Abstract
Background
Current recommendations for the management of axial spondyloarthritis (SpA) and psoriatic arthritis are to monitor disease activity and adjust therapy accordingly. However, treatment targets and timeframes of change have not been defined. An international expert panel has been convened to develop ‘treat-to-target’ recommendations, based on published evidence and expert opinion.
Objective
To review evidence on targeted treatment for axial and peripheral SpA, as well as for psoriatic skin disease.
Methods
We performed a systematic literature search covering Medline, Embase and Cochrane, conference abstracts and studies in http://www.clinicaltrials.gov.
Results
Randomised comparisons of targeted versus routine treatment are lacking. Some studies implemented treatment targets before escalating therapy: in ankylosing spondylitis, most trials used a decrease in Bath Ankylosing Spondylitis Disease Activity Index; in psoriatic arthritis, protocols primarily considered a reduction in swollen and tender joints; in psoriasis, the Modified Psoriasis Severity Score and the Psoriasis Area and Severity Index were used. Complementary evidence correlating these factors with function and radiographic damage at follow-up is sparse and equivocal.
Conclusions
There is a need for randomised trials that investigate the value of treat-to-target recommendations in SpA and psoriasis. Several trials have used thresholds of disease activity measures to guide treatment decisions. However, evidence on the effect of these data on long-term outcome is scarce. The search data informed the expert committee regarding the formulation of recommendations and a research agenda.
Keywords: Spondyloarthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Treatment
Introduction
Recommendations from the Ankylosing Spondylitis Assessment Study (ASAS)/European League Against Rheumatism (EULAR) for the management of ankylosing spondylitis (AS)1 and from EULAR for the management of psoriatic arthritis (PsA)2 are to monitor the disease,1 2 adjust treatment appropriately,2 and adapt the frequency of monitoring depending on the course and severity of the disease.1
However, no evidence that a guided treatment strategy is as effective for AS and PsA as it is for rheumatoid arthritis (RA)3 has yet been established. This is partly due to the heterogeneity of the presentations of these and related diseases, which some would group under the broader term, spondyloarthritis (SpA). In fact, it has been suggested that the terms axial SpA and peripheral SpA could be considered rather than the traditional names.4 To address this issue, an international panel of expert rheumatologists and patients convened to discuss recommendations on a ‘treat-to-target’ (T2T) concept for SpA. In line with respective recommendations by EULAR,5 a systematic literature review of the current state of evidence was deemed necessary. In the following, we present this systematic literature review, which served as the background for generating the recommendations document.6
Methods
We performed a systematic literature search of the Medline, Embase and Cochrane databases. This search was based on a PICO (population, intervention, control and outcome) strategy and search terms developed in the course of discussions of the task force's steering committee. Box 1 shows the PICO strategy, and online supplementary table S1 lists the search terms.
Box 1. PICO strategy.
Population: adult patients with axial or peripheral SpA or psoriasis
Intervention: targeted use of NSAIDs, synthetic DMARDs or biologicals
Control: routine treatment
Outcome: the applied definition of a therapeutic target; parameters of disease activity that serve as surrogates for clinical, functional or radiographic success
Design: ‘strategy trial’: interventional, prescheduled therapeutic adaptation; RCT, open-label controlled, or single-arm study
Duration: any given follow-up
Excluded:
DX: degenerative and dialysis-associated SpA, psoriasis, spondylodiscitis
TX: intervention other than drugs (surgery, physiotherapy, balneotherapy, hydrotherapy, exercise, radon, cryotherapy, mud bath), excluded drugs (bisphosphonates, antidepressants, complementary and alternative medicine (CAM)) and excluded applications (intra-articular injections, intravascular steroids)
Study setting: non-interventional (ie, observational/retrospective)
Publication form: letters, editorials, narrative reviews
CAM, ; DMARD, disease-modifying antirheumatic drug; DX, diagnosis; NSAID, non-steroidal anti-inflammatory drug; RCT, randomised controlled trial; PICO, population, intervention, control, outcome; SpA, spondyloarthritis; TX, treatment.
We retrieved publications from each database's inception to September 2011. We also screened 2010 and 2011 EULAR and American College of Rheumatology (ACR) conference abstracts7 8 and accessed the US National Institutes of Health (NIH) database on clinical trials.9 We selected eligible studies according to our inclusion criteria (see box 1 and online supplementary table S1) and compiled the applied measures of disease activity and the thresholds and timelines that guided the decision to change therapy in the respective study protocols. The primary aim of the search was retrieval of strategic studies that compared a therapy steered towards a prespecified treatment target versus a conventional, non-steered approach, as is available for RA.10 Secondly, we reviewed ancillary literature providing circumstantial evidence that a steered therapy might be beneficial during long-term follow-up.
Results
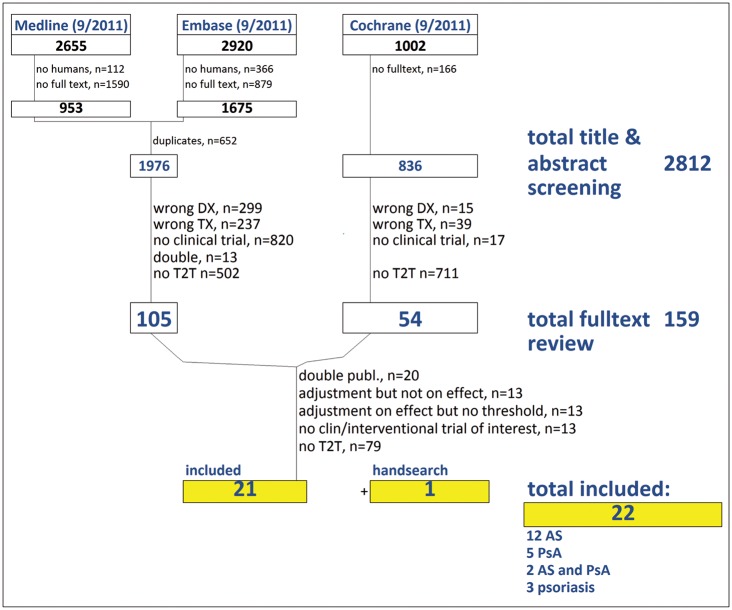
We initially retrieved 1976 publications in Medline and Embase, and 1002 in Cochrane. By title and abstract screening, we selected 159 of these for full-text review, and finally included 21 papers plus one additional publication found by hand-search. Of these, 12 trials enrolled patients with AS, five included patients with PsA, and two studies included both AS and PsA patients (table 1). No studies on peripheral SpA were obtained; three studies addressed patients with psoriasis. No conference abstracts and no trials from the NIH database provided data on treatment targets. Figure 1 illustrates the search and selection process.
Table 1.
Treatment targets and timeline definition in trials of ankylosing spondylitis and psoriatic arthritis
| Measure of disease activity | Target definition | Assessment after | Study (drug) |
|---|---|---|---|
| Ankylosing spondylitis | |||
| ASAS | ≥20% response | Week 12 (OLE) | ATLAS (ADA)*11 |
| BASDAI | <3 at both current and prior assessment | Week 36 | ASSERT (IFX)†12 |
| ASAS | ≥40% response | Week 12 | Haibel (ADA)*14 |
| BASDAI | ≥50% reduction, or ≤3 | Week 22 and 38 | CANDLE (IFX)†15 |
| BASDAI | ≥20% reduction | Month 3 | Jois (IFX)17 |
| ≥50% reduction | Month 6 | ||
| BASDAI | ≥40% reduction | Week 14 | Cherouvim (IFX)*18 |
| ESR | ≥1 mm reduction per week: escalate ≤20 (women)/≤10 (men) mm/h for step down Remission: ESR ≤10 (men ≤5) and BASDAI, BASFI, BASG, BASMI scores mean <1: taper |
Weekly for escalation Month 6 for step down |
Darmawan (IS)†21 |
| Combined/alternative targets | |||
| Total back pain (VAS), MST (min) | ≥20% reduction in both back pain and MST | Week 16 | GO-RAISE (GOL)†13 |
| BASDAI, IFX serum level | <40 and 5.0 μg/ml |
After 4th IFX (∼22 weeks) | Meric (IFX)*16 |
| BASDAI, ESR/CRP | <4 (BASDAI) or <30 mm/h ESR and <5 mg/l CRP |
Week 38 | Collantes (IFX)†19 |
| MST (VAS), pain (VAS), ESR | ≥20% reduction in 2/3 | Week 4 | Van Denderen (mesalazine)*20 |
| BASDAI, ESR/CRP | ≥2 patients. BASDAI reduction and ≥20% ESR/CRP reduction |
Week 2, then 6-weekly | Cheung (IFX)22 |
| Q1: disease has remained under control? Q2: disease has been worsening? VAS pain, BASDAI |
No relapse; definition: Q1 ‘Yes’ and Q2 ‘No’ and either <2/10 pain increase and <1/10 BASDAI increase |
≥4 weeks after stopping for on-demand week 40 for dose escalation |
Breban (IFX)†23 |
| BASMI, PhysGA | No relapse; definition: ≤4 BASMI and ≤4 PhysGA |
26 weeks after stop | Braun (IFX)†24 |
| Psoriatic arthritis | |||
| TJC and SJC | ≥20% reduction | 12 weeks | ADEPT (ADA)25 |
| TJC and SJC | ≥10% reduction | 16 weeks | GO-REVEAL (GOL)26 |
| TJC and SJC combined N | ≥20% reduction | 38 and 46 weeks | IMPACT 2 (IFX)27 |
| Joint count ‘actively inflamed’ | ≥30% reduction | 14 weeks | Feletar (IFX)28 |
| Joint count | ≥40% reduction | 3 months | Rahman (SSZ)29 |
| PGA | ≥40% reduction | 14 weeks | Cherouvim (IFX)18 |
| BASDAI, ESR/CRP | <4 (BASDAI) or <30 mm/h ESR and <5 mg/l CRP |
Week 38 (cave diff AB 30/text38) | Collantes (IFX)†19 |
| Psoriasis | |||
| MPSS | MPSSpresent visit >MPSSprevious visit−0.2* (MPSSprevious visit−MPSSbaseline) | Max 18 weeks | De Jong (MTX)37 |
| PASI | Improvement >25% | 6 weeks | Beissert (CsA, MMF)38 |
| PASI | Improvement ≥75% | 12 weeks | Nevin (CsA)39 |
*Target measure is identical with primary end point measure.
†Target measure is not identical with primary end point measure.
ADA, adalimumab; ADEPT, Adalimumab Effectiveness in Psoriatic Arthritis Trial; ASAS, Ankylosing Spondylitis Assessment Study; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASG, Bath Ankylosing Spondylitis Global Score; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C-reactive protein; CsA, ciclosporin; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; GOL, golimumab; IFX, infliximab; IS, immunosuppressant therapy (consisting of combined DMARDs); mesa, mesalazine; MMF, mycophenolate mofetil; MPSS, Modified Psoriasis Severity Score; MST, morning stiffness; MTX, methotrexate; OLE, open label extension; PASI, Psoriasis Area Severity Index; PGA, Patient global assessment of disease activity; PhysGA, physician global assessment; Q1, Q2, question 1 and 2; SJC, swollen joint count; SSZ, sulfasalazine; TJC, tender joint count; VAS, visual analogue scale.
Figure 1.
Search and selection process. AS, ankylosing spondylitis; clin, clinical; DX, diagnosis; PsA, psoriatic arthritis; publ., publication; T2T, treatment to target; TX, treatment.
The most important result of the search was the failure to find any randomised comparison evaluating a T2T approach versus routine treatment. However, several publications report on targets and timelines that were used as thresholds before escalating therapy.
Axial SpA (including AS and non-radiographic axial SpA)
Overall, we found 14 studies11–24 with predetermined treatment targets in AS that were suitable for inclusion. Table 1 specifies the measures of disease activity or function and timelines as well as cut-off points used as indication of (in)sufficient response. The baseline characteristics of the study populations were comparable with regard to inclusion criteria, disease activity, function, age and disease duration (online supplementary table S2 lists details of the included studies and baseline characteristics of the patients).
Definitions of treatment targets and timelines
The majority of studies used the Bath AS Disease Activity Index (BASDAI) at follow-up for treatment ‘escalation’ until a prespecified outcome was achieved.12 15 17 18 This outcome was defined as BASDAI<3 at two consecutive assessments starting from weeks 30 and 36 in one trial,12 while in most studies, a percentage reduction from baseline was required, being either ≥20% after 12 weeks,17 ≥40% after 14 weeks18 or ≥50% after 6 months.15 17 Two protocols required a decline of ≥20%11 or ≥40%14 in the response criteria of the ASAS after 12 weeks. One study21 based treatment decisions on the erythrocyte sedimentation rate (ESR) at follow-up and required a ≥1 mm reduction per week. One trial that included AS and PsA patients18 required a ≥40% reduction in patient global assessment of disease activity (PGA) after 14 weeks, otherwise infliximab (IFX) frequency was increased from an 8-weekly to a 4-weekly schedule (table 1).
Several authors used combined targets, mostly combinations of the BASDAI19 22 or the Bath AS Metrology Index (BASMI)24 with either acute phase reactants19 22 or the physician global assessment (PhysGA).24 Meric et al16 measured serum IFX levels after four infusions to customise infusion schedules previously determined according to the BASDAI. Reductions in morning stiffness and pain were used to adjust golimumab therapy13 and—expanded by the ESR—also to guide dose escalations of mesalazine.20 Cheung et al22 reported therapeutic outcomes using Australian Pharmaceutical Benefit Schedule standards, which only reinforce ‘continuation’ of IFX after decline of BASDAI by ≥2 points and ≥20% improvement in ESR and/or C-reactive protein (CRP) (table 1). Several studies tested the efficacy of ‘on-demand’ treatment in the case of relapse after cessation of IFX.23 24 The definition of relapse was based on a short questionnaire in combination with BASDAI and an increase in acute phase reactants (table 1),23 or an absolute BASMI or PhysGA of ≥4.24 Therapy was tapered according to ESR,21 BASDAI and serum IFX levels16 (table 1).
In AS, prospective analyses to identify the predictive value of the above measures for long-term functional and radiographic outcomes have not been carried out.
Psoriatic arthritis
Seven studies fulfilled our inclusion criteria for PsA.18 19 25–29 Table 1 details their treatment targets. Online supplementary table S2 shows study details and patients’ baseline characteristics.
In the majority, the treatment target was a reduction in swollen and tender joint counts.26–29 The prespecified decrease for a treatment to be considered sufficiently effective was a reduction in joint counts of ≥10% after 16 weeks,26 ≥20% after 38 and 46 weeks,27 29 ≥30% after 14 weeks28 or ≥40% after 3 months.29 Two trials18 19 included mixed SpA populations and used ≥40% reduction in PGA after 14 weeks18 or ESR and CRP19 (table 1). Some prospective studies investigated the correlation between clinical symptoms and progression of radiographic damage and reported a predictive capacity of synovitis,30–32 dactylitis33 and CRP,34 while other authors did not observe these associations.35 Serological markers that can predict long-term outcome in PsA are under investigation.36
There were no trials available that specifically investigated targeted treatment in other peripheral SpAs or contributed evidence on correlation with long-term outcomes.
Psoriasis
In psoriasis also, there are no randomised controlled trials available to compare T2T with routine treatment. The Modified Psoriasis Severity Score (MPSS) was used to titrate weekly dosage of methotrexate,37 and the Psoriasis Area and Severity Index (PASI) was used to titrate ciclosporin38 39 or mycophenolate mofetil38 (table 1 and online supplementary table S2). Other than that, there has been no defined target to guide treatment escalation, although some studies used thresholds to decide whether to pause therapy—for example, to pause etanercept as soon as a target of PGA of ≤2 (clear, almost clear or mild) was reached.40
Discussion and conclusion
We present a systematic review of targeted treatment for SpA and psoriasis that informed the consensus-finding process of the expert committee for T2T-SpA recommendations.
Randomised trials designed to compare targeted treatment with another type of care are not available, but evidence can be derived from studies that apply target-oriented treatment adaption. The majority of designs suggest use of the BASDAI to evaluate therapeutic response in AS (but other composite measures such as ASDAS41 42 seem to be increasingly used), swollen and tender joint counts for PsA, and MPSS and PASI for psoriasis. In many studies, response was evaluated after 12–14 weeks, while others stretched out to 36 weeks. Importantly, no information on long-term outcomes is available. Composite measures of disease activity have not yet been formally evaluated for PsA. Likewise, no such studies are available for other peripheral spondyloarthritides including reactive arthritis. Some trials for reactive arthritis used antibiotic therapy (reviewed by Hannu43). These studies are not included here because they did not use criteria for insufficient response.
The definition of pertinent treatment targets for SpA is challenging because of the heterogeneity of the disease, including axial, peripheral and extra-articular/extraspinal manifestations. Moreover, no data on a positive effect on physical function and radiographic damage resulting from a T2T strategy have been published for SpA.
The data presented informed the task force on the current state of evidence and clearly reveal that further research is needed. In particular, clinical trials comparing the value of treatment steered by levels of disease activity versus conventional therapy in SpA, both axial and peripheral, are needed.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it was published Online First. The fourth author affiliation has been amended.
Contributors: MS performed the search, and all authors contributed to the manuscript and finally approved it.
Funding: Supported by an unrestricted grant from Abbott/Abbvie.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 2012;71:4–12 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, Bijlsma JW, et al. for the T2T Expert Committee. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83 [DOI] [PubMed] [Google Scholar]
- 5.Dougados M, Betteridge N, Burmester GR, et al. EULAR standardised operating procedures for the elaboration, evaluation, dissemination, and implementation of recommendations endorsed by the EULAR standing committees. Ann Rheum Dis 2004;63:1172–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Braun J, Dougados M, et al. ; for the T2T Expert Committee Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Submitted for publication [DOI] [PMC free article] [PubMed]
- 7.http://www.abstracts2view.com/eular/[Online]
- 8.http://www.rheumatology.org/publications/acr_arhp_annual_meeting.asp [Online]
- 9.http://www.clinicaltrials.gov [Online]
- 10.Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010;69:638–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijde D, Kivitz A, Schiff MH, et al. ; for the ATLAS Study Group Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46 [DOI] [PubMed] [Google Scholar]
- 12.Van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91 [DOI] [PubMed] [Google Scholar]
- 13.Inman RD, Davis JC, van der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402–12 [DOI] [PubMed] [Google Scholar]
- 14.Haibel H, Rudwaleit M, Listing J, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91 [DOI] [PubMed] [Google Scholar]
- 15.Inman RD, Maksymowych WP; for the CANDLE Study Group A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010;37:1203–10 [DOI] [PubMed] [Google Scholar]
- 16.Meric JC, Mulleman D, Ducourau E, et al. Therapeutic drug monitoring of infliximab in spondyloarthritis: an observational open-label study. Ther Drug Monit 2011;33:411–16 [DOI] [PubMed] [Google Scholar]
- 17.Jois RN, Leeder J, Gibb A, et al. Low-dose infliximab treatment for ankylosing spondylitis–clinically- and cost-effective. Rheumatol (Oxford) 2006;45:1566–9 [DOI] [PubMed] [Google Scholar]
- 18.Cherouvim EP, Zintzaras E, Boki KA, et al. Infliximab therapy for patients with active and refractory spondyloarthropathies at the dose of 3 mg/kg: a 20-month open treatment. J Clin Rheumatol 2004;10:162–8 [DOI] [PubMed] [Google Scholar]
- 19.Collantes-Estevez E, Munoz-Villanueva MC, Zarco P, et al. Effectiveness of reducing infliximab dose interval in non-responder patients with refractory spondyloarthropathies. An open extension of a multicenter study. Rheumatology (Oxford) 2005;44:1555–8 [DOI] [PubMed] [Google Scholar]
- 20.Van Denderen JC, van der Horst-Bruinsma I, Bezemer PD, et al. Efficacy and safety of mesalazine (Salofalk) in an open study of 20 patients with ankylosing spondylitis. J Rheumatol 2003;30:1558–60 [PubMed] [Google Scholar]
- 21.Darmawan J, Nasution AR, Chen SL, et al. Excellent endpoints from step-down bridge combination therapy of 5 immunosuppressants in NSAID-refractory ankylosing spondylitis: 6 year international study in Asia—WHO-ILAR COPCORD stage II treatment of the autoimmune diseases. J Rheumatol 2006;33:2484–92 [PubMed] [Google Scholar]
- 22.Cheung PPM, Tymms KE, Wilson BJ, et al. Infliximab in severe active ankylosing spondylitis with spinal ankylosis. Intern Med J 2008;38:396–401 [DOI] [PubMed] [Google Scholar]
- 23.Breban M, Ravaud P, Claudepierre P, et al. Maintenance of infliximab treatment in ankylosing spondylitis: results of a one-year randomized controlled trial comparing systematic versus on-demand treatment. Arthritis Rheum 2008;58:88–97 [DOI] [PubMed] [Google Scholar]
- 24.Braun J, Baraliakos X, Listing J, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis 2008;67:340–5 [DOI] [PubMed] [Google Scholar]
- 25.Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 2009;68:702–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86 [DOI] [PubMed] [Google Scholar]
- 27.Kavanaugh A, Krueger GG, Beutler A, et al. ; for the IMPACT 2 Study Group Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007;66:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feletar M, Brockbank JE, Schentag CT, et al. Treatment of refractory psoriatic arthritis with infliximab: a 12 month observational study of 16 patients. Ann Rheum Dis 2004;63:156–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman P, Gladman DD, Cook RJ, et al. The use of sulfasalazine in psoriatic arthritis: a clinical experience. J Rheumatol 1998;25:1957–61 [PubMed] [Google Scholar]
- 30.Simon P, Pfoehler C, Bergner R, et al. Swollen joint count in psoriatic arthritis is associated with progressive radiological damage in hands and feet. Clin Exp Rheumatol 2012;30:45–50 [PubMed] [Google Scholar]
- 31.Cresswell L, Chandran V, Farewell VT, et al. Inflammation in an individual joint predicts damage to that joint in psoriatic arthritis. Ann Rheum Dis 2011;70:305–8 [DOI] [PubMed] [Google Scholar]
- 32.Bond SJ, Farewell VT, Schentag CT, et al. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brockbank JE, Stein M, Schentag CT, et al. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis 2005;64:188–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladman DD, Mease PJ, Choy EH, et al. Risk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPT. Arthritis Res Ther 2010;12:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane D, Stafford L, Bresnihan B, et al. Prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatol (Oxford) 2003;42:1460–8 [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald O, Chandran V. Update on biomarkers in psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J Rheumatol 2012;39:427–30 [DOI] [PubMed] [Google Scholar]
- 37.De Jong EMGJ, Mork NJ, Seijger MMB, et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: results of a multicentre placebo-controlled randomized trial. Br J Dermatol 2003;148:318–25 [DOI] [PubMed] [Google Scholar]
- 38.Beissert S, Pauser S, Sticherling M, et al. A comparison of mycophenolate mofetil with ciclosporine for the treatment of chronic plaque-type psoriasis. Dermatology 2009;219:126–32 [DOI] [PubMed] [Google Scholar]
- 39.Nevin RJ, Schulz EJ. Treatement of psoriasis with cyclosporine. S Afr Med J 1995;85:1165–8 [PubMed] [Google Scholar]
- 40.Dauden E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol 2009;23:1374–82 [DOI] [PubMed] [Google Scholar]
- 41.Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24 [DOI] [PubMed] [Google Scholar]
- 42.Machado P, Landewe R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53 [DOI] [PubMed] [Google Scholar]
- 43.Hannu T. Reactive arthritis. Best Pract Res Clin Rheum 2011;25:347–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.