Abstract
Objective
To investigate the safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole protocol for fertility preservation in breast cancer patients.
Design
Retrospective cohort study.
Setting
Academic fertility preservation center.
Patient(s)
Seventy-eight women ≤ 45 years, diagnosed with stage ≤ 3 breast cancer, who desired fertility preservation
Intervention(s)
Two consecutive (2C) vs. single (1C) ovarian stimulation cycle with the Letrozole-FSH protocol.
Main Outcome Measurement(s)
Embryo or oocyte cryopreservation outcomes, time interval from surgery to chemotherapy (CT), and breast cancer recurrence rates.
Results
Sixty-one patients underwent 1C while 17 had 2C. The mean total number of oocytes harvested (16.1 ± 13.2 vs. 9.1 ± 5.2) and embryos generated (6.4 ± 2.9 vs. 3.7 ± 3.1) were significantly higher in patients in 2C vs. 1C. The time interval from surgery to CT was similar between the 2C and 1C groups (63.7 ± 7.7 vs. 58.0 ± 12.1 days). After a mean follow up of 58.5 ± 13.6 months, recurrence rates were similar between 2C (0/17) and 1C (2/49) patients.
Conclusion
It appears to be safe and feasible to perform two consecutive ovarian stimulation cycles to increase the oocyte/embryo yield for fertility preservation.
Keywords: Letrozole, breast cancer, fertility preservation, consecutive cycles, ovarian stimulation
Introduction
Breast cancer is the most prevalent malignancy among reproductive women in the United States (1). With improvements in diagnostic and therapeutic strategies, breast cancer mortality rates significantly declined over the past years (2). At present, breast cancer survivors represent the largest group of cancer survivors in the United States (3). New challenges that these survivors now face are chemotherapy-induced ovarian reserve damage, premature ovarian failure and infertility.
Reproductive endocrinologists should counsel and provide information about fertility preservation options to breast cancer patients who will face such gonadotoxic treatments. Embryo, oocyte and ovarian tissue cryopreservation are the three main options to preserve fertility, although ovarian tissue cryopreservation is still experimental (4). Both embryo and oocyte cryopreservation require controlled ovarian hyperstimulation (COH). However, traditional COH regimens are associated with higher levels of estrogen and as a result are not recommended for breast cancer patients. To protect the patients from the potential deleterious effects of elevated estrogen levels during ovarian stimulation for fertility preservation, protocols using aromatase inhibitors were developed (5). Letrozole, a potent and highly selective third-generation aromatase inhibitor, has been shown to reduce estrogen exposure when combined with gonadotropin for ovarian stimulation in breast cancer patients (6). Letrozole reduces serum estrogen levels by aromatase inhibition. Yet because of the reflex increase in the endogenous follicle stimulating hormone (FSH), aromatase inhibitors also result in ovarian stimulation.
Women with breast cancer typically have an interval of 6–8 weeks between surgery and the initiation of adjuvant chemotherapy. However, studies have shown no effect on survival or recurrence rates in patients with early stage breast cancer if chemotherapy is initiated 12 weeks after breast surgery (7, 8). Early fertility preservation referral enables earlier counseling and initiation of cryopreservation cycles before chemotherapy and, when appropriate, two consecutive ovarian stimulation cycles may be completed without any delay in chemotherapy treatment. For instance, patients who have had oocyte retrieval within 4 weeks of the breast surgery are able to complete a second ovarian stimulation cycle within 8 weeks of surgery. Because of this early counseling and initiation of fertility preservation, these women are at an advantage since a greater number of oocytes and embryos can be cryopreserved. Thus, the chemotherapy-induced ovarian reserve loss may be better compensated by cryopreserving a higher number of oocyte and embryos. Supporting this notion, in a recent individual patient data metaanalysis, we showed that the live birth probability from IVF increases with the increasing number of oocytes frozen from individuals, regardless of age (9).
In this study, our primary aim was to investigate the safety and feasibility of performing two consecutive ovarian stimulation cycles for fertility preservation prior to the initiation of chemotherapy among breast cancer patients. Our secondary aim was to analyze interval differences in time of surgery and the initiation of chemotherapy between groups, those receiving two versus one ovarian stimulation cycle.
Materials and Methods
Institutional Review Board approval was obtained at New York Medical College. Data was generated using secondary analysis of a prospectively database of all women diagnosed with breast cancer who underwent assisted reproductive technology (ART) treatment at our Institution. Exclusion criteria included: age > 45 years, breast cancer stage > 3, previous chemotherapy or radiotherapy, and history of ovarian surgery or infertility (Supplemental figure 1).
Recruitment within the two groups, single versus two consecutive cycles therapy was not randomized. The decision of undergoing single cycle or two consecutive cycles was based on several factors which included: amount of time available from referral to chemotherapy start date, patients’ desire to increase total number of oocytes/embryos, and after concordance of the patients’ respective oncologists.
Letrozole protocol used for ovarian stimulation was identical for both groups. Letrozole 5mg/day was started on cycle day 2 or 3. Daily injections of FSH (150–300 IU/day) was added beginning 2 days after and until trigger administration. To prevent premature luteinizing hormone surge, a gonadotropin-releasing hormone (GnRH) antagonist (250 µg/d) was administered when the lead follicle size reached 14-mm in mean diameter. Serum FSH and estrogen levels were monitored throughout the cycle. Oocyte maturation was triggered by hCG (5000–10,000 IU HCG - Organon) or 250 µg recombinant hCG (Serono) or GnRHa (leuprolide acetate 1 mg - Ferring Pharmaceuticals) when at least two follicles reached the mean diameter of 20 – 21 mm. Letrozole was discontinued on the day of trigger administration. Transvaginal ultrasound guided oocyte retrieval was performed 35 hours after the trigger. The estradiol (E2) measurement was repeated 3 days after the oocyte retrieval in patients triggered by hCG and if the E2 level was more than 250 pg/mL, letrozole was continued about 3 to 6 days until E2 levels decreased to less than 50 pg/ml (10). The same procedure was performed for the patients who had a second cycle. All cycles were initiated after breast surgery.
Total gonadotropin and letrozole dose received, number of oocytes retrieved, number of mature and fertilized oocytes and number of embryos frozen following intracytoplasmic sperm injection were assessed and compared between both groups. Follow-up information was collected during return office visits, phone interview, or by contacting the referring oncologist. Recurrence for breast cancer was defined as the detection of regional tumor, distant metastases, or contralateral invasive breast cancer, and recurrence rates were compared between groups.
Statistical analysis was performed with the SPSS 15 for Windows package (SPSS, Chicago, IL). Continuous data (presented as mean ±standard deviation) was analyzed using student t test and ANOVA as appropriate. Variables were complied using visual (histograms, probability plots) and analytical methods (Kolmogorov Simirnov/Shapiro-Wilks test) to determine the normality of distributions. Log rank was used to compare the survival curves (time interval from surgery to chemotherapy) between the groups. Chi-square, and where appropriate Fischer’s exact tests, was used to compare proportions of different groups, with p-value less than 0.05 considered statistically significant. For multivariate analyses, a multiple linear regression model was further entered to control for the multiple factors. The model fit was assessed using appropriate residual and goodness of fit statistics.
Results
A total of 157 patients with breast cancer underwent ovarian stimulation with 78 meeting inclusion criteria. Baseline patient characteristics are listed in Table 1. Of those, 61 patients received single cycle versus 17 patients who received two consecutive ovarian stimulation cycles. The two groups were similar with respect to age at diagnosis, body mass index, breast cancer diagnosis, tumor characteristics (tumor size, number of positive lymph nodes, grade, HER-2/neu overexpression and vascular space invasion) and BRCA status (Table 1). Multiple factors such as age, FSH levels, antral follicle count, tumor size, lymph node involvement, tumor grade, number of oocyte retrieved and embryo generated in the first cycle did not influence whether patients chose to do one cycle or two after multivariate analyses.
Table 1.
The comparison of patients’ demographics and tumor characteristics
| Two cycle (n=17) | Single cycle (n=61) | P value | |
|---|---|---|---|
| Age at diagnosis (years) | 35.7 ± 0.4 | 35.9 ± 0.6 | NS |
| BMI (kg/m2) | 23.1 ± 4.3 | 22.7 ± 2.7 | NS |
| FSH levels on day 2 (IU/mL) | 9.6 ± 3.2 | 9.3 ± 3.2 | NS |
| Antral follicle count | 10.9 ± 9.8 | 8.2 ± 4.7 | NS |
| Tumor characteristics | |||
| Histologic Grade Grade 1–2 Grade 3 |
16/17 (94.2%) 1/17 (5.8%) |
58/61 (95.1%) 3/61 (4.9%) |
NS NS |
| Nodal Status Negative Positive |
9/17 (64.8%) 6/17 (35.2%) |
39/61 (63.4%) 22/61 (36.6%) |
NS NS |
| Lymph vascular invasion (+) | 3/17 (17.6%) | 15/61 (24.5) | NS |
| Tumor size (cm) | 1.8 ± 0.9 | 1.7 ± 0.1 | NS |
| ER (+) | 11/17 (64.7%) | 35/61 (69.3%) | NS |
| PR (+) | 11/17 (64.7%) | 33/61 (65.3%) | NS |
| HER-2/neu (+) | 4/17 (23.5%) | 13/61 (22.4%) | NS |
| BRCA (+) | 3/17 (17.6%) | 9/61 (12.2%) | NS |
| Family history of cancer (+) | 10/17 (58.8%) | 34/61 (65.3%) | NS |
Values for continuous variables are mean ± SD. Values for categorical variables are number/total number of cases (%) (ER: Estrogen receptor; PR: progesterone receptor). A p-value less than 0.05 was considered to show a statistically significant data
Significant differences were seen regarding timing of referral to fertility counseling. The majority of two cycle patients were referred prior to surgery (82.3 %) compared to only 34.4 % of single cycle patients (p=0.001).
The majority of patients were triggered by hCG in both groups (12/17 vs 38/61; p=0.528). While 3 out of 17 patients underwent only oocyte cryopreservation after two consecutive cyles, the number of patients performed only oocyte cryopreservation was 11 out of 61 patients in single cycle group (p=0.97) Between each ovarian stimulation cycle, the mean number of oocytes harvested (7.7 ± 5.4 vs. 8.4 ± 9.6 vs. 9.1 ± 5.2) and embryos frozen (3.5 ± 2.6 vs. 3.4 ± 2.6 vs. 3.7 ± 3.1) were not statistically different (Table 2). As expected, for patients receiving two consecutive stimulation cycles, the cumulative mean number of oocyte retrieved (16.1 ± 13.2 vs. 9.1 ± 5.2; p value = 0.008) and embryos frozen (6.4 ± 2.9 vs. 3.7 ± 3.1; p value = 0.019) were significantly higher compared to those who only received a single cycle (Table 3).
Table 2.
Fertility preservation cycle outcomes of patients with breast cancer prior to initiation of treatment
| Single cycle (n=61) |
Two cycles (n=17) |
P value | ||
|---|---|---|---|---|
| First cycle | Second cycle | |||
| Total letrozole dose (mg) | 50.3 ± 9.9 | 53.2 ± 13.4 | 51.1 ± 10.0 | NS |
| Total rFSH dose (IU) | 2320.9 ± 889.9 | 2369.5 ± 833.4 | 2522.7 ± 931.7 | NS |
| Peak E2 levels (pg/mL) | 535.0 ± 448.0 | 374.0 ± 196.0 | 337.5 ± 185.1 | NS |
| Oocytes (n) | 9.1 ± 5.2 | 7.7 ± 5.4 | 8.4 ± 9.6 | NS |
| Mature oocytes (n) | 6.2 ± 3.0 | 5.1 ± 3.1 | 5.5 ± 4.7 | NS |
| Inseminated oocytes (n) | 6.0 ± 3.9 | 4.9 ± 3.8 | 4.9 ± 3.0 | NS |
| Fertilized oocytes (n) | 5.4 ± 2.3 | 4.4 ± 2.8 | 4.1 ± 3.7 | NS |
| Embryos (n) | 3.7 ± 3.1 | 3.5 ± 2.6 | 3.4 ± 2.6 | NS |
Results were given as mean ± SD. A p-value less than 0.05 was considered to show a statistically significant data.
Table 3.
The comparison of fertility preservation cycle outcomes of patients with breast cancer after performing two cycles
| Single cycle (n=61) | Two cycles (n=17) | P value | |
|---|---|---|---|
| Oocytes (n) | 9.1±5.2 | 16.1±13.2 | 0.008 |
| Mature oocytes (n) | 6.2±3.0 | 10.3±7.7 | 0.004 |
| Inseminated oocytes (n) | 6.0±3.9 | 9.8±5.5 | 0.002 |
| Fertilized oocytes (n) | 5.4± 2.3 | 7.4±3.9 | 0.040 |
| Embryos (n) | 3.7±3.1 | 6.4±2.9 | 0.019 |
Results were given as mean ± SD. A p-value less than 0.05 was considered to show a statistically significant data
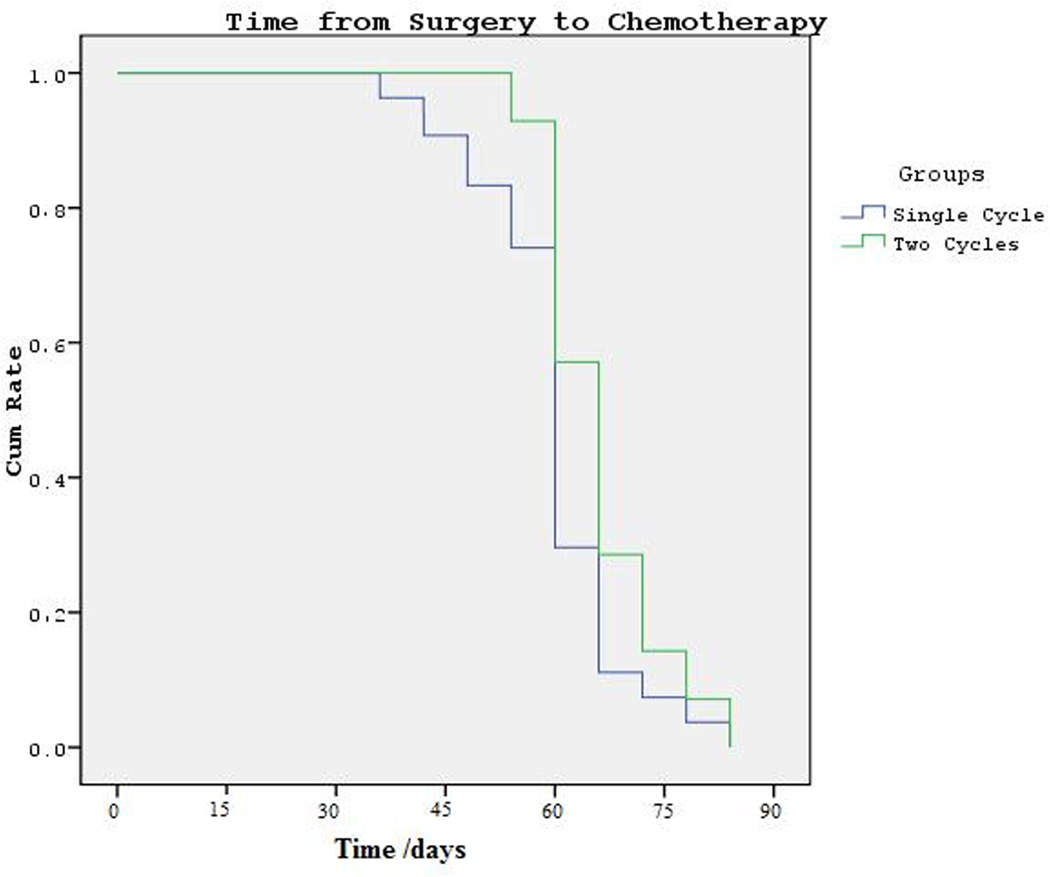
When mean time intervals from breast surgery to chemotherapy was considered, no statistical difference was seen with 63.7 ± 7.7 compared to 58.0 ± 12.1 days in patients undergoing two cycles vs. single cycle respectively. Chemotherapy was initiated on average 5.7 days earlier in performing single cycle compared to performing two cycles, though this difference did not reach statistical significance (p= 0.176) (Fig. 1). Furthermore, the mean time intervals from initial diagnosis (ID) to first ovarian stimulation and ID to chemotherapy were 36.8 ± 6.4 vs. 43.6 ± 13.4 and 81.3 ± 10.2 vs. 76.1 ± 10.4 days in patients undergoing two cycle and single cycle respectively (p= 0.057; p=0.096).
Figure 1.
Time interval from surgery to chemotherapy in patients undergoing two cycles vs. single cycle. After log rank test, no difference was found between two groups (p=0,176)
In terms of patient follow-up after cycle stimulation, the difference in mean follow-up time for single cycle patients was 56.6 ± 8.4 months vs. 67.5 ± 14.4 months for two cycle patients. Mean follow-up period for both groups was 58.5 ± 13.6 months. Twenty percent (n=12) of single cycle patients were lost to follow-up; however, baseline characteristics of these patients were consistent with lower risk of recurrence. Specifically they all had tumor size less than 2 cm with grade 1–2, and only 2 out of 12 patients had lymph node involvement. No breast cancer recurrence was observed in patients undergoing two cycles (0/17), compared to two recurrences in single cycle patients (2/49). This was not a statistically significant difference (p=0.548). One of these recurrences was local, while the other one was distant.
Discussion
The main principle of fertility preservation counseling should be to recommend and utilize the most successful and the least experimental approach for young women who are diagnosed with cancer (11). However, concerns related to estrogen exposure and the potential delay in the initiation of chemotherapy limit the access of women diagnosed with breast cancer to established procedures requiring ovarian stimulation, i.e. embryo cryopreservation and oocyte cryopreservation. We previously described that breast cancer recurrence rates are not increased after ovarian stimulation with the letrozole-gonadotropin protocol (12). The current study investigated the feasibility of performing 2 consecutive cycles prior to breast cancer treatment. In the present study, no recurrence in breast cancer was found in breast cancer patients undergoing two consecutive cycles of ovarian stimulation during 67.5 ± 14.4 months of follow-up. Nonetheless, future studies should be planned to compare the recurrence rates between single cycle and multiple cycles including longer time period of follow-up and larger numbers of patients.
Women with breast cancer typically have an interval of 6–8 weeks between surgery and the initiation of adjuvant chemotherapy and several studies demonstrated that chemotherapy could be delayed up to 12 weeks after surgery in patients with early stage breast cancer (7, 8). In the present study, the time interval from breast surgery to chemotherapy in patients undergoing two consecutive cycles of ovarian stimulation was 63.7 ± 7.7 days. Although this time period was longer when compared to performing single cycle (on average 5.7 days), the difference was not statistically significant and may not be considered meaningful clinically relevant. If patients are referred as early as possible after initial diagnosis, fertility preservation cycles can be initiated sooner and the time interval from surgery to chemotherapy is reduced (13). In the present study, 82.3 % of patients having two cycles were referred before breast cancer surgery whereas only 34.4 % of patients having single cycle were referred before surgery. Thus, early referral can be one of the major factors in determining the number of ovarian stimulation cycles performed. We have recently reported the importance of early referral (13). In that study, 93 patients with breast cancer were divided into two groups (before or after breast surgery) according to their referral time to reproductive specialist. While 9 of 35 patients who were in the pre-surgery group had a chance to undergo two consecutive ovarian stimulation cycles, only one of 58 patients in the post-surgery group had a chance to undergo two consecutive cycles. At the end of the study we have shown that the patients in the pre-surgery group initiated chemotherapy on average 24 days earlier compared with patients in the post-surgery group.
The American Society of Clinical Oncology (14) and the American Society for Reproductive Medicine recommends attention to the impact of cancer treatments on infertility. Despite the fact that 57% of patients reported substantial concern at diagnosis about infertility specialists (15), recent studies have indicated that still less than half of physicians routinely refer reproductive aged patients with cancer to reproductive specialist (16). As well as the reproductive specialist, breast surgeons and medical oncologist should be involved in the management of these patients to increase the likelihood of success rate of fertility preservation.
Small sample size and short follow-up time may be the limitations of this study. Additional studies are necessary, including those with a larger number of patients and longer follow-up period, to further evaluate these findings.
In conclusion, our study suggests that performing two consecutive cycles of ovarian stimulation with letrozole and gonadotropin protocol for fertility preservation prior to chemotherapy is feasible and can provide a larger number of oocytes and embryos for cryopreservation. Early referral may provide additional time for women with breast cancer to undergo two consecutive cycles without significant delay in the initiation of chemotherapy.
Supplementary Material
Supplemental figure 1 - Configuration of all breast cancer patients in this study
Acknowledgment
The authors thank Adanna Linda Anyikam (MD, MPH) for her contributions.
Funding: This work is partially funded by NICHD grants R01 HD053112 and R21 HD061259
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 2012;9:163–173. doi: 10.7150/ijms.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Wallberg KA, Oktay K. Options on fertility preservation in female cancer patients. Cancer Treat Rev. 2012;38:354–361. doi: 10.1016/j.ctrv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Checa Vizcaino MA, Corchado AR, Cuadri ME, Comadran MG, Brassesco M, Carreras R. The effects of letrozole on ovarian stimulation for fertility preservation in canceraffected women. Reprod Biomed Online. 2012;24:606–610. doi: 10.1016/j.rbmo.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 7.Cold S, During M, Ewertz M, Knoop A, Moller S. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG) Br J Cancer. 2005;93:627–632. doi: 10.1038/sj.bjc.6602734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 9.Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100:492–499. doi: 10.1016/j.fertnstert.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. 2005 Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99:1496–1502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28:4683–4686. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. Fertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. doi: 10.1200/JCO.2013.49.2678. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 16.Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–5957. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1 - Configuration of all breast cancer patients in this study