Abstract
Ubiquitination cascade plays an important role in the assembly of repair and signaling proteins at sites of double-strand DNA breaks (DSBs). E3 ubiquitin ligase RNF8 triggers initial ubiquitination at DSBs, whereas substained ubiquitination requires downstream E3 ligase RNF168. It is not yet known whether RNF8 and RNF168 have discrete substrates and/or form different ubiquitin chains. Here we show that RNF168 acts with E2 enzyme UBC13 and specifically synthesizes lysine 63 (K63)-linked chains, whereas RNF8 forms mainly K48-linked chains on chromatin, promoting substrate degradation. We also find that RNF8 regulates the abundance of NHEJ repair protein KU80 at sites of DNA damage, and RNF8 depletion resulted in prolonged retention of KU80 at damage sites and impaired non-homologous end joining (NHEJ) repair. These findings reveal a distinct feature of RNF8 and indicate the involvement of ubiquitination-mediated degradation pathway in DNA damage repair.
Keywords: RNF8, ubiquitin, non-homologous end joining, DNA damage
INTRODUCTION
Ubiquitin is a highly conserved 76-amino-acid protein that is covalently linked to its substrates by a three-step enzymatic cascade carried out by ubiquitin activation enzyme (E1), ubiquitin conjugating enzyme (E2), and ubiquitin ligase (E3) (see review1). Seven lysine residues of ubiquitin (K6, K11, K27, K29, K33, K48, and K63) and the amino-terminal methionine (M1) can be linked to the C-terminal glycine for the assembly of polyubiquitin chains. The conformation of different linkage specific chains varies, with that K63-linked or linear chains adopt open conformation2, whereas K48-linked chains are tightly packed with each other3. The topology of ubiquitin conjugates usually determines their biological outcomes. A chain of at least four ubiquitins linked via K48 or K11 linkage leads for protein degradation by the 26S proteasome4,5, whereas monoubiquitination or K63-linked chains have been implicated in non-proteolytic pathways such as receptor trafficking, signal transduction, autophagy and DNA repair (see reviews6–8).
Most E3 ubiquitin ligases can be classified into two subfamilies: HECT (homology to E6AP C-terminus) domain-containing E3s and RING (really interesting new gene) domain-containing E3s. The HECT E3s possess intrinsic catalytic activity as they can form ubiquitin-thioester intermediate via catalytic cysteine residue. Therefore, the linkage specificity of the product is largely determined by E3s. On the contrary, RING-domain E3s lack a catalytic site. They promote the modification of their targets by facilitating the direct transfer of ubiquitin from E2-ubiquitin intermediate to substrate, and thus the linkage specificity is determined by the nature of E2–E3 pairs. Some E2s build ubiquitin chains of a specific linkage even in the absence of an E3. In these cases the chain specificity is an intrinsic property of E2s. For example, UBC13-MMS2 heterodimer only assembles K63-linked polyubiquitin chains9. In other cases, RING E3s could also influence ubiquitin chain topology. For example, a non-specific E2 enzyme UBCH5 predominantly synthesizes K6-linked chains when it acts with BRCA1-BARD110,11. So far, how RING domain-containing E3s achieve their linkage specificity is still poorly understood.
Ubiquitination plays a critical role in orchestrating cellular response to double-strand DNA breaks (DSBs), especially in mammalian systems11,12. The first E3 ubiquitin ligase that acts in the DNA damage signaling pathway is the RING finger protein 8 (RNF8)13–15. RNF8 rapidly accumulates at DSBs via the interaction of its forkhead-associated (FHA) domain with the ATM-phosphorylated TQXF motifs on MDC1. The E3 ligase activity of RNF8 is required for the IR-induced foci formation (IRIF) of 53BP1 and BRCA1, and thus RNF8 acts downstream of MDC1 but upstream of 53BP1 and BRCA1 in DNA damage signaling pathway. A second E3 ligase RNF168 has been discovered later16,17, which functions downstream of RNF8 but also upstream of 53BP1 and BRCA1. Unlike the phosphorylation-dependent accumulation of RNF8 at DSBs, RNF168 foci formation depends on its two ubiquitin binding domains termed MIUs (motif interacting with ubiquitin)16,17. The current model is that RNF8 and RNF168 act with E2 enzyme UBC13 to synthesize K63-linked polyubiquitin chains at DSB sites. RNF8 is required for di-ubiquitination of histone H2A/H2AX. Subsequently RNF168 recognizes RNF8-synthesized di-ubiquitinated H2A/H2AX and other yet-to-be-identified substrates via its MIU motifs and further elongate K63-linked polyubiquitin chains to reach a threshold that is required for sustained assembly of 53BP1, BRCA1 and other DNA damage repair proteins at DSB sites13,15–18. Here we expanded this model and showed that RNF8 and RNF168 assemble functionally distinct polyubiquitin chains and thereby assign different fates to their substrates.
RESULTS
RNF8 and RNF168 synthesize different ubiquitin chains at DSBs
It has been shown that epitope-tagged K6-only or K63-only ubiquitin, but not K48-only ubiquitin (all other lysines mutated to arginines) form foci following DNA damage19, suggesting that K6-linked and K63-linked polyubiquitin chains are enriched at DSB sites. Furthermore, accumulation of endogenous K63-linked, but not K48-linked ubiquitin chains at DSB sites have been observed by the use of linkage-specific antibodies16,17,20. However, we felt that the involvement of K48-linked polyubiquitin chains in DNA damage signaling pathway could not be ruled out because K48-linked polyubiquitin chains are extreme unstable4.
To understand what types of ubiquitin chains are formed by RNF8 and RNF168, we expressed RNF8 or RNF168 at modest levels, which exhibited normal IRIF (IR-induced foci) after exposure to ionizing radiation. RNF8 readily formed foci at DNA break sites and co-localized with ubiquitin conjugates detected by FK2 antibody21, however it had little effect on the brightness of FK2 foci (Fig. 1a, left panel). Surprisingly, using linkage specific anti-ubiquitin antibodies, we showed that RNF8 induced K48-linked polyubiquitin chain formation at DSB sites marked by γH2AX (Fig. 1a, left panel and Supplementary Fig. 1a), whereas RNF168 did not markedly affect the intensity or the diffuse distribution of K48 staining (Fig. 1a, middle panel and 1c). The ability to promote K48 staining relies on the E3 ligase activity of RNF8, since an inactive mutant of RNF8 could not enhance K48 staining despite its normal localization to DSBs (Supplementary Fig. 1b). In contrast, expression of RNF168 resulted in dramatically increased staining for FK2, as well as K63 (Fig. 1a, middle panel), which is consistent with the observation that FK2 and K63 foci formation largely depend on RNF16816,17. Together these data suggest that RNF8 and RNF168 promote the accumulation of ubiquitin conjugates at DSB sites, with a preference of RNF8 and RNF168 for, respectively, K48- and K63-linked ubiquitin chain formation.
Fig 1. RNF8 and RNF168 promote the accumulation of different linkage specific ubiquitin chain formation at sites of DNA breaks.
(a) HeLa cells were transiently transfected with plasmids encoding Flag-tagged RNF8, RNF168 or MIU domains (residues 100–500) of RNF168 as indicated. Cells were treated with 5 Gy ionizing radiation (IR), fixed 4 hours later, and immunostained with indicated antibodies. (b) HeLa cells were tansfected with Flag-UIM domains of RAP8022 and processed as described in (a). (c) Quantification of K48 or K63 foci formation promoted by indicated constructs as described in (b). More than 50 Flag-positive cells with IRIF were counted. All data are represented as mean ± SD (n=3). (d) In vitro pull-down assay was performed using polyUb2–7 linked by K48 (left) or K63 (right) and GST-MIU fusion proteins of RNF168 (residues 100–500). Immunoblotting was conducted using anti-Ub antibody. F-T: flow-through.
The likely reason that we and others failed to detect endogenous K48-linked ubiquitin chain formation at DSB sites is the rapid turnover of K48 chains. To overcome this problem, we used the MIU domains of RNF168 (residues 100–500), which binds ubiquitin conjugates at DNA breaks generated by RNF8 and RNF16816,17 without any marked preference for K48- and K63-linkages (Fig. 1d). We speculated that ubiquitin binding ability of RNF168 MIUs could stabilize ubiquitin chains at DSB sites by reducing the accessibility of deubiquitinating enzymes (DUBs) and/or the 26S proteasome, and thus might allow us to detect endogenous K48-linked ubiquitin chains at DSB sites. Indeed, expression of RNF168 MIUs markedly promoted the foci formation of K48- as well as K63-linked ubiquitin chains (Fig. 1a, right panel), suggesting that K48-linked ubiquitin chains form at DSB sites. Unlike MIUs of RNF168, which binds to both K48- and K63-linked ubiquitin chains, the two UIM domains of RAP80 specifically associate with K63- but not K48-linked ubiquitin chains19,22,23. Accordingly, the expression of RAP80 UIMs increased K63-linked ubiquitin chains at DSB sites but had no effect on K48 staining (Fig. 1b and c). To further rule out any possible cross-reactions of linkage specific antibodies, we used 293T cells stably expressing Flag-tagged K48-only or K63-only mutant of ubiquitin and confirmed the above results (Supplementary Fig. 1c). In addition, RNF8 did not promote lysine-less ubiquitin mutant K0 to form foci in response to IR (Supplementary Fig. 1d), and neither RNF8 nor K48 could form foci in the absence of IR (Supplementary Fig. 1e). Together, these data suggest that K48-linked ubiquitin chains normally accumulate at DSB sites, but they are hard to detect due to their rapid turnover.
UBC13 is only required for K63-linked ubiquitin chain formation
UBC13 is the only known E2 ubiquitin conjugating enzyme that specifically assembles K63-linked chains9. Previous studies have shown that depletion of either RNF8 or UBC13 abolished ubiquitin conjugates (FK2) and K63 foci formation at DSB sites12,13,15. Moreover, UBC13-RNF8 interaction was revealed by yeast-two hybrid assay24. While RNF8 and UBC13 may in part work together, it is unlikely that UBC13 would be involved in RNF8-dependent K48 chain formation.
To directly test the requirement of UBC13 for these E3 ligases, we expressed RNF8 or RNF168 in wild-type and Ubc13−/− MEF cells. Not surprisingly, RNF168 failed to enhance the formation of either K63-linked chains (Fig. 2a, upper panel) or total ubiquitin conjugates detected by FK2 antibody (Fig. 2a, lower panel) in Ubc13−/− MEFs, whereas the ability of RNF8 to promote K48 chain formation occurred independent of Ubc13 (Fig. 2b). Therefore, UBC13 is a physiological E2 partner for RNF168, but it is dispensable for RNF8 to catalyze K48-linked ubiquitin chain formation.
Fig 2. RNF8 promotes K48-linked ubiquitin chain formation independent of UBC13.
Wild-type or Ubc13−/− MEFs were transfected with constructs encoding Flag-tagged RNF168 (a) or RNF8 (b). Experiments were carried out as those described in Fig. 1a.
RNF8 promotes KU80 and CHK2 degradation
A typical feature of most E3 ligases is the ability to catalyze their own ubiquitination25,26. We found that RNF8 could auto-ubiquitinate and degrade itself through its RING domain both in vivo and in vitro (Supplementary Fig. 2 and 3), and the auto-ubiqutination chains formed in vivo are K48-linked (Supplementary Fig. 2f). These data further substantiated the notion that RNF8 mainly synthesizes K48-linked ubiquitin chains, which lead to protein degradation by the proteasome-dependent pathway. However, it is unlikely that the sole function of RNF8 is to remove itself from DSB sites. We suspected that there should be other RNF8 substrates that are important for DNA damage response. Thus, we tried to identify physiological substrate(s) of RNF8, which might be unstable at chromatin.
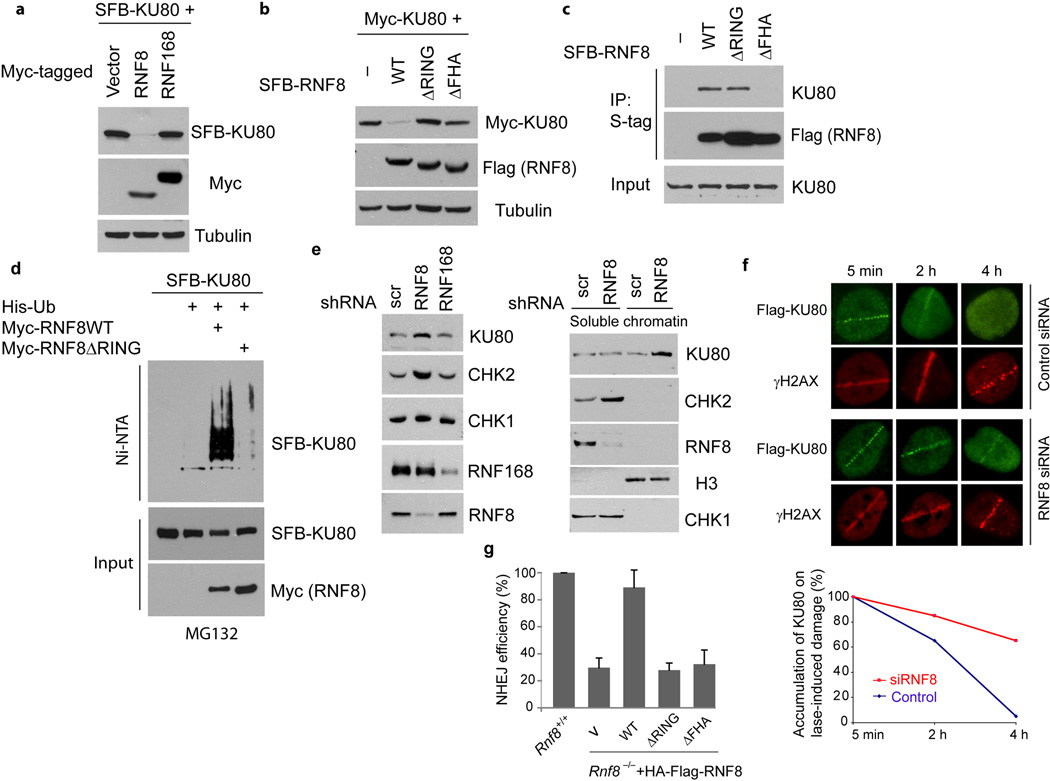
A recent study showed that KU80 is ubiquitinated through K48-linkage at DSB sites and this ubiquitination of KU80 is required for the removal of KU80 from DNA27. This finding prompted us to test the possibility that RNF8 might trigger the degradation of KU80. Indeed, expression of RNF8 dramatically reduced the protein level of epitope-tagged KU80, while the expression of RNF168 did not affect KU80 protein level (Fig. 3a). Furthermore, deletion of RING domain as well as deletion of FHA domain abrogated the ability of RNF8 to degrade KU80 (Fig. 3b). In addition, both wild-type and the ΔRING mutant of RNF8, but not the FHA deletion mutant, bound to KU80 (Fig. 3c), implying that the binding of KU80 to RNF8 is required for its de-stabilization. Moreover, RNF8 efficiently promoted ubiquitination of KU80 in vivo, which requires the intact RING domain of RNF8 (Fig. 3d). Depletion of endogenous RNF8 in HeLa cells increased the protein level of KU80 (Fig. 3e, left panel), again confirming that RNF8 promotes KU80 degradation.
Fig 3. RNF8 regulates the expression of KU80 and CHK2 in vivo.
(a) RNF8, but not RNF168, mediates the degradation of KU80. HeLa cells were transiently transfected with plasmids encoding S-Flag-SBP (SFB)-tagged KU80 in the presence or absence of plasmids encoding Myc-RNF8 or Myc-RNF168. Cell lysates were immunoblotted with antibodies as indicated. (b) Both RING domain and FHA domain of RNF8 are required for the degradation of KU80 by RNF8. HeLa cells were transiently transfected with plasmids encoding SFB-KU80 along with empty vector or constructs for SFB-tagged wild-type (WT), ΔRING or ΔFHA mutants of RNF8. Cells were harvested and extracts were examined by immunoblotting as indicated. (c) RNF8 binds to KU80. 293T cells were transiently transfected with plasmids encoding various proteins as indicated. Immunoprecipitation (IP) was conducted with S beads and probed with anti-KU80 antibody. (d) RNF8 ubiquitinates KU80 in vivo. HeLa cells were transfected with expression constructs encoding His-tagged ubiquitin (Ub), SFB-KU80 and Myc-RNF8 or ΔRING mutant of RNF8 as indicated. Pulldown and immunoblotting analysis was conducted as indicated. (e) Depletion of RNF8 but not of RNF168 increases the levels of KU80 and CHK2. Control, RNF8 and RNF168 stable knockdown cells were harvested. Total protein levels (left panel) or soluble and chromatin fractions of cell lysates (right panel) were analyzed by immunoblotting using indicated antibodies. (f) Persistence of KU80 proteins at laser induced DNA damage sites in the absence of RNF8. U2OS cells stably expressing Flag-KU80 were transfected with control or RNF8 siRNAs. 72 hrs later, cells were laser micro-irradiated and fixed at indicated times. Quantification was shown in the bottom panel and more than 25 γH2AX-positive cells were counted per condition. (g) Non-homologous end joining (NHEJ) efficiencies in Rnf8+/+ or Rnf8−/− MEFs reconstituted with empty vector (V), wild-type RNF8, ΔRING or ΔFHA mutants were determined and presented as mean ± SD (n=3).
Next we explored the physiological significance of KU80 degradation promoted by RNF8. KU forms ring-like structure and completely encircles the DNA double-strand break ends, which may translocate inward or be removed to provide room for DNA-PKcs and other repair factors that process the DNA ends prior to ligation (see review28). Fractionation experiments showed that the levels of KU80 increased in chromatin fraction in RNF8-depleted cells (Fig. 3e, right panel), suggesting that RNF8 may specifically target KU degradation or removal at DSB sites. To test this possibility, we used laser-induced DNA damage for the visualization of KU80 accumulation at DNA damage sites. As previously reported29,30, immediate but transient accumulation of KU80 at damage sites was observed following laser microirradiation treatment (Fig. 3f). However, depletion of RNF8 markedly prolonged the retention of KU80 at laser-induced DNA damage sites (Fig. 3f), implying that the degradation of KU80 by RNF8 is required for the removal of KU80 from DSBs. Consistent with the requirement of RNF8 for KU80 turnover at damage sites, Rnf8−/− cells displayed reduced non-homologous end joining (NHEJ) repair as assessed by a previously established plasmid-based end-joining assay31, and only reintroduction of wild-type RNF8, but not RING- or FHA-deletion mutants, was able to rescue this NHEJ repair deficiency (Fig. 3g). Together, these data suggest that RNF8 may target the removal of KU80 at sites of DNA damage and thus facilitate NHEJ.
Besides KU80, CHK2 is another possible substrate of RNF8 based on its up-regulation in T cells from Rnf8-deficient mice32. CHK2 protein level was also elevated in human RNF8 knockdown cells, whereas as the expression of CHK1 was unperturbed (Fig. 3e). Moreover, depletion of RNF168 did not affect KU80 or CHK2 levels (Fig. 3e), suggesting that RNF8 has a distinct set of substrates that are independent of RNF168. Similar to KU80, CHK2 also associated with RNF8 and was ubiquitinated by RNF8 in vivo (Supplementary Fig. 4a–c). Furthermore both abundance and activation of CHK2 in response to IR were elevated in RNF8 depleted cells (Supplementary Fig. 4d). Collectively, these data support that RNF8 has two additional physiological substrates: KU80 and CHK2.
Kinetics of distinct ubiquitin chain formation at DNA damage sites
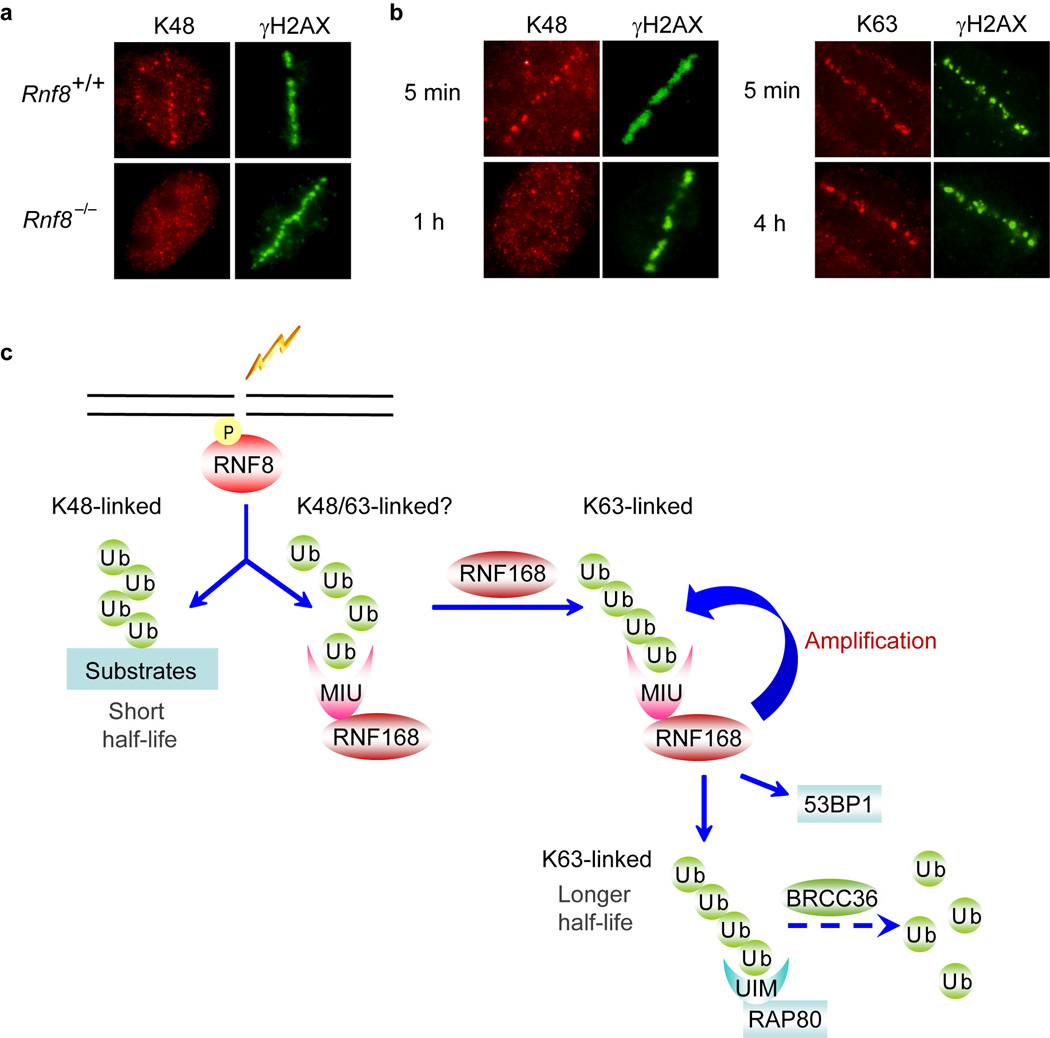
The localization of K48-linked ubiquitin chains at ionizing radiation (IR)–induced damage sites could not be cytologically detected without ectopically expression of RNF8 or MIU domains of RNF168 (Fig. 1), which might be caused by subdetectable amount of these ubiquitin chains at sites of DNA breaks. In order to detect endogenous K48-linked ubiquitin chain formation at DNA damage sites, we employed laser microirradiation method. Our results revealed that endogenous K48-linked ubiquitin chains accumulated at the laser-induced damage sites and this accumulation is RNF8-dependent (Fig. 4a). These data support our conclusion that RNF8 generates K48-linked ubiquitin chains at DSBs. Next we studied the kinetics of different ubiquitin chain formation at DNA damage sites. As expected, the formation of K48-linked ubiquitin chains was transient, since it appeared rapidly at laser-induced damage sites and then quickly diminished, became undetectable one hour after irradiation (Fig. 4b). In contrast, the K63-linked ubiquitin chains remained at laser-induced DNA damage sites 4 hours post-microirradiaton (Fig. 4b). These data agree with the notion that K48 linkage serves as proteolytic signal and targets substrates for degradation, while K63 linkage is involved in non-proteolytic pathway and mainly plays a role as signaling molecules in DNA damage response.
Fig 4. Accumulation of K48-linked and K63-linked ubiquitin chains at DNA damage sites.
(a) Rnf8+/+ and Rnf8−/− MEF cells were treated with laser mirco-irradiation and immunostained 5 minutes later using indicated antibodies. (b) Time course analysis of K48-linked and K63-linked ubiquitin chain formation at DNA damage sites. U2OS cells were fixed at the indicated time points after microirradiation. (c) Working model of the possible roles of RNF8 and RNF168 in DNA damage response.
DISCUSSION
Ubiquitination modification plays an important role in DNA damage response. In this study we showed that the two RING finger E3 ubiquitin ligases involved in DNA damage pathway differ significantly in the specific ubiquitin linkages they catalyze. RNF168 acts with E2 conjugating enzyme UBC13 to synthesize K63-linked ubiquitin chains. On the other hand, RNF8 mainly promotes the assembly of K48-linked ubiquitin chains, which are independent of UBC13. While we cannot rule out the possibility that RNF8 may also have limited roles in UBC13-dependent assembly of K63-linked ubiquitin chains and/or promote mono- or di-ubiquitination of some of its substrates, our data clearly demonstrate that RNF8 has an unexpected role in promoting protein degradation.
Indeed, the specificities of ubiquitin chain linkage assembled by RNF8 and RNF168 correlate with different biological effects on their substrates. RNF168 is the major E3 ligase that catalyzes K63-linked ubiquitination of histone H2A/H2AX, which are recognized by the tandem UIM domains of RAP80 and thereby recruiting the BRCA1-A complex to sites of DNA lesions33–35. The di-ubiquitinated H2A (K63-linked) is abundant and relatively stable in chromatin following DNA damage18, which is consistent with the general idea that K63-linked ubiquitin chains are associated with non-proteolytic functions such as signal transduction. At sites of DNA damage, the K63-linked chains are slowly hydrolyzed by a K63-specific deubiquinating enzyme BRCC36, a component of BRCA1-A complex18. The exact function of this slow turn-over of K63-linked ubiquitin chains at DSB sites remains to be determined.
The major function of RNF8 is to promote the assembly of K48-linked ubiquitin chains. We showed this by the use of K48 linkage specific antibody (Figs. 1 and 2) and a cell line expressing an epitope-tagged K48 only ubiquitin mutant (Supplementary Fig. 1c). In addition, we were able to demonstrate the existing of endogenous K48 chain formation at DSB sites by the use of RNF168 MIU domains (Fig. 1a), which bind to K48-linked ubiquitin chains36 and stabilize them in the cell. Furthermore the accumulation of endogenous K48-linked ubiquitin chains can be detected by laser micro-irradiation (Fig. 4a,b). K48 specific polyubiquitination normally targets substrates for degradation. In support of our working hypothesis that RNF8 promotes K48-linked ubiquitin chain formation, we showed that RNF8 regulates the degradation of several of its substrates, including KU80 and CHK2, both are highly dynamic at DSB sites and do not show discernible IRIF29,37. Similar to other NHEJ repair proteins, the accumulation of KU at laser-induced DNA damage regions is very rapid but transient (29,30 and Fig. 3g). KU forms a ring-like structure and its central channel is large enough to encircle a single duplex of DNA38. It was speculated that KU should be removed from DSB ends, since KU trapped on DNA would impair not only DNA repair but also basic cellular processes such as transcription and replication. However, the factor(s) responsible for the removal of KU from DNA ends has not been identified. Our discovery, that RNF8 regulates the turnover of KU80 at damage sites and loss of RNF8 profoundly impairs NHEJ efficiency, suggests that RNF8 is at least one of the factors involved in the removal of KU from DNA ends. In addition, we identified checkpoint protein CHK2 as another substrate of RNF8. Depletion of RNF8 increased the abundance and activity of CHK2 following DNA damage (Supplementary Fig. 4d). CHK2 is phosphorylated by ATM at DSBs and then rapidly diffuses throughout the nucleus to transmit checkpoint signals37. The functional significance of RNF8-dependent CHK2 turnover is not clear, since unique substrates of CHK2 remain elusive39.
Lok et al. have recently published a study demonstrating that RNF8 catalyzes K48-linked ubiquitin chain formation and targets itself for degradation40. This study agrees with an earlier study41 and our observations here, particularly the data presented in Supplementary Figures 2 and 3. It remains unclear whether or not RNF8 also promotes K63-linked ubiquitin chain formation. Based on the observations that RNF8 overexpression did not enhance K63 staining in vivo (Fig. 1a and Fig. 2b), we speculate that the major ligase activity of RNF8 is to promote K48-linked, but not K63-linked, polyubiquitination. However, we could not rule out the possibility that RNF8 might have limited activity towards K63-linked ubiquitin chains, which may be sufficient to recruit RNF168 and initiate RNF168-dependent DNA damage signaling pathway. Nevertheless, RNF168 is the major E3 ligase that promotes K63-linked ubiquitin chain formation at DNA damage sites. RNF168 recognizes both K48- and K63-linked ubiquitin chains (Fig. 1d). This property of RNF168 allows it to recognize its own products and thus set off a positive feedback loop to further promote the accumulation of K63-linked ubiquitin chain formation at DSB sites (Fig. 4c), which is important for the accumulation of 53BP1, BRCA1, and other damage repair proteins. In this context, RNF8 plays two distinct roles at DNA damage sites: one is to recruit other DNA damage repair proteins to DSB sites via the action of RNF168; the other is to remove a number of proteins from DNA damages sites via RNF8-dependent K48-specific ubiquitin chain formation. Both of these roles of RNF8 are important in DNA damage signaling and repair pathways (Fig. 4c).
METHODS
Antibodies and reagents
The following antibodies were used in this study: anti-Myc and anti-CHK1 antibodies (Santa Cruz), anti-Flag and anti-α-tubulin antibodies (Sigma), anti-KU80 antibodies (Cell Signaling), anti-FK2 and anti-ubiquitin antibodies (Upstate), anti-GAPDH, anti-K48 (clone Apu2), and anti-K63 (clone Apu3) antibodies (Millipore). Anti-53BP1, CHK2 and γH2AX antibodies were described previously43, while anti-RNF8 and RNF168 antibodies were gifts from Michael S.Y. Huen (The University of Hong Kong). Poly-ubiquitin chains (Ub2–7, K48-linked or K63-linked) were purchased from Boston Biochem. Plasmids encoding His-tagged wild-type Ub, K48 and K63 only mutants were gifts from Richard Baer (Columbia University).
Cell culture and transfection
The culture of human cells has been described previously18. Mouse embryonic fibroblasts (MEFs) derived from Rnf8 knockout mice were cultured in DMEM supplemented with 15% FBS and maintained in 5% CO2 at 37°C. To reconstitute Rnf8−/− MEFs with wild-type or mutant RNF8, BOSC23 cells were transfected with pCL-ampho and HA-Flag-tagged expression constructs. Viral supernatant was collected 48 hours post-transfection and was used for infection. Stable clones of infected MEFs were selected in the presence of 2 µg ml−1 puromycin. Transient transfection of HeLa and 293T cells was performed with the polyethylenimine (25 kDa) method and cells were routinely collected 24 to 48 hours post-transfection for further analysis. SiRNA transfection was performed using Oligofectamine (Invitrogen) following the manufacturer’s instruction. Transfection was repeated three times with an interval of 24 hours to achieve maximal RNAi effect.
siRNA, shRNAs and stable knockdown cell lines
The sequence for RNF8 siRNA was previously described13; shRNAs targeting RNF8 and RNF168 were cloned in the pLKO.1 vector (Open Biosystems). Sequences of scramble shRNA or shRNAs against RNF8 and RNF168 are 5’-CCTAAGGTTAAGTCGCClCTCG-3’, 5’-CCAAAGAATGACCAAATGATA-3’ and 5’-GCAGTCAGTTAATAGAAGAAA-3’, respectively. To generate stable knockdown cells, shRNAs were packaged into lentiviruses by co-transfecting with packaging plasmids pMD2G and pSPAX2 (kindly provided by Professor Songyang Zhou, Baylor College of Medicine) into 293T cells. 48 hours post-transfection, the supernatant was collected and was used for infection. Stable pools were selected with medium containing 2 µ g ml−1 puromycin.
GST pull-down assay
GST-MIU domains of RNF168 (residues100–500) fusion proteins immobilized onto Glutathione Sepharose 4B beads (GE Healthcare) were incubated with 0.25 µg synthetic polyUb2–7 linked by K48 or K63 in NETN buffer (20 mM Tris-HCl, pH8.0, 100 mM NaCl, 1 mM EDTA, 0.5 mM DTT, 0.5% NP-40 and 1 mM PMSF) for 4 h at 4 °C, both bound and unbound (flow-through, F-T) proteins were collected and resolved on 12.5% SDS-PAGE, followed by immunoblotting.
In vivo ubiquitination assay
In vivo ubiquitination assays were performed as described previously18. Briefly, HeLa or 293T cells were transfected with the indicated plasmids. 36 hours after transfection, cells were treated with 20 µM MG132 for additional 10 hours before they were collected and lysed in denaturing buffer (6M guanidine-HCl, 0.1 M Na2HPO4/NaH2PO4, pH8.0, 10 mM imidazole), followed by affinity purification with nickel-nitrilotriacetic acid (Ni-NTA) resins (Sigma) and immunoblotting analysis.
Non-homologous end-joining (NHEJ) assay
DNA repair by NHEJ was assessed by a cell-based plasmid-integration assay31. Briefly, MEF cells were transfected with BamH1/Xho1 linearized pcDNA3.1/hygro (Invitrogen) along with the pEGFP-C1 plasmid by electroporation (220V, 950 µF). 24 hrs later cells were collected, counted and plated on two plates. One day after plating, one plate of cells were fixed to determine transfection efficiency by EGFP expression and the other plate were incubated in selective media containing 100 µg ml−1 hygromycin for 14 days at 37 °C to allow colony formation. Colonies were stained with 2% methylene blue and the numbers of colonies were determined. Random-plasmid integration events were normalized for transfection and plating efficiencies.
Laser micro-irradiation
Cells were seeded on 35 mm glass bottom dishes (MatTek Corporation) for overnight prior to visualize with a Nikon Elipse TE2000-U inverted microsope and micro-irradiated with a Micropoint Ablation System (Photonics Instruments, St. Charles, IL, USA). The laser output was set to 35%, which was the lowest power that reproducibly gave a focused γH2AX- stripe. Typically an average of 25 cells were microirradiated within 5 min.
Histone extraction, Immunoprecipitation and Immunofluorescence microscopy
These assays have been described previously18,34. Of note, for all the K48 staining, cells were pre-extracted in extraction buffer (20 mM HEPES, pH7.4, 50 mM NaCl, 3 mM MgCl2, 0.3 M sucrose and 0.5% Triton X-100) for 8 min on ice, then fixed in 4% paraformaldehyde for 15 min on ice.
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues in the Chen’s laboratory for insightful discussions and technical assistance. We also thank Xiaoshan Zhang and Henry P. Adams for technical assistance on laser microirradiation. This work was supported in part by grants from the National Institutes of Health (CA089239 and CA092312 to J.C.). J.C. is a recipient of an Era of Hope Scholar award from the Department of Defense (W81XWH-05-1-0470) and a member of MD Anderson Cancer Center (CA016672).
Footnotes
AUTHOR CONTRIBUTIONS
L.F. designed and carried out the experiments. J.C. advised on the design of the experiments.
L.F. and J.C. were responsible for the preparation of the manuscript.
Please see Supplementary Methods section for additional information.
REFERENCES
- 1.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 2.Komander D, et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddins MJ, Varadan R, Fushman D, Pickart CM, Wolberger C. Crystal structure and solution NMR studies of Lys48-linked tetraubiquitin at neutral pH. J Mol Biol. 2007;367:204–211. doi: 10.1016/j.jmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 4.Chau V, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto ML, et al. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell. 39:477–484. doi: 10.1016/j.molcel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 7.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 11:479–489. doi: 10.1038/nrm2921. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 10.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem. 2003;278:34743–34746. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa H, et al. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:3916–3924. doi: 10.1074/jbc.M308540200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao GY, et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolas NK, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mailand N, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Doil C, et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Stewart GS, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Feng L, Wang J, Chen J. The Lys63-specific deubiquitinating enzyme BRCC36 is regulated by two scaffold proteins localizing in different subcellular compartments. J Biol Chem. 285:30982–30988. doi: 10.1074/jbc.M110.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobhian B, et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science. 2007;316:1198–1202. doi: 10.1126/science.1139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Fujimuro M, Sawada H, Yokosawa H. Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett. 1994;349:173–180. doi: 10.1016/0014-5793(94)00647-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Chen J, Yu X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science. 2007;316:1202–1205. doi: 10.1126/science.1139621. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plans V, et al. The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation. J Cell Biochem. 2006;97:572–582. doi: 10.1002/jcb.20587. [DOI] [PubMed] [Google Scholar]
- 25.Lorick KL, et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci U S A. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 27.Postow L, et al. Ku80 removal from DNA through double strand break-induced ubiquitylation. J Cell Biol. 2008;182:467–479. doi: 10.1083/jcb.200802146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, et al. Independent and sequential recruitment of NHEJ and HR factors to DNA damage sites in mammalian cells. J Cell Biol. 2005;170:341–347. doi: 10.1083/jcb.200411083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mari PO, et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci U S A. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanty Y, et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009;462:935–939. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, et al. Rnf8 deficiency impairs class switch recombination, spermatogenesis, and genomic integrity and predisposes for cancer. J Exp Med. 207:983–997. doi: 10.1084/jem.20092437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao G, et al. MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 2009;23:740–754. doi: 10.1101/gad.1739609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng L, Huang J, Chen J. MERIT40 facilitates BRCA1 localization and DNA damage repair. Genes Dev. 2009;23:719–728. doi: 10.1101/gad.1770609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Hurov K, Hofmann K, Elledge SJ. NBA1, a new player in the Brca1 A complex, is required for DNA damage resistance and checkpoint control. Genes Dev. 2009;23:729–739. doi: 10.1101/gad.1770309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penengo L, et al. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell. 2006;124:1183–1195. doi: 10.1016/j.cell.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol. 2003;5:255–260. doi: 10.1038/ncb945. [DOI] [PubMed] [Google Scholar]
- 38.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 39.Antoni L, Sodha N, Collins I, Garrett MD. CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat Rev Cancer. 2007;7:925–936. doi: 10.1038/nrc2251. [DOI] [PubMed] [Google Scholar]
- 40.Lok GT, et al. Differential regulation of RNF8-mediated Lys48- and Lys63-based poly-ubiquitylation. Nucleic Acids Res. doi: 10.1093/nar/gkr655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bekker-Jensen S, et al. HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat Cell Biol. 12:80–86. doi: 10.1038/ncb2008. sup pp 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.