Abstract
Objective
To estimate age-specific probabilities of live-birth with oocyte cryopreservation in non-donor (ND) egg cycles.
Design
Individual patient data (IPD) meta-analysis.
Setting
Assisted reproduction centers.
Patients
Infertile patients undergoing ND mature oocyte cryopreservation.
Interventions
PubMed was searched for the clinical studies on oocyte cryopreservation from January 1996 through July 2011. Randomized and non-randomized studies that used ND frozen-thawed mature oocytes with pregnancy outcomes were included. Authors of eligible studies were contacted to obtain IPD.
Main outcome measures
Live-birth probabilities based on age, cryopreservation method, and the number of oocytes thawed, injected, or embryos transferred.
Results
Original data from 10 studies including 2265 cycles from 1805 patients were obtained. Live-birth success rates declined with age regardless of the freezing technique. Despite this age-induced compromise, live-births continued to occur as late as to the ages of 42 and 44 with slowly-frozen (SF) and vitrified (VF) oocytes, respectively. Estimated probabilities of live-birth for VF oocytes were higher than those for SF.
Conclusions
The live-birth probabilities we calculated would enable more accurate counseling and informed decision of infertile women who consider oocyte cryopreservation. Given the success probabilities, we suggest that policy-makers should consider oocyte freezing as an integral part of prevention and treatment of infertility.
Keywords: oocyte cryopreservation, slow freezing, vitrification, meta-analysis, individual patient data
INTRODUCTION
After embryo cryopreservation, oocyte cryopreservation is the second most commonly used method of fertility preservation for medical indications (1,2). In addition, oocyte cryopreservation can be considered when there are ethical, legal, and/or religious obstacles to embryo cryopreservation (3), and more controversially, for defying reproductive aging (4,5). The technique can also be used to establish donor oocyte banks (6-8) as well as to minimize the risk of ovarian hyperstimulation syndrome.
More than 50% of the assisted reproductive technology (ART) clinics in the United States currently offer oocyte cryopreservation (5) and “elective use” to defer childbearing is cited as the most common indication (64%), followed by IVF (18%) and medical reasons (18%) (5). After much debate, American Society of Reproductive Medicine (ASRM) has recently removed oocyte freezing from the experimental category for the patients who are unable to cryopreserve embryos and facing infertility due to chemotherapy or other gonadotoxic therapies, but not for the sole purpose of circumventing reproductive aging in healthy women (9). However oocyte cryopreservation is still considered experimental by the health insurance industry, as a result, a non-covered service for women’s health (10).
One of the most critical questions unanswered about oocyte cryopreservation is the success rates among different age groups, especially later reproductive years. Lack of age-specific success rate information is one of the likely reasons for the overall reluctance to accept this technique as a standard treatment. Despite the fact that no study reported on age-specific success rates, the majority of the clinics consider age greater than 38 years to be acceptable for elective oocyte cryopreservation (5). Age-specific live-birth success information is essential in evidence-based medicine to be able to properly counsel women prior to oocyte cryopreservation so that they can weigh alternatives (such as embryo freezing) for fertility preservation and to determine the feasibility and utility of performing oocyte cryopreservation at a given age.
The main methods of oocyte cryopreservation are slow freezing (SF) and vitrification (VF), with the latter gaining more popularity in recent years. In an earlier traditional meta-analysis based on summary statistics published, we investigated the overall success of oocyte cryopreservation by SF and VF, and compared it to that of IVF with fresh oocytes (11). In the current study, we collected individual cycle data from 2265 oocyte cryopreservation freeze-thaw cycles in 1805 patients, and performed a novel individual patient data (IPD) meta-analysis. Our goal was to determine the probability of live birth as a function of age, cryopreservation method (SF vs. VF), and, number of oocytes thawed, injected, or embryos transferred in non-donor oocyte (NDO) cycles of infertile patients.
MATERIALS AND METHODS
This study was planned as a meta-analysis of IPD from randomized and non-randomized studies on oocyte cryopreservation. Oocyte donors are younger and do not adequately represent infertile population (7,12); hence we did not include donor oocyte (DO) cycles in the present study. We did not restrict our meta-analysis to randomized controlled trials (RCT), as there has been only one RCT with NDO, when the study was planned (13). The Institutional Review Board at New York Medical College exempted this study as de-identified existing databases were utilized for the purpose of the meta analysis.
Eligibility Criteria
We requested IPD from all identified studies published in peer-reviewed journals which: [1] utilized frozen-thawed mature oocytes without prescreening for aneuploidy, followed by ICSI for IVF, and [2] provided pregnancy outcomes information. Case reports were not included in this analysis. We detailed the inclusions and exclusions in Figure 1, and summarized the key characteristics of the studies in Table 1.
Figure 1.
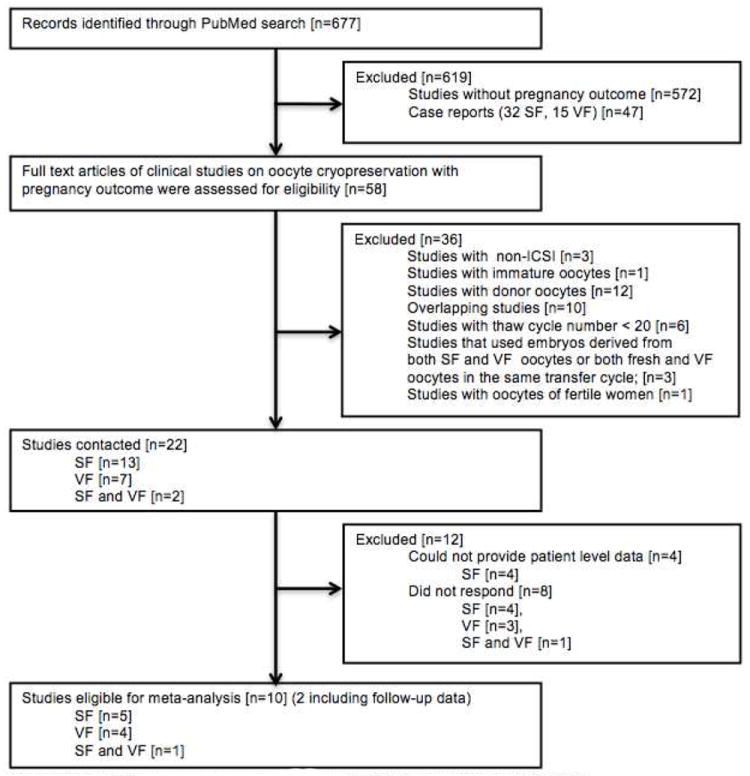
Selection of studies eligible for meta-analysis
Reasons for exclusion describe the first reason for exclusion that was encountered during the review process. Several articles had multiple reasons for exclusions. SF:Slow freezing, VF: Vitrification
Table 1.
Characteristics and success rates of the studies from which IPD was available and used for meta-analysis.
| Age | Year | Author | Study Type | Method | Patient # | Thawed oocytes | Thaw cycles | SR (%) | FR (%) | IR (%) | CP/T (%) | LB/T (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 31.7±4.7 | Taiwan | Chen S-U/2005 (17) | prospective | SF | 21 | 159 | 21 | 76.1 | 66.1 | 10.7 | 33.3 | 33.3 |
| 31.7±4.1 | US | Boldt J/2006 (18) | retrospective | SF | 82 | 556 | 87 | 55.4 | 65.4 | 12.6 | 26.5 | 19.1 |
| 32.0±3.6 | US | Boldt J/2006a | VF | 25 | 168 | 28 | 78.0 | 76.7 | 11.5 | 29.2 | 20.8 | |
| 32.3±3.6 | Hungary | Konc J/2008 (19) | retrospective | SF | 54 | 215 | 64 | 80.0 | 84.3 | 11.0 | 20.3 | 15.6 |
| 35.7±5.7 | Italy | Albani E/2008 (20) | retrospective | SF | 949 | 7584 | 1280 | 58.1 | 70.2 | 6.9 | 12.8 | 8.4 |
| 34.1±3.9 | Italy | Parmegiani L/2009 (21) | retrospective | SF | 424 | 2608 | 510 | 70.7 | 82.6 | 8.2 | 16.3 | 8.8 |
| 34.5±3.8 | Korea | Yoon TK/2003 (22) | prospective | VF | 34 | 474 | 34 | 68.6 | 71.7 | 5.6 | 22.2 | 22.2 |
| 33.6±4.3 | Colombia | Lucena E/2006 (23)b | retrospective | VF | 37 | 179 | 37 | 81.0 | 85.8 | 3.5 | 10.8 | 10.8 |
| 32.5±5.8 | Korea | Yoon TK/2007 (24) | prospective | VF | 28 | 364 | 30 | 83.0 | 77.1 | 14.2 | 43.3 | 36.7 |
| 35.7±4.9 | Italy | Fadini R/2009 (25) | retrospective | VF | 46 | 285 | 59 | 78.9 | 72.8 | 9.3 | 19.2 | 11.5 |
| 31.9±4.5 | Italy | Ubaldi Fc/2010 (26) | prospective | VF | 105 | 487 | 115 | 89.7 | 85.4 | 16.2 | 31.5 | 26.1 |
| Total | 1805 | 13079 | 2265 |
For age, mean±SD. The success rates of IPD available studies were recomputed from the data obtained.
SR: survival rate; FR: fertilization rate; IR: implantation rate (sacs/embryos transferred); CP/T: clinical pregnancy/transfer; LB/T: live-birth/transfer, RCT: Randomized controlled trial.
follow-up data of reference 18, written in a different row because different cryopreservation method is used;
includes follow-up data;
includes RCT data from a previous study by the same group (27).
Outcome Measures
The primary aim of this study was to develop three live-birth probability models based on age, cryopreservation method, and 1) the number of oocytes thawed, 2) the number of oocytes injected, or 3) the number of embryos transferred, where 1)-3) were separately modeled due to high correlations.
The secondary outcomes were success rates for survival, fertilization, and implantation.
Search Strategy
We used oocyte cryopreservation, slow freezing, and vitrification as the keywords for the title and abstract search by PubMed. The search strategy is summarized as a flow chart in Figure 1. Studies were identified for the period from January 1986 (when the first pregnancy from oocyte cryopreservation was reported) until July 2011, as well as by directly contacting experts in the field. We also obtained unpublished follow up data on pregnancy outcomes via personal communications (Drs. J. Boldt and E. Lucena).
Data collection process and time frame of the study
After identifying the studies eligible to be included, we contacted the majority of the authors by email and a few others by phone. To consider the author as non-responsive, we made at least 3 additional attempts to contact the author. Thus the study ran from Oct 2009 until July 2011, when the last contact was made.
Data items
Data extraction sheet which included the summary of information of their studies were sent to all the authors. With this information given, the authors were asked to verify to check if we had retrieved the right information from their studies and show if any of their study data covered a previous one (overlapping data). After obtaining and checking the raw data sent, if there was a mismatch or if there was missing or abstruse data, the author was contacted again. Any disagreement was resolved unanimously by discussion. If the author did not reply to resolve the disagreement, the data under discussion were excluded.
Statistical Analysis
Summary statistics were used to describe individual studies and thaw cycle characteristics, such as mean, standard deviation (SD) and range for continuous variables, and percentage for categorical variables.
The associations between the covariates and the outcomes are modeled via generalized estimating equations (GEE), accounting for 2-level clustering (i.e., within study, and within patient within study) (14,15). In the GEEs, compound symmetry was assumed for within-cluster correlation. For binary outcome, the logit link was used, and for continuous outcome (e.g., survival rate), the normal link was used. Information Criterion for GEEs, QIC, was computed as the model fit statistic (16), where lower value indicates improved fit. To asses the ability to discriminate successes vs. failurees using covariates, the area under the ROC curve (AUC) was computed from standard logistic regression model. AUC=1 means perfect discriminination between events vs. non-events, and 0.5 means noninformative, random discrimination.
Different age cut-off points were examined in terms of discriminatory ability, with subdividing the dataset into two by each age (i.e., 25 to 42 by 1 year increment). In this task, AUCs from simple (age as the sole covariate) and multiple (further adjusting other covariates) regression were used.
As an ancillary analysis, we repeated regression analyses, restricting the study sample to data from the first thaw attempt. Because we achieved results qualitatively similar to what we obtained from the analysis based on all available data, we elected not to report the results from these ancillary analyses.
Two-sided tests were used for inference. P-values and confidence intervals were not adjusted for multiple comparisons. Analyses were performed by SAS 9.3 (Cary, NC) and graphs were made by Microsoft Excel.
Of note, our study is not an ordinary meta-analysis which aims to estimate treatment effects or associational measures, and we did not conduct sensitivity analyses and bias assessments concerning unmeasured confounders and/or unavailable data. If they had been included in our analysis, the results could be potentially affected and selection bias (e.g., publication or non-response bias) is not avoidable. Yet, using raw data, we could control key covariates in individual levels.
RESULTS
Study Exclusions and Inclusions
The search in PubMed database yielded 677 potential records. After the exclusions shown in Figure 1, 22 reports remained (17-38). Of those, we were able to obtain the IPD from ten studies (17-26), two of which included unpublished updated data (18,23) (Table 1). Of the ten, four were prospective (17,22,24,26), one of which (26) also included the data from a randomized controlled trial on NDO cycles (27). The remaining six were retrospective (18-21,23,25). In one study, where both NDO and DO cycles were reported (23), we only used data from NDO cycles. In one study where both SF and VF cycles were reported, we could not verify SF data and hence only VF cycles were included (25).
This amounted to 2265 thawing/warming cycles from 1805 patients in the final dataset. All studies utilized surplus oocytes after IVF cycle, which were cryopreserved either because embryo freezing was legally forbidden or the patients did not wish to freeze embryos. All embryo transfers were done on day 2 or 3. Mean±SD ages of the patients at freezing were 33.8±4.0 (range: 20-48) and 34.1±4.7 (20-51) for SF and VF, respectively. Of the thawing/warming cycles, 1962 and 303 were after SF and VF. These cycles involved 11122 SF and 1957 VF oocytes originally retrieved and frozen between 1997 and 2009.
Overall Number of Pregnancies Resulting from SF and VF Oocytes
In the final dataset, there were 328 clinical pregnancies resulting in 281 singletons; 43 twins and four triplets or higher order pregnancies. Of the 328 clinical pregnancies, 253 were from SF and 75 from VF. Of SF and VF cycles, 14.2% and 14.7% of the clinical pregnancies were multiple pregnancies. These pregnancies resulted in a total of 224 live births; 163 after SF and 61 after VF. Included in the live births were few ongoing pregnancies (4 SF and 1 VF).
Description of Studies that IPD was Unavailable
Overall, we were able to retrieve 40% and 55.5% of SF and VF cycles from NDO studies published, respectively. The 12 studies that we were not able to obtain IPD included eight retrospective SF (28-35), three prospective VF studies (36-38), and one RCT comparing SF vs. VF (39). The one RCT included 30 thawing and 48 warming cycles. The mean age range among these studies was 32.3-35.5.
The reported success rates of the studies from which IPD was available vs. unavailable are given in Supplemental figure 1.
Thaw Cycle Characteristics
In 13.5%, 21.1%, 19.2%, 19.4%, 18% of the cycles, 3,4,5,6 and >6 oocytes were thawed, respectively. There were no cycles with single-oocyte thaw and only 17 cycles (0.8%) with 2-oocyte thaw. In 5.5%, 14.2%, 64.3%, 4.6%, 3.0%, 2.0% and 3.2% of the cycles, 1, 2, 3, 4, 5, 6 and >6 oocytes were injected, respectively. In majority of the cycles, either 2 (33.3%) or 3 embryos (32.6%) were transferred. Single (17.8%) and supernumerary embryo (4.2%) transfers were less common.
Mean numbers of thawed, survived, injected, fertilized oocytes, and embryos transferred were significantly different between the SF and VF cycles (Supplemental table 1). In none of the studies embryos generated from thawed oocytes were frozen for future use.
After adjusting for age and method, a higher percentage of cycles were cancelled with SF, compared to VF (12.9% vs. 7.3%; p=0.006). Thaw cycle cancellation rates increased with age for both SF and VF (p=0.009), indicating age-induced decline in oocyte reserve and quality.
Age-specific Success Rates After SF and VF
Survival and fertilization rates
Overall survival and fertilization rates were lower after SF (65% and 74%), compared to VF (85% and 79%) (p< 0.001). However, age was not significantly associated with oocyte survival (p=0.24) and fertilization success rates (p=0.56) for both SF and VF.
Implantation rate
Implantation rates were higher after VF (p=0.002) and showed a decline with age for both SF and VF (p<0.0001). For women under age 30, the likelihood of an embryo deriving from SF oocytes to implant was >8.9%. This probability declined to 4.3% after age 40, but live births – despite lower frequencies – continued to occur with SF until 42 years of age. Success of implantation also declined from 13.2% for age 30 to 8.6% for age 40 with VF, but live-births continued to occur until age 44.
Miscarriage rates
Miscarriage rates were higher after SF (p=0.005) and showed slight age-related trends, 36%-41% and 19%-22%, between ages 30-40 for SF and VF, respectively.
Age-specific probabilities of live-birth based on number of thawed, injected oocytes or embryos transferred
From GEEs fitted (Supplemental table 2), the probability of live-birth (in Y-axis) as a function of age (in X-axis) were derived with the method and the number of oocytes fixed at some values. Specifically, we presented plots to give probabilities of live-birth for 2,4,6 thawed and 2,4,6 injected oocytes, and 1,2,3 embryos transferred (Figures 2A-C). Of note, there were no cycles with single-oocyte thaw and only 17 cycles (0.8%) with 2-oocyte thaw. We limited age at freezing up to 42 in the plots because the reliability of predictions could be limited as there were only 29 cycles from patients who were older than 42 when their oocytes were frozen (of those two were 48 and one was 51 years old). Median [interquartile range] of the number of thawed, injected oocytes and embryos transferred was 5 [4-7], 3 [3-3] and 2 [1-3], respectively. For example, the probability of live-birth for a 30-year-old woman who has 2 to 6 oocytes to thaw ranges between 9.1-10.5% and 21.4-24.1% after SF and VF, respectively. If the same individual has 2 to 6 oocytes to be injected and 1 to 3 embryos to be transferred, her chances of having a live-birth would likely range between 9.1-15.4% and 18.9-29.9% for injected oocytes and 5.5-15.3% and 9.7-24.9% for embryos transferred after SF and VF, respectively.
Figure 2.
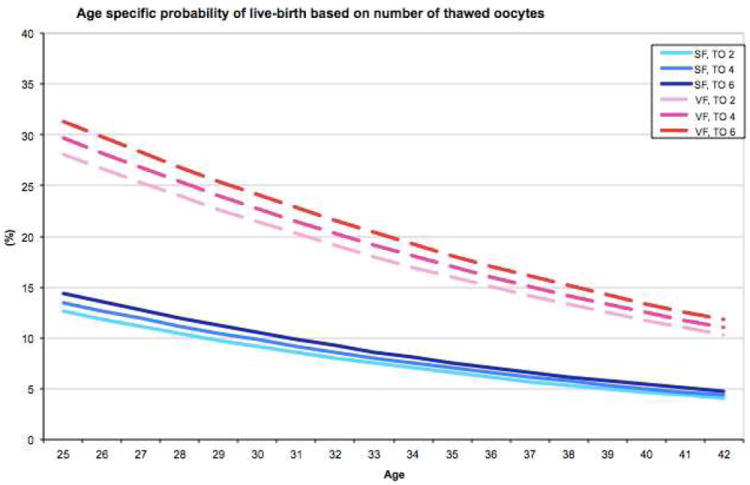
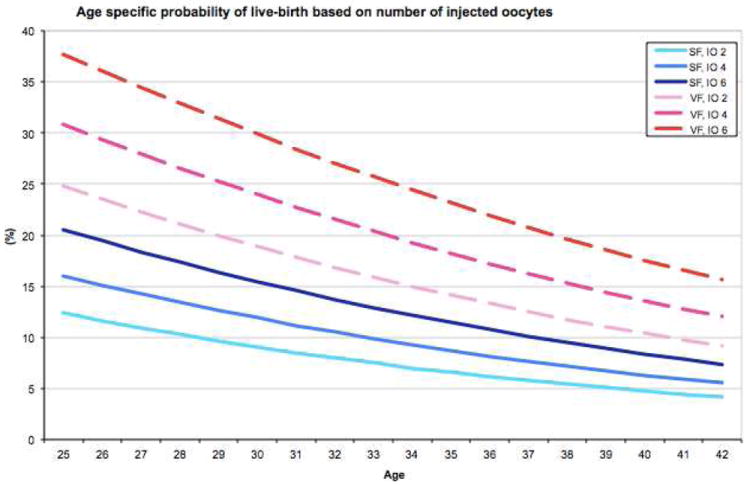
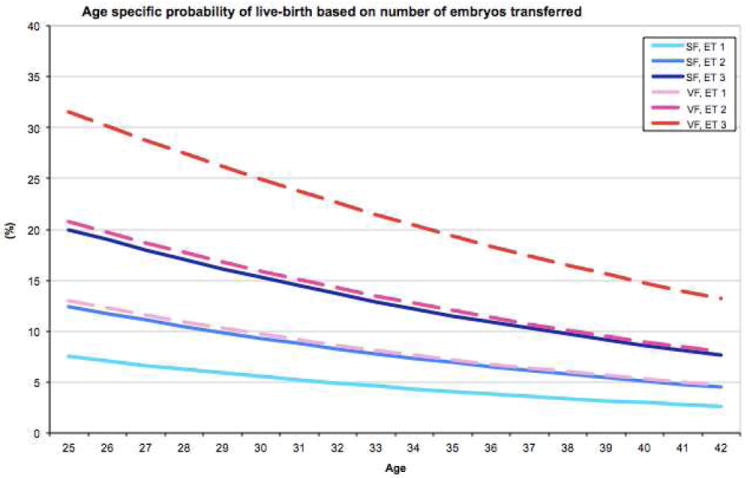
Age-specific probabilities of live-birth based on oocyte cryopreservation method and the number of oocytes thawed (A), number of oocytes injected (B), or number of embryos transferred (C).
SF: slow freezing, VF: vitrification, TO: thawed oocytes, IO: injected oocytes, ET: embryos transferred
In addition, selected probabilities of live-births (e.g., for ages 25-42 based on 2-6 oocytes thawed and injected, 1-3 embryos transferred) are tabulated in Table 2, which may be used for patient counseling or self-assessment.
Table 2.
Representative probabilities (%) of live-birth for ages 25-42 based on number of oocytes thawed, injected, or embryos transferred
| SLOW FREEZING | VITRIFICATION | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Oocytes Thawed | Oocytes Injected | Embryos Transferred | Oocytes Thawed | Oocytes Injected | Embryos Transferred | |||||||||||||
|
| ||||||||||||||||||
| Age | 2 | 4 | 6 | 2 | 4 | 6 | 1 | 2 | 3 | 2 | 4 | 6 | 2 | 4 | 6 | 1 | 2 | 3 |
| 25 | 12.6 | 13.5 | 14.4 | 12.4 | 16.0 | 20.5 | 7.5 | 12.4 | 20.0 | 28.1 | 29.7 | 31.3 | 24.8 | 30.9 | 37.7 | 13.0 | 20.7 | 31.5 |
| 26 | 11.8 | 12.7 | 13.5 | 11.6 | 15.1 | 19.4 | 7.0 | 11.8 | 19.0 | 26.7 | 28.2 | 29.8 | 23.5 | 29.4 | 36.0 | 12.2 | 19.7 | 30.1 |
| 27 | 11.1 | 11.9 | 12.7 | 10.9 | 14.3 | 18.4 | 6.6 | 11.1 | 18.0 | 25.3 | 26.8 | 28.3 | 22.3 | 28.0 | 34.5 | 11.6 | 18.7 | 28.8 |
| 28 | 10.4 | 11.2 | 11.9 | 10.3 | 13.4 | 17.3 | 6.2 | 10.5 | 17.1 | 24.0 | 25.4 | 26.8 | 21.1 | 26.6 | 32.9 | 10.9 | 17.7 | 27.4 |
| 29 | 9.8 | 10.5 | 11.2 | 9.6 | 12.6 | 16.4 | 5.9 | 9.9 | 16.2 | 22.7 | 24.0 | 25.4 | 20.0 | 25.2 | 31.4 | 10.3 | 16.8 | 26.2 |
| 30 | 9.1 | 9.8 | 10.5 | 9.1 | 11.9 | 15.4 | 5.5 | 9.3 | 15.3 | 21.4 | 22.7 | 24.1 | 18.9 | 24.0 | 29.9 | 9.7 | 15.9 | 24.9 |
| 31 | 8.6 | 9.2 | 9.8 | 8.5 | 11.2 | 14.6 | 5.2 | 8.8 | 14.5 | 20.2 | 21.5 | 22.8 | 17.8 | 22.7 | 28.5 | 9.2 | 15.0 | 23.7 |
| 32 | 8.0 | 8.6 | 9.2 | 8.0 | 10.5 | 13.7 | 4.9 | 8.3 | 13.7 | 19.1 | 20.3 | 21.6 | 16.8 | 21.5 | 27.1 | 8.6 | 14.2 | 22.6 |
| 33 | 7.5 | 8.1 | 8.6 | 7.5 | 9.9 | 12.9 | 4.6 | 7.8 | 12.9 | 18.0 | 19.2 | 20.4 | 15.9 | 20.4 | 25.7 | 8.1 | 13.5 | 21.5 |
| 34 | 7.0 | 7.5 | 8.1 | 7.0 | 9.3 | 12.1 | 4.3 | 7.3 | 12.2 | 17.0 | 18.1 | 19.2 | 15.0 | 19.3 | 24.4 | 7.7 | 12.7 | 20.4 |
| 35 | 6.6 | 7.0 | 7.6 | 6.6 | 8.7 | 11.4 | 4.0 | 6.9 | 11.5 | 16.0 | 17.0 | 18.1 | 14.1 | 18.2 | 23.1 | 7.2 | 12.0 | 19.3 |
| 36 | 6.1 | 6.6 | 7.1 | 6.2 | 8.2 | 10.7 | 3.8 | 6.5 | 10.9 | 15.0 | 16.0 | 17.1 | 13.3 | 17.2 | 21.9 | 6.8 | 11.3 | 18.3 |
| 37 | 5.7 | 6.2 | 6.6 | 5.8 | 7.7 | 10.1 | 3.6 | 6.1 | 10.3 | 14.1 | 15.1 | 16.1 | 12.5 | 16.2 | 20.8 | 6.4 | 10.7 | 17.4 |
| 38 | 5.4 | 5.8 | 6.2 | 5.4 | 7.2 | 9.5 | 3.4 | 5.7 | 9.7 | 13.3 | 14.2 | 15.1 | 11.8 | 15.3 | 19.6 | 6.0 | 10.1 | 16.5 |
| 39 | 5.0 | 5.4 | 5.8 | 5.1 | 6.7 | 8.9 | 3.1 | 5.4 | 9.1 | 12.5 | 13.3 | 14.2 | 11.1 | 14.4 | 18.6 | 5.6 | 9.5 | 15.6 |
| 40 | 4.7 | 5.0 | 5.4 | 4.7 | 6.3 | 8.3 | 3.0 | 5.1 | 8.6 | 11.7 | 12.5 | 13.4 | 10.4 | 13.6 | 17.5 | 5.3 | 9.0 | 14.8 |
| 41 | 4.4 | 4.7 | 5.0 | 4.4 | 5.9 | 7.8 | 2.8 | 4.8 | 8.1 | 11.0 | 11.8 | 12.6 | 9.8 | 12.8 | 16.6 | 5.0 | 8.5 | 14.0 |
| 42 | 4.1 | 4.4 | 4.7 | 4.1 | 5.5 | 7.3 | 2.6 | 4.5 | 7.6 | 10.3 | 11.0 | 11.8 | 9.2 | 12.0 | 15.6 | 4.7 | 8.0 | 13.2 |
Determining the Potential Age Threshold for Live-Birth Outcome
We found that age 36 (≥36 vs. <36) showed the highest discrimination capability for success vs. failure (AUC=0.72) after adjusting for the method and number of embryos transferred, while age 35 showed the highest AUC without adjustment.
DISCUSSION
This unique IPD meta-analysis is the first to report age-specific probabilities of live-birth for oocyte cryopreservation after SF and VF. In this study, all measurements of successful outcome declined with patient age, regardless of the freezing method used, which is highly expected. When the number of thawed, injected oocytes and embryos transferred were controlled, probability of live-birth after VF was higher than SF across all age groups.
Meta-analyses based on IPD are still scarce in medicine, though they are likely to replace conventional meta-analysis whenever feasible in near future (40). IPD meta-analysis offer numerous advantages over the conventional meta-analysis (15) or modeling based on hypothetical data or simulation. Most importantly, access to IPD enabled us to account for patient characteristics. There are also a few disadvantages of IPD meta-analysis. The newest data may not be included as obtaining, processing and analyzing raw data takes time – for example, our study included studies until 2010. Also, some authors may not share their raw data. Yet, our meta-analysis and models can be naturally updated as more raw data will be available.
Although the most popular applications of oocyte freezing are for cancer patients or for patients undergoing oocyte cryopreservation electively, the majority of available data in the literature reflecting clinical success are from infertile patients. Hence, our results may not be generalizable to excluded populations, e.g., cancer patients or to patients pursuing elective oocyte freezing. New studies and models are warranted for these populations in the future.
Due to a small number of RCTs available, the comparison of SF vs. VF may be biased and our analysis should be understood as “as observed” rather than “intent to treat”. As our primary goal is to estimate the probability of the live birth as a function of patient age rather than treatment assigned, age-based probability derived from predominantly observational studies can be still valuable. We hope to update our models when a sufficient number of RCTs will be available or a good combination of RCTs and observational studies can be assembled.
An important question for current and future patients and clinicians is: What is the upper age limit to offer oocyte cryopreservation? First, if one considers the possibility of live birth, this age seems to be 42 for SF and 44 for VF, according to the data presented here. However, if one considers “reasonable” chance of conception, such a cut off is less clear. Our analysis revealed 36 as age cutpoint provides the best discrimination between successes vs. failures hence the optimal results may be expected in patients who are younger than this age threshold. Nevertheless, as one cannot place an absolute value on childbearing, upper age limit for considering oocyte cryopreservation may vary based on individual preferences, values, and available resources.
Though we found that oocyte cryopreservation was performed in women aged 20-51 years in clinics across the world, we limited the probability plots for the age range 25-42 years because there were few cycles outside this range (1.3% of all cycles above or below the range). While it is unlikely that the live birth probabilities would be higher for those younger than 25, our data does not bring light into the efficiency of oocyte cryopreservation after age 42. Further studies are needed to understand the feasibility to offer oocyte freezing to women age >42 years.
Of note, the raw data utilized in our analysis are from infertile patients. It is probable that the success rates are more favorable with fertile individuals undergoing elective cryopreservation before cancer treatments or elective reasons. Furthermore, even though we showed that VF results in significantly higher success rates compared to SF, the latter protocol is still undergoing evolution, and its efficiency may catch up with VF. Recently, Bianchi et al. reported higher success rates using a modified SF protocol showing that future studies are likely to have enhanced success with SF (41). This feature will be accounted in newer or updated models as more data will be available. Nevertheless, the pregnancy rates presented in our IPD-meta-analysis maybe sufficiently high for the policy makers to argue for the acceptance of oocyte cryopreservation into the routine practice of infertility treatment and fertility preservation.
In conclusion, this IPD meta-analysis shows that VF success rates are superior to SF (based on mostly observational evidence) and the success rates with either technique may begin to decline meaningfully after the age of 36. Age induced decline of live birth probability after oocyte cryopreservation is highly anticipated but has not been estimated empirically using raw data to date. Although an upper age limit could not be specified with the available data, it may be safe that we do not recommend oocyte cryopreservation in women over 45 years of age. Though it is generally preferred that each center generates its own model with important predictors, most clinics currently do not have the critical mass to provide that information to their patients. For the majority of centers in the US and around the world and infertile patients who consider or choose oocyte freezing, the age-based success rates estimated using best available empirical data and statistical modeling would provide an important tool for informed decision making and counseling that is currently unavailable. A future direction would be that more specific or individualized models will be developed for specific populations, such as for cancer patients or women pursuing oocyte cryopreservation electively. Finally, we surmise that it is time for the managed care companies to consider oocyte cryopreservation as an integral part of the treatment and prevention of infertility.
Supplementary Material
Clustered column charts of implantation and live-birth rates per transfer from studies that IPD was available vs. unavailable.
Comparison of thaw cycle characteristics between SF and VF.
Generalized Estimating Equation (GEE) models for the outcome of live-birth
Acknowledgments
The authors would like to thank all authors who shared their valuable data for the purpose of this meta-analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts J, Oktay K. Fertility preservation: a comprehensive approach to the young woman with cancer. J Natl Cancer Inst Monogr. 2005;34:57–9. doi: 10.1093/jncimonographs/lgi014. [DOI] [PubMed] [Google Scholar]
- 2.Cobo A, Domingo J, Perez S, Crespo J, Remohi J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10:268–73. doi: 10.1007/s12094-008-0196-7. [DOI] [PubMed] [Google Scholar]
- 3.Cobo A, Bellver J, Domingo J, Pérez S, Crespo J, Pellicer A, et al. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod Biomed Online. 2008;17:68–72. doi: 10.1016/s1472-6483(10)60295-7. [DOI] [PubMed] [Google Scholar]
- 4.Stoop D, Nekkebroeck J, Devroey P. A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Hum Reprod. 2011;26:655–61. doi: 10.1093/humrep/deq367. [DOI] [PubMed] [Google Scholar]
- 5.Rudick B, Opper N, Paulson R, Bendikson K, Chung K. The status of oocyte cryopreservation in the United States. Fertil Steril. 2010;94:2642–6. doi: 10.1016/j.fertnstert.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 6.Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remoh J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–64. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Cobo A, Meseguer M, Remoh J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective randomized controlled clinical trial. Hum Reprod. 2010;25:2239–46. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 8.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92:520–6. doi: 10.1016/j.fertnstert.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of American Society for Reproductive Medicine. Practice Committee of Society for Assisted Reproductive Technology. Mature Oocyte Cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Fertil Hope. [September 16, 2012]; http://www.fertilehope.org/financial-assistance/egg-and-embryo-freezing.cfm#q1.
- 11.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–85. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Fioravanti J, Alegretti JR, Hassun PA, Motta ELA, Serafini PC, Smith GD. Prospective Randomized Comparison of Human Oocyte Freezing and Vitrification: An Update. Fertil Steril. 2007;88(Supp. 1):S13. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 14.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 15.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2012;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 16.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen SU, Lien YR, Chen HF, Chang LJ, Tsai YY, Yang YS. Observational clinical follow-up of oocyte cryopreservation using a slow-freezing method with 1.2-propanediol plus sucrose followed by ICSI. Hum Reprod. 2005;20:1975–80. doi: 10.1093/humrep/deh884. [DOI] [PubMed] [Google Scholar]
- 18.Boldt J, Tidswell N, Sayers A, Kilani R, Cline D. Human oocyte cryopreservation: 5-year experience with a sodium-depleted slow freezing method. Reprod Biomed Online. 2006;13:96–100. doi: 10.1016/s1472-6483(10)62021-4. [DOI] [PubMed] [Google Scholar]
- 19.Konc J, Kanyo K, Varga E, Kriston R, Cseh S. Oocyte cryopreservation: the birth of the first Hungarian babies from frozen oocytes. J Assist Reprod Genet. 2008;25:349–52. doi: 10.1007/s10815-008-9235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albani E, Barbieri J, Novara PV, Smeraldi A, Scaravelli G, Levi Setti PE. Oocyte cryopreservation. Placenta. 2008;29(Suppl B):143–6. doi: 10.1016/j.placenta.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Parmegiani L, Bertocci F, Garello C, Salvarani MC, Tambuscio G, Fabbri R. Efficiency of human oocyte slow freezing: results from five assisted reproduction centres. Reprod Biomed Online. 2009;18:352–9. doi: 10.1016/s1472-6483(10)60093-4. [DOI] [PubMed] [Google Scholar]
- 22.Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2003;79:1323–6. doi: 10.1016/s0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 23.Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril. 2006;85:108–11. doi: 10.1016/j.fertnstert.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Yoon TK, Lee DR, Cha SK, Chung HM, Lee WS, Cha KY. Survival rate of human oocytes and pregnancy outcome after vitrification using slush nitrogen in assisted reproductive technologies. Fertil Steril. 2007;88:952–6. doi: 10.1016/j.fertnstert.2006.12.071. [DOI] [PubMed] [Google Scholar]
- 25.Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19:171–80. doi: 10.1016/s1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 26.Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod. 2010;25:1199–205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Sala GB, Nicoli A, Villani MT, Pescarini M, Gallinelli A, Blickstein I. Outcome of 518 salvage oocyte-cryopreservation cycles performed as a routine procedure in an in vitro fertilization program. Fertil Steril. 2006;86:1423–7. doi: 10.1016/j.fertnstert.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Chamayou S, Alecci C, Ragolia C, Storaci G, Maglia E, Russo E, et al. Comparison of in-vitro outcomes from cryopreserved oocytes and sibling fresh oocytes. Reprod Biomed Online. 2006;12:730–6. doi: 10.1016/s1472-6483(10)61085-1. [DOI] [PubMed] [Google Scholar]
- 30.Borini A, Lagalla C, Bonu MA, Bianchi V, Flamigni C, Coticchio G. Cumulative pregnancy rates resulting from the use of fresh and frozen oocytes: 7 years’ experience. Reprod Biomed Online. 2006;12:481–6. doi: 10.1016/s1472-6483(10)62002-0. [DOI] [PubMed] [Google Scholar]
- 31.De Santis L, Cino I, Rabellotti E, Papaleo E, Calzi F, Fusi FM, et al. Oocyte cryopreservation: clinical outcome of slow-cooling protocols differing in sucrose concentration. Reprod Biomed Online. 2007;14:57–63. doi: 10.1016/s1472-6483(10)60764-x. [DOI] [PubMed] [Google Scholar]
- 32.Borini A, Bianchi V, Bonu MA, Sciajno R, Sereni E, Cattoli M, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod Biomed Online. 2007;15:175–81. doi: 10.1016/s1472-6483(10)60706-7. [DOI] [PubMed] [Google Scholar]
- 33.Ferraretti AP, Lappi M, Magli MC, Muzzonigro F, Resta S, Gianaroli L. Factors affecting thawed oocyte viability suggest a customized policy of embryo transfer. Fertil Steril. 2010;94:1308–13. doi: 10.1016/j.fertnstert.2009.05.088. [DOI] [PubMed] [Google Scholar]
- 34.Borini A, Levi Setti PE, Anserini P, De Luca R, De Santis L, Porcu E, et al. Multicenter observational study on slow-cooling oocyte cryopreservation: clinical outcome. Fertil Steril. 2010;94:1662–8. doi: 10.1016/j.fertnstert.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Azambuja R, Petracco A, Okada L, Michelon J, Badalotti F, Badalotti M. Experience of freezing human oocytes using sodium-depleted media. Reprod Biomed Online. 2011;22:83–7. doi: 10.1016/j.rbmo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 37.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online. 2007;14:72–9. doi: 10.1016/s1472-6483(10)60766-3. [DOI] [PubMed] [Google Scholar]
- 38.Almodin CG, Minguetti-Camara VC, Paixao CL, Pereira PC. Embryo development and gestation using fresh and vitrified oocytes. Hum Reprod. 2010;25:1192–8. doi: 10.1093/humrep/deq042. [DOI] [PubMed] [Google Scholar]
- 39.Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94:2088–95. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 40.Broeze KA, Opmeer BC, van der Veen F, Bossuyt PM, Bhattacharya S, Mol BW. Individual patient data meta-analysis: a promising approach for evidence synthesis in reproductive medicine. Hum Reprod Update. 2012;16:561–7. doi: 10.1093/humupd/dmq043. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi V, Lappi M, Bonu MA, Borini A. Oocyte slow freezing using a 0.2-0.3 M sucrose concentration protocol: is it really the time to trash the cryopreservation machine? Fertil Steril. 2012;97:1101–7. doi: 10.1016/j.fertnstert.2012.01.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clustered column charts of implantation and live-birth rates per transfer from studies that IPD was available vs. unavailable.
Comparison of thaw cycle characteristics between SF and VF.
Generalized Estimating Equation (GEE) models for the outcome of live-birth


