.40 | |
| Preauricular Tag | 0.28 | 0 | 0.01 | 0.27 | |
| Rib | 0.85 | 0.02 | 0.11 | 0.72 | |
| Pellet | 0.01 | 0 | 0 | 0.01 | |
| PLGA | 0.02 | 0 | 0 | 0.02 |
All native tissues demonstrated similar extracellular glycosaminoglycan by alcian blue staining (Figure 2); the pellet and PLGA samples stained less intensely, but were uniformly positive. Native auricular cartilage (conchal bowl, preauricular tags, microtia sample) stained similarly for elastin, with less intense staining noted in TE cartilage and none in costal cartilage. Fibrillin I stains were similar in all stained tissue samples, with the exception of costal cartilage, where staining was lighter. All native cartilage types stained negative for fibrillin III; however, both TE cartilage samples were positive for this immature fibrillin.
Figure 2.
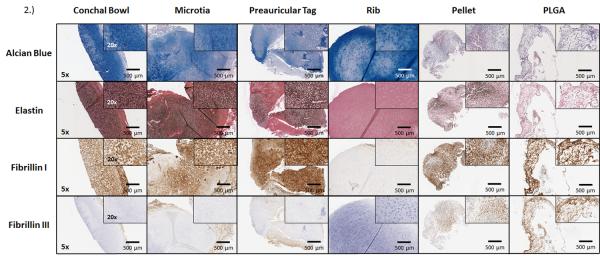
Histochemical analysis of cartilage. Images are representative of alcian blue, elastin, fibrillin I, and III staining of frozen adult conchal bowl, pediatric microtia, pediatric preauricular cartilage remnants, and adult costal cartilage. All samples demonstrated glycosaminoglycans in the extracellular matrix. The TE and auricular cartilage showed similar elastin and fibrillin I distributions, with rib minimally demonstrating these proteins. Fibrillin III was positively expressed in TE samples, while not present in endogenous tissues.
Elastin staining in cartilage from the conchal bowl, microtia, and preauricular cartilage were similar to the TE pellet, but higher than the PLGA-supported cartilage and rib cartilage (Table 2). Fibrillin I stain quantification was similar in the native auricular samples (> 96% positive), lower in the TE cartilage samples (90%) and lowest in rib cartilage (41%). Fibrillin III stain was minimally present in conchal bowl, preauricular, and microtic cartilage, and absent in rib cartilage. Both the TE pellets (55%) and PLGA-supported cartilage (92%) demonstrated extensive fibrillin III staining.
Table 2.
Semi-quantitative image analysis of elastin, fibrillin I, and III. Total positivity of the stain was analyzed using Spectrum software. Positivity was then quantitated as strong, medium, or weak, depending on intensity/darkness of each pixel. Increased elastin and fibrillin I were seen in all samples, with the exception of rib cartilage. Fibrillin III was present in the TE samples (PLGA-supported greater than pellet), with absent staining in endogenous tissues.
| Stain | Tissue | % Positive | %Strong+ | %Medium+ | % Weak+ |
|---|---|---|---|---|---|
| Elastin | Conchal bowl | 49 | 1 | 18 | 29 |
| Microtia | 47 | 1 | 15 | 31 | |
| Preauricular Tag | 38 | 1 | 11 | 26 | |
| Rib | 10 | 2 | 3 | 5 | |
| Pellet | 48 | 2 | 14 | 32 | |
| PLGA | 30 | 1 | 5 | 25 | |
| Fibrillin I | Conchal bowl | 97 | 26 | 46 | 24 |
| Microtia | 96 | 22 | 47 | 27 | |
| Preauricular Tag | 99 | 48 | 41 | 10 | |
| Rib | 41 | 1 | 12 | 27 | |
| Pellet | 89 | 29 | 39 | 22 | |
| PLGA | 90 | 23 | 42 | 24 | |
| Fibrillin III | Conchal bowl | 1 | 0 | 0 | 1 |
| Microtia | 5 | 0 | 1 | 4 | |
| Preauricular Tag | 7 | 0 | 0 | 7 | |
| Rib | 0 | 0 | 0 | 0 | |
| Pellet | 55 | 6 | 20 | 29 | |
| PLGA | 92 | 11 | 50 | 30 |
Discussion
TE elastic cartilage is a potential solution for current limitations in microtia reconstruction. This cartilage will overcome the rigidity and lack of deformability of the reconstructed ear seen in both costal cartilage and synthetic implant strategies, eliminate the surgical risks associated with rib harvest, and result in a non-immunogenic construct. However, before clinical utility can be established, there must be demonstration that this cartilage is phenotypically similar to native auricular cartilage, and will maintain an elastic cartilage phenotype when implanted. Because conchal cartilage is the true normal in our set of comparisons, initial comparisons need to be established between TE and conchal cartilage. Both of the TE cartilage samples (2-D and PLGA supported) expressed similar levels of collagen I, X, and fibrillin I compared to conchal bowl cartilage. However, some notable differences also existed, including lower levels of elastin and collagen II in the PLGA nanofiber-supported cartilage. Both of these findings will eventually need to be remedied in our TE cartilage prior to implantation.
An interesting finding in TE cartilage is the presence of fibrillin III (to the best of our knowledge, ours is the first report of this immature fibrillin in TE cartilage). Lower levels of elastin in the TE PLGA-supported cartilage may in part be explained by the immaturity of TE cartilage, demonstrated by the presence of fibrillin III. The TE cartilage pellet exhibited 55% fibrillin III positivity, while PLGA-supported TE cartilage exhibited higher positivity (92%), indicating that the TE cartilage is more primitive, and may have similarities to pre-natal cartilage. In the human embryo, fibrillin III is thought to be instrumental in the initial formation of microfibril elastic cores; fibrillin III then disappears during maturation of the embryo, and fibrillin I becomes the primary extracellular matrix protein for elastic microfibrils17. In order for this fibrillin III to convert to fibrillin I, TE cartilage may need to be housed in vitro for longer time periods, or may need to be reassessed following implantation. Further studies are required to determine if fibrillin III expression in TE cartilage changes over time. While 40 weeks (analogous to human gestation) is probably not needed for maturation of in vitro-generated TE cartilage, time periods greater than the current 21 days will likely be required13.
Further indication of the immaturity of the TE cartilage can be seen in the relative distribution of collagen I and II in both the nanofiber construct and pellet. In mature cartilage, collagen I is normally expressed by chondrocytes in a thin layer covering the surface, whereas collagen II is expressed more interiorly. However, seen in the staining of the both the nanofiber-supported and pellet TE cartilage, collagen I seems to not only be expressed by chondrocytes in the periphery, but also throughout the entirety of the construct. The collagen II-producing chondrocytes are found in a central location, as would be expected in native cartilage. The wide distribution of collagen I is a possible indication of TE cartilage immaturity. This hypothesis is further strengthened because the staining pattern of the PLGA and pellet constructs seems to more closely resemble the preauricular cartilage remnants (a more immature cartilage) than the conchal bowl staining.
A second goal of this work was to assess potential cell sources for the purpose of engineering elastic cartilage. The most readily available sources, in descending order, are preauricular cartilage remnants (generally excised and discarded, and often done at an early age), microtic cartilage (easily accessible during surgery for microtia repair and discarded), rib cartilage (potentially discarded after multiple procedures, including microtia repair), and normal ears (rarely discarded). Histological staining of these native cartilage sources reinforces the known differences between costal (hyaline cartilage) and auricular cartilage (elastic cartilage)18. Elastic cartilage 19 is populated with larger chondrocytes, higher expression of ECM components, specifically elastin and collagen II, and faster proliferation in vitro 20. The use of rib cartilage as a stem cell source for auricular chondrocytes would be expected to lead to insufficient elastic generation, and secondarily to an incorrect collagen II to I ratio. This is supported by work with bovine chondrocytes harvested from several anatomical locations, including costal cartilage. These cells were seeded on poly (l-lactide-ε-caprolactone) scaffolds, and implanted for 40 weeks in nude mice. At study completion, the costal chondrocyte scaffold was rigid, exhibiting calcified nodules21, and did not appear to be suitable for generating elastic cartilage.
Conchal, preauricular, and microtia cartilage samples all exhibited similar staining for elastin, fibrillin I and III, as well as collagen I and II. Microtic cartilage was the only cartilage sample, however, that stained positive for calcium, suggesting abnormalities in this cartilage that make unsuitable as a stem cell source for TE cartilage. Among these samples, the best source for TE cartilage may be preauricular cartilage remnants (given the similarity in staining patterns to conchal cartilage, a relatively high incidence among patients (which may be as high as 1% of the population)22, and the fact that they are surgically excised relatively early in life and discarded). Preauricular cartilage also expressed a higher fibrillin III level among the endogenous cartilage tested, suggesting that it may represent an immature auricular cartilage, which could be advantageous for TE cartilage. However, the amount of cartilage present within the preauricular remnants is variable (sometimes absent altogether) which presents a challenge as a cartilage cell source.
The umbilical cord as a discarded source of MSCs capable of undergoing chondrogenesis appears to be an ideal source of stem cells for TE cartilage but there continue to be biochemical hurdles that must be overcome in order to replicate normal auricular cartilage including maintaining Collagen II/I ratio above one, controlling elastin and fibrillin content, minimizing collagen X content and preventing calcium deposition.
Summary
TE cartilage using hUCMSCs on PLGA nanofiber-supported scaffolds results in cartilage that is similar to conchal bowl in staining for collagen I, X, and fibrillin I. However, elastin and collagen II expression levels are lower and the presence of fibrillin III suggests immaturity. TE cartilage needs to have higher levels of elastin and collagen II, lower levels of fibrillin III and be less deformable in order to justify use in microtia repair. An on-going, and unanswered question regarding TE cartilage, is how to find a balance between the needs for construct flexibility and a sufficient rigidity to withstand the deforming forces of the overlying skin envelope. Though this study sets the stage for creating a flexible elastic cartilage phenotype, significant further studies will be required to determine efficacy in vivo.
Acknowledgements
We would like to thank Stryker CMF for donating Delta Polymer in order to electrospin PLGA nanofibers.
Potential Conflicts of Interest This work was funded in part by a Plastic Surgery Foundation Grant entitled “Elusive Deformable Tissue Engineered Cartilage: Focus on Elastin” and a KL2 Career Development Grant (5KL2RR025746-03)
Footnotes
Statement of Authorship Andrew Pappa, intellectual design of all experiments, collection and analysis of the data, drafting of the manuscript, and revising and approval of the final manuscript. Monstserrat Caballero, assistance in intellectual design of experiments, collection and interpretation of data, drafting of the manuscript, critically revising the manuscript, and approval of the final draft. Robert Dennis, assistance in intellectual design of the experiment, with critical review and revising of the manuscript, and approval of the final draft. Matthew Skancke, assistance with experiments, with revising of the manuscript, and with final approval of the draft. Roger Narayan, intellectual concept and design of the experiments, understanding of the findings, and with review and approval of the manuscript. John Dahl, assistance in intellectual of design of experiments that were based on his previous work, assistance running experiments, and review/approval of the final draft. John van Aalst, assistance in intellectual design of the experiments, with drafting/revision of the manuscript, and with final approval of the manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luquetti DV, Heike CL, Hing AV, Cunningham ML, Cox TC. Microtia: Epidemiology and genetics. Am J Med Genet A. 2011 Nov 21; doi: 10.1002/ajmg.a.34352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiamei D, Jiake C, Hongxing Z, Wanhou G, Yan W, Gaifen L. An investigation of psychological profiles and risk factors in congenital microtia patients. J Plast Reconstr Aesthet Surg. 2008;61(Suppl 1):S37–43. doi: 10.1016/j.bjps.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Sivayoham E, Woolford TJ. Current opinion on auricular reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2012 Aug;20(4):287–290. doi: 10.1097/MOO.0b013e328355b1d9. [DOI] [PubMed] [Google Scholar]
- 4.Quatela VC, Thompson SK, Goldman ND. Microtia reconstruction. Facial Plast Surg Clin North Am. 2006 May;14(2):117–127. vi. doi: 10.1016/j.fsc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Romo T, 3rd, Presti PM, Yalamanchili HR. Medpor alternative for microtia repair. Facial Plast Surg Clin North Am. 2006 May;14(2):129–136. vi. doi: 10.1016/j.fsc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Brent B. Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases. Clin Plast Surg. 2002 Apr;29(2):257–271. vii. doi: 10.1016/s0094-1298(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 7.Wellisz T. Clinical experience with the Medpor porous polyethylene implant. Aesthetic Plast Surg. 1993 Fall;17(4):339–344. doi: 10.1007/BF00437109. [DOI] [PubMed] [Google Scholar]
- 8.Bichara DA, O’Sullivan NA, Pomerantseva I, et al. The Tissue-Engineered Auricle: Past, Present, and Future. Tissue Eng Part B Rev. 2011 Oct 4; doi: 10.1089/ten.TEB.2011.0326. [DOI] [PubMed] [Google Scholar]
- 9.Dahl JP, Caballero M, Pappa AK, Madan G, Shockley WW, van Aalst JA. Analysis of Human Auricular Cartilage to Guide Tissue-Engineered Nanofiber-Based Chondrogenesis: Implications for Microtia Reconstruction. Otolaryngol Head Neck Surg. 2011 Sep 9; doi: 10.1177/0194599811419092. [DOI] [PubMed] [Google Scholar]
- 10.Marlovits S, Hombauer M, Truppe M, Vecsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004 Mar;86(2):286–295. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]
- 11.Rock MJ, Cain SA, Freeman LJ, et al. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J Biol Chem. 2004 May 28;279(22):23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- 12.Quondamatteo F, Reinhardt DP, Charbonneau NL, Pophal G, Sakai LY, Herken R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002 Dec;21(8):637–646. doi: 10.1016/s0945-053x(02)00100-2. [DOI] [PubMed] [Google Scholar]
- 13.Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011 Jan;30(1):43–52. doi: 10.1016/j.matbio.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Yang XF, He X, He J, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. doi: 10.1186/1423-0127-18-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed CR, Han L, Andrady A, et al. Composite tissue engineering on polycaprolactone nanofiber scaffolds. Ann Plast Surg. 2009 May;62(5):505–512. doi: 10.1097/SAP.0b013e31818e48bf. [DOI] [PubMed] [Google Scholar]
- 17.Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004 Mar;83(3):461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Rubin R, editor. Clinicopathologic Foundations of Medicine. 5 ed. Wolters Luwer; Baltimore, MD: 2008. Rubin’s Pathology. [Google Scholar]
- 19.Huber M, Trattnig S, Lintner F. Anatomy, biochemistry, and physiology of articular cartilage. Invest Radiol. 2000 Oct;35(10):573–580. doi: 10.1097/00004424-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Nabzdyk C, Pradhan L, Molina J, Perin E, Paniagua D, Rosenstrauch D. Review: auricular chondrocytes - from benchwork to clinical applications. In Vivo. 2009 May-Jun;23(3):369–380. [PubMed] [Google Scholar]
- 21.Kusuhara H, Isogai N, Enjo M, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009 Jan-Feb;17(1):136–146. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 22.Roth DA, Hildesheimer M, Bardenstein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008 Oct;122(4):e884–890. doi: 10.1542/peds.2008-0606. [DOI] [PubMed] [Google Scholar]


