Schmallenberg virus (SBV) is a recently emerged Orthobunyavirus of ruminants originally discovered in 2011 near the town of Schmallenberg in Germany (Hoffmann and others 2012). SBV appears to have entered Europe in the summer of 2011 and has since spread rapidly across much of central and northern Europe. Seroprevalence in some areas has been reported close to 100 per cent (Tarlinton and others 2012, Meroc and others 2013). Viruses closely related to SBV are known to be transmitted by Culicoides biting midges (Jennings and Mellor 1989), and field studies have shown the presence of SBV RNA in Culicoides species in several affected countries (Rasmussen and others 2012, Elbers and others 2013).
SBV infection is associated with abortion and malformations in cattle and sheep, and has been shown to be neurotropic in lambs and calves infected in utero (van den Brom and others 2012, Varela and others 2013). In dairy cattle, an ‘acute’ form of the disease associated with a drop in milk yield, diarrhoea and mild pyrexia has also been observed. Here we report a within-herd study on a typical dairy farm located in southern England during 2012. The farm runs a dairy herd comprising approximately 230 Holstein cows, approximately 150 of which represent a milking herd. Importantly, no animals were imported onto the farm during the period of this study.
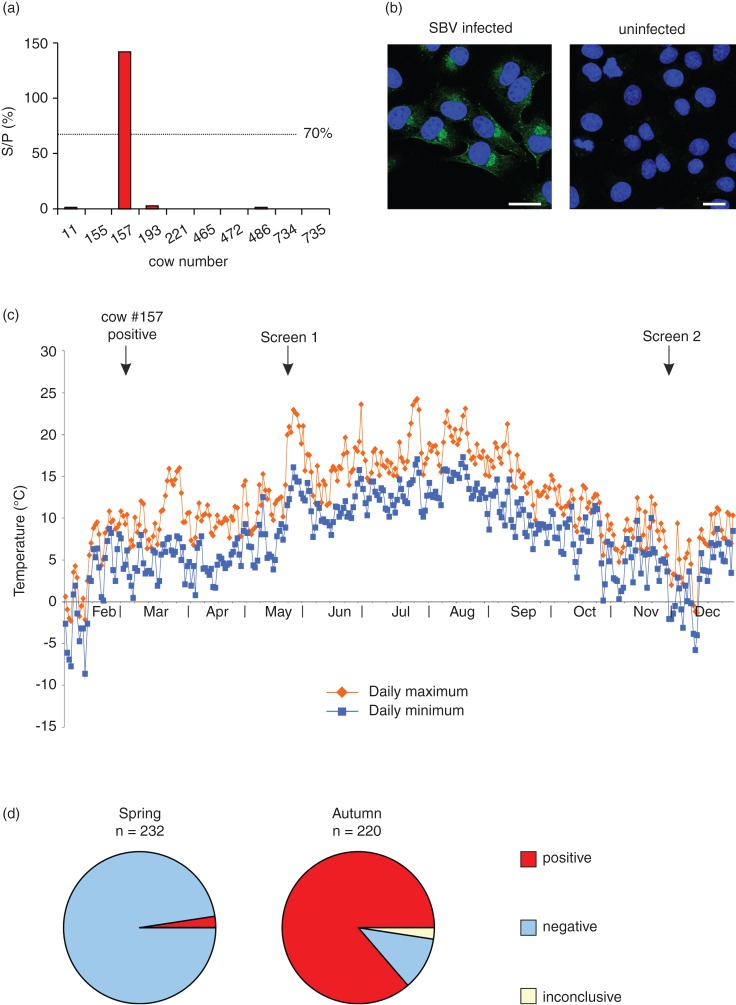
In February 2012, a cow (#157) aborted close to term. Suspecting SBV as the cause of abortion, we sampled the affected cow as well as nine additional animals using an indirect SBV antibody ELISA (IDvet). All animals were seronegative for SBV with the exception of cow #157 (Fig 1a). An identical result was obtained upon repeat testing using a second set of blood samples. We further confirmed the presence of anti-SBV antibodies by virus neutralisation assay (Loeffen and others 2012) (data not shown), and immunofluorescence using SBV or mock-infected BHK21 cells and sera from cow #157 (Fig 1b). Fluorescent signal was only observed in cells infected with SBV, while no cross-reaction was observed in uninfected cells. Together, these data indicate that SBV infection was present at least as far north as 51.5°N in the UK by February 2012, merely six to eight months after its first recorded appearance in Germany (Hoffmann and others 2012).
FIG 1:
(a) Sera of a selection of cattle tested for the presence of antibodies to Schmallenberg virus (SBV) in February 2012. Sample-to-positive ratios (S/P) are expressed as percentages and values >70 per cent are regarded as positive. All animals were negative with the exception of cow #157. (b) SBV infected (left) and uninfected (right) BHK21 cells were immunolabelled with sera from cow #157 and analysed by immunofluorescence microscopy. Fluorescent signal was only observed in cells infected with SBV. Bar=10μm. (c) Maximum and minimum daily temperatures for the period February–December 2012 at the nearest weather station (Lyneham, approximately 4.8 km from the farm described in this study), with arrows indicating dates of significance during this study. Data were obtained from the UK Meteorological Office, Met Office Integrated Data Archive System (MIDAS) Land and Marine Surface Stations Data (1853-current), NCAS British Atmospheric Data Centre, 2012. ‘Screen 1′ and ′Screen 2’ represent the points of whole-herd screening for SBV antibodies. (d) Seroprevalence was determined for the entire herd in the spring (May) and autumn (November) 2012, and was found to rise from 1.7 per cent in the spring to 89.1 per cent in the autumn.
Considering arbovirus replication within insect vectors, and the biting activity of the midges required for transmission is inherently reliant upon the ambient temperature, it is interesting to note that in the period immediately prior to the sampling of cow #157, the maximum temperature only reached approximately 10°C (Fig 1c). Using isotype-specific antibody ELISAs, we found IgG but not IgM antibodies in the serum of cow #157 (data not shown). These data suggest cow #157 had been infected for more than 10–14 days prior to sampling, although it is difficult to speculate the exact time of infection.
In May 2012 we screened the entire herd for the presence of SBV antibodies. Seroprevalence among all the animals tested in May was 1.7 per cent (n=232). Subsequently, we retested the herd in November 2012 (towards the end of the midge season), whereupon seroprevalence had risen to 89.1 per cent (n=220, Fig 1e).
During the period between spring and autumn samplings, numerous clinical cases similar to the ‘acute’ form of SBV infection were observed in the herd, with a sudden drop in milk yield for up to a week, followed by recovery, as described in other herds experiencing SBV infection. Similarly, a general increase in diarrhoea was observed among the herd during the summer period, although this observation is difficult to measure, may be multifactorial and retains an element of subjectivity. The dispersed nature of the episodes of acute disease suggests that the spread of infection proceeded over a protracted period of time, although it is difficult to retroactively diagnose acute SBV infection.
Interestingly, the heavy rainfall during the summer period resulted in the milking herd being at pasture on only two occasions (in total four days between May 31 and June 2, and between June 21 and 23) in the whole of 2012. In the remaining time, the herd was housed in open-plan sheds used commonly in the UK, with openings that make them freely accessible to insects.
The study reported here has uncovered valuable insights not necessarily revealed by national serosurveillance screens, and largely concurs with data from other studies of SBV ((EFSA) 2013, Meroc and others 2013, Wernike and others 2013). However, in contrast with some studies, we found a large increase in SBV herd prevalence during a period in which many of the animals were housed (Tarlinton and Daly 2013). For exophilic species of midge, for example, Culicoides imicola (Kieffer), stabling during periods of vector activity has historically been used as a way in which to reduce transmission risk (Meiswinkel and others 2007, 2000). This study, as well as previous reports during the recent Bluetongue virus epizootics, reaffirms that housing animals in farm buildings typical of those in the UK during periods of vector activity is not an effective measure against Culicoides-borne arbovirus infections in northern Europe, where the predominant Culicoides species are those of the C obsoletus species complex (Meiswinkel and others 2008, Baylis and others 2010, Viennet and others 2012). Heavy rain has been shown to suppress outdoors rather than indoors Culicoides activity (Baylis and others 2010), and it is therefore reasonable to suggest that substantial Culicoides-borne SBV transmission occurred inside the sheds during the summer of 2012. Clearly, outbreaks of arboviral diseases can still occur even when animals are not at pasture. It remains possible that significant ‘midge-proofing’ of buildings may offer some protection from midge-borne transmission, although this is likely to additionally depend upon climatic variables, the local landscape and husbandry practices. Further work is required to determine whether such measures are economically viable and the contexts in which they are effective.
Interestingly, the first case (cow #157) identified at this farm was diagnosed in February 2012. Therefore, cow #157 must have been infected either during the winter, when the outside temperature was never above 10°C, or in the summer/autumn of 2011 before or soon after the discovery of SBV in Germany.
Acknowledgments
The authors wish to thank the farm staff at Bishops Farm for their help in collecting samples. This work was funded by the BBSRC and the Wellcome Trust.
References
- Baylis M., Parkin H., Kreppel K., Carpenter S., Mellor P. S., Mcintyre K. M. (2010) Evaluation of housing as a means to protect cattle from Culicoides biting midges, the vectors of bluetongue virus. Medical and Veterinary Entomology 24, 38–45 [DOI] [PubMed] [Google Scholar]
- (EFSA), E. F. S. A. (2013) Technical report: “Schmallenberg” virus: analysis of the epidemiological data [Google Scholar]
- Elbers A. R., Meiswinkel R., Van weezep E., Sloet van oldruitenborgh-oosterbaan M. M., Kooi E. A. (2013) Schmallenberg virus in Culicoides spp. Biting midges, the Netherlands, 2011. Emerging Infectious Diseases 19, 106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Scheuch M., Hoper D., Jungblut R., Holsteg M., Schirrmeier H., Eschbaumer M., Goller K. V., Wernike K., Fischer M., Breithaupt A., Mettenleiter T. C., Beer M. (2012) Novel orthobunyavirus in Cattle, Europe, 2011. Emerging Infectious Diseases 18, 469–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M., Mellor P. S. (1989) Culicoides: biological vectors of Akabane virus. Veterinary Microbiology 21, 125–131 [DOI] [PubMed] [Google Scholar]
- Loeffen W., Quak S., De Boer-Luijtze E., Hulst M., Van der poel W., Bouwstra R., Maas R. (2012) Development of a virus neutralisation test to detect antibodies against Schmallenberg virus and serological results in suspect and infected herds. Acta Veterinaria Scandinavica 54, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiswinkel R., Baldet T., De deken R., Takken W., Del'ecolle J. C., Mellor P. S. (2007) Epidemiological Analysis of the 2006 Bluetongue Virus Serotype 8 Epidemic in North-Western Europe: Distribution and Dynamics of Vector Species. Parma: European Food Safety Authority [Google Scholar]
- Meiswinkel R., Baldet T., De deken R., Takken W., Delecolle J. C., Mellor P. S. (2008) The 2006 outbreak of bluetongue in northern Europe-the entomological perspective. Preventive Veterinary Medicine 87, 55–63 [DOI] [PubMed] [Google Scholar]
- Meiswinkel R., Baylis M., Labuschagne K. (2000) Stabling and the protection of horses from Culicoides bolitinos (Diptera: Ceratopogonidae), a recently identified vector of African horse sickness. Bulletin of Entomological Research 90, 509–515 [DOI] [PubMed] [Google Scholar]
- Meroc E., Poskin A., Van loo H., Quinet C., Van driessche E., Delooz L., Behaeghel I., Riocreux F., Hooyberghs J., De regge N., Caij A. B., Van den berg T., Van der stede Y. (2013) Large-scale cross-sectional serological survey of schmallenberg virus in belgian cattle at the end of the first vector season. Transbound Emergency Disease 60, 4–8 [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Kristensen B., Kirkeby C., Rasmussen T. B., Belsham G. J., BØdker R., BØtner A. (2012) Culicoids as vectors of schmallenberg virus. Emerging Infectious Diseases 18, 1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton R., Daly J. (2013) Testing for Schmallenberg virus. The Veterinary Record 172, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton R., Daly J., Dunham S., Kydd J. (2012) The challenge of Schmallenberg virus emergence in Europe. Veterinary Journal 194, 10–18 [DOI] [PubMed] [Google Scholar]
- Van den brom R., Luttikholt S. J., Lievaart-peterson K., Peperkamp N. H., Mars M. H., Van der poel W. H., Vellema P. (2012) Epizootic of ovine congenital malformations associated with Schmallenberg virus infection. Tijdschrift Voor Diergeneeskunde 137, 106–111 [PubMed] [Google Scholar]
- Varela M., Schnettler E., Caporale M., Murgia C., Barry G., Mcfarlane M., Mcgregor E., Piras I. M., Shaw A., Lamm C., Janowicz A., Beer M., Glass M., Herder V., Hahn K., Baumgartner W., Kohl A., Palmarini M. (2013) Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathogens 9, pe1003133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennet E., Garros C., Rakotoarivony I., Allene X., Gardes L., Lhoir J., Fuentes I., Venail R., Crochet D., Lancelot R., Riou M., Moulia C., Baldet T., Balenghien T. (2012) Host-seeking activity of bluetongue virus vectors: endo/exophagy and circadian rhythm of culicoides in Western Europe. PLoS ONE 7, pe48120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernike K., Kohn M., Conraths F. J., Werner D., Kameke D., Hechinger S., Kampen H., Beer M. (2013) Transmission of Schmallenberg Virus during Winter, Germany. Emerging Infectious Diseases 19, 1701–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]