Abstract
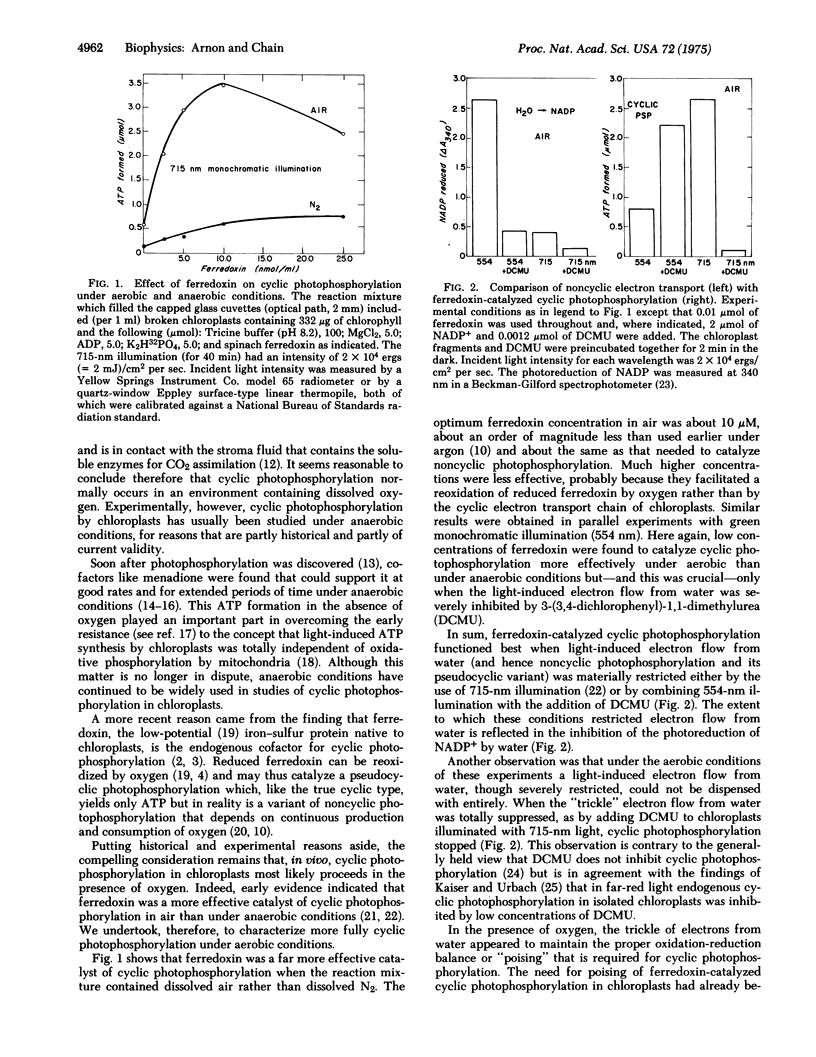
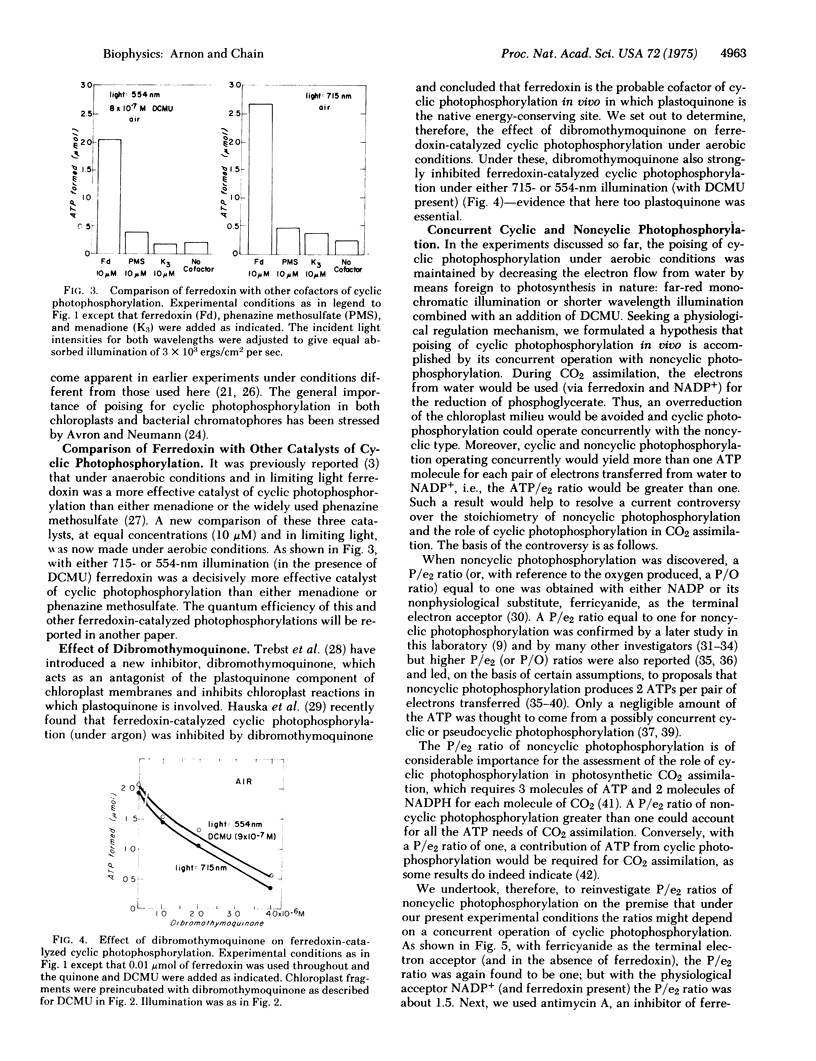
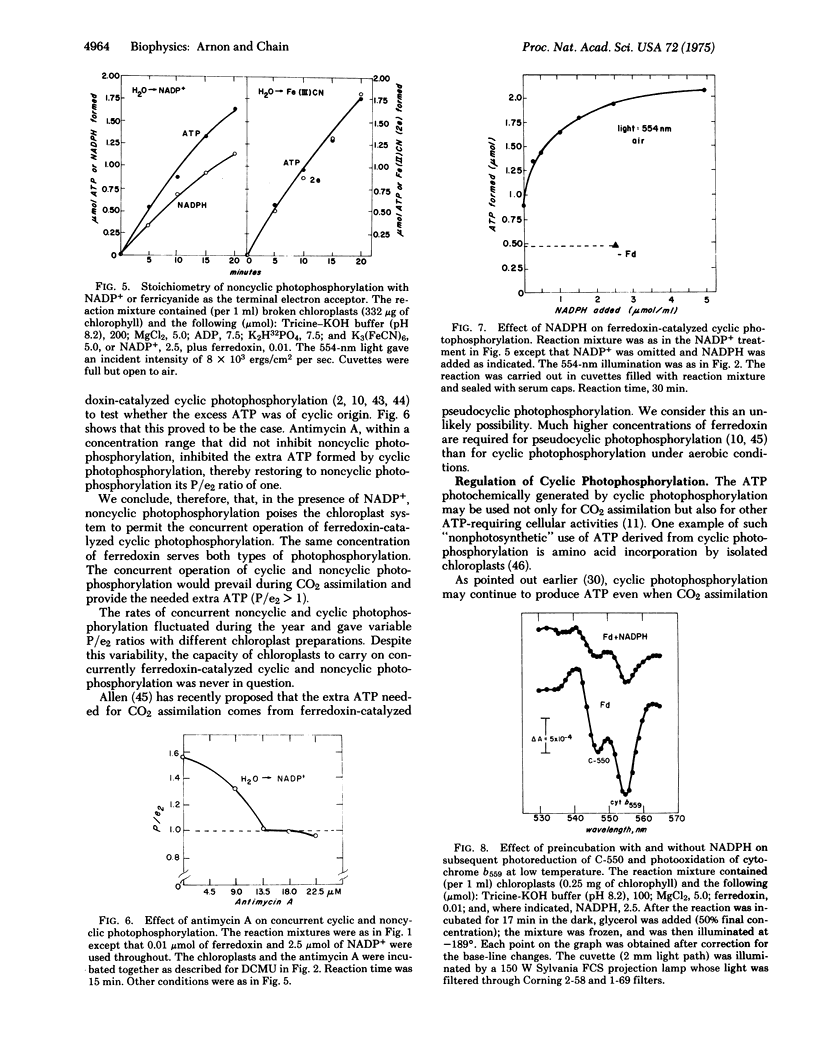
Under aerobic conditions that are likely to prevail in chloroplasts in vivo, the optimal concentration of ferredoxin for cyclic photophosphorylation was found to be equal to that required for NADP reduction and about one-tenth of that needed for cyclic photophosphorylation under anaerobic conditions. In the presence of ferredoxin and NADP, cyclic photophosphorylation operated concurrently with noncyclic photophosphorylation, producing an ATP: NADPH ratio of about 1.5. The effective operation of ferredoxin-catalyzed cyclic photophosphorylation by itself required a curtailment of the electron flow from water which was accomplished experimentally by the use of either an inhibitor or far-red monochromatic light. An unexpected discovery was that the operation of cyclic photophosphorylation by itself was also regulated by a back reaction of NADPH and ferredoxin with two components of chloroplast membranes, component C550 and cytochrome b559. The significance of these findings to photosynthesis in vivo is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B., WHATLEY F. R., ARNON D. I. Photosynthesis by isolated chloroplasts. VI. Rates of conversion of light into chemical energy in photosynthetic phosphorylation. Biochim Biophys Acta. 1958 Jan;27(1):16–23. doi: 10.1016/0006-3002(58)90288-9. [DOI] [PubMed] [Google Scholar]
- ARNON D. I., ALLEN M. B., WHATLEY F. R. Photosynthesis by isolated chloroplasts. Nature. 1954 Aug 28;174(4426):394–396. doi: 10.1038/174394a0. [DOI] [PubMed] [Google Scholar]
- ARNON D. I., LOSADA M., WHATLEY F. R., TSUJIMOTO H. Y., HALL D. O., HORTON A. A. Photosynthetic phosphorylation and molecular oxygen. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1314–1334. doi: 10.1073/pnas.47.9.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNON D. I. The chloroplast as a complete photosynthetic unit. Science. 1955 Jul 1;122(3157):9–16. doi: 10.1126/science.122.3157.9. [DOI] [PubMed] [Google Scholar]
- ARNON D. I., WHATLEY F. R., ALLEN M. B. Vitamin K as a cofactor of photosynthetic phosphorylation. Biochim Biophys Acta. 1955 Apr;16(4):607–608. doi: 10.1016/0006-3002(55)90295-x. [DOI] [PubMed] [Google Scholar]
- AVRON M., JAGENDORF A. T. Interactions of catalytic cofactors for photosynthetic phosphorylation with Hill reaction oxidants. J Biol Chem. 1959 May;234(5):1315–1320. [PubMed] [Google Scholar]
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Whatley F. R., Allen M. B. Assimilatory Power in Photosynthesis: Photosynthetic phosphorylation by isolated chloroplasts is coupled with TPN reduction. Science. 1958 May 2;127(3305):1026–1034. doi: 10.1126/science.127.3305.1026. [DOI] [PubMed] [Google Scholar]
- Del Campo F. F., Ramírez J. M., Arnon D. I. Stoichiometry of photosynthetic phosphorylation. J Biol Chem. 1968 May 25;243(10):2805–2809. [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- HAGIHARA B., LARDY H. A. A method for the separation of orthophosphate from other phosphate compounds. J Biol Chem. 1960 Mar;235:889–894. [PubMed] [Google Scholar]
- Hall D. O., Reeves S. G., Baltscheffsky H. Photosynthetic control in isolated spinach chloroplasts with endogenous and artificial electron acceptors. Biochem Biophys Res Commun. 1971 Apr 16;43(2):359–366. doi: 10.1016/0006-291x(71)90761-3. [DOI] [PubMed] [Google Scholar]
- Hauska G., Reimer S., Trebst A. Native and artificial energy-conserving sites in cyclic photophosphorylation systems. Biochim Biophys Acta. 1974 Jul 25;357(1):1–13. doi: 10.1016/0005-2728(74)90106-6. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. The stoichiometric relation of phosphorylation to electron transport in isolated chloroplasts. Biochim Biophys Acta. 1968 Oct 1;162(3):380–391. doi: 10.1016/0005-2728(68)90124-2. [DOI] [PubMed] [Google Scholar]
- JAGENDORF A. T., AVRON M. Cofactors and rates of photosynthetic phosphorylation by spinach chloroplasts. J Biol Chem. 1958 Mar;231(1):277–290. [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci U S A. 1969 Jul;63(3):963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J. M., Campo F. F., Arnon D. I. Photosynthetic phosphorylation as energy source for protein synthesis and carbon dioxide assimilation by chloroplasts. Proc Natl Acad Sci U S A. 1968 Feb;59(2):606–612. doi: 10.1073/pnas.59.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S. G., Hall D. O. The stoichiometry (ATP-2e- ratio) of non-cyclic photophosphorylation in isolated spinach chloroplasts. Biochim Biophys Acta. 1973 Jul 26;314(1):66–78. doi: 10.1016/0005-2728(73)90064-9. [DOI] [PubMed] [Google Scholar]
- SHIN M., ARNON D. I. ENZYMIC MECHANISMS OF PYRIDINE NUCLEOTIDE REDUCTION IN CHLOROPLASTS. J Biol Chem. 1965 Mar;240:1405–1411. [PubMed] [Google Scholar]
- STILLER M., VENNESLAND B. Photophosphorylation accompanying the Hill reaction with ferricyanide. Biochim Biophys Acta. 1962 Jul 16;60:562–579. doi: 10.1016/0006-3002(62)90875-2. [DOI] [PubMed] [Google Scholar]
- Saha S., Good N. E. Products of the photophosphorylation reaction. J Biol Chem. 1970 Oct 10;245(19):5017–5021. [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B., Arnon D. I. Role of cyclic photophosphorylation in photosynthetic carbon dioxide assimilation by isolated chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):111–124. doi: 10.1016/0005-2728(72)90143-0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. ANALYSIS OF PHOTOSYNTHETIC REACTIONS BY THE USE OF MONOCHROMATIC LIGHT. Nature. 1963 Sep 28;199:1247–1252. doi: 10.1038/1991247a0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci U S A. 1963 Apr;49:567–572. doi: 10.1073/pnas.49.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. SEPARATION BY MONOCHROMATIC LIGHT OF PHOTOSYNTHETIC PHOSPHORYLATION FROM OXYGEN EVOLUTION. Proc Natl Acad Sci U S A. 1963 Sep;50:544–549. doi: 10.1073/pnas.50.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]
- WHATLEY F. R., ALLEN M. B., ROSENBERG L. L., CAPINDALE J. B., ARNON D. I. Photosynthesis by isolated chloroplasts. V. Phosphorylation and carbon dioxide fixation by broken chloroplasts. Biochim Biophys Acta. 1956 Jun;20(3):462–468. doi: 10.1016/0006-3002(56)90340-7. [DOI] [PubMed] [Google Scholar]
- West K. R., Wiskich J. T. Evidence for two phosphorylation sites associated with the electron transport chain of chloroplasts. Biochim Biophys Acta. 1973 Jan 18;292(1):197–205. doi: 10.1016/0005-2728(73)90264-8. [DOI] [PubMed] [Google Scholar]
- Winget G. D., Izawa S., Good N. E. The stoichiometry of photophosphorylation. Biochem Biophys Res Commun. 1965 Dec 9;21(5):438–443. doi: 10.1016/0006-291x(65)90401-8. [DOI] [PubMed] [Google Scholar]


