Abstract
Objectives
To report the effect of different imputation methodologies on the assessment of radiographic progression in clinical trials.
Methods
The 216-week RAPID-psoriatic arthritis (PsA) (NCT01087788) trial of certolizumab pegol (CZP) in patients with active PsA was double-blind and placebo-controlled until week 24. A primary end point was change from baseline in modified Total Sharp Score(s) (mTSS). Prespecified imputation methodology in patients with fewer than two analysable mTSS used minimum observed baseline score for missing baseline values and maximum observed week 24 score for missing week 24 values. Post hoc analyses used alternative methods of imputation in patients with fewer than two analysable mTSS. mTSS non-progressors were defined as patients with ≤0 (predefined) or ≤0.5 (post hoc) change in mTSS from baseline to week 24. Baseline mTSS and C-reactive protein levels as predictors of radiographic progression were investigated.
Results
409 patients were randomised. Baseline demographics were similar between groups. Prespecified imputation analysis inappropriately overestimated radiographic progression (least squares mean placebo, 28.9; CZP, 18.3; p≥0.05). Multiple post hoc analyses demonstrated that CZP inhibited radiographic progression compared with placebo, particularly in patients with high baseline mTSS and C-reactive protein levels. mTSS non-progression rate was higher in CZP than placebo groups in all analyses.
Conclusions
Inappropriate prespecified imputation methodology resulted in an unrealistic assessment of progression in all arms. Methodologies for imputing missing radiographic data can greatly affect assessment and reporting of mTSS progression.
Keywords: Anti-TNF, Psoriatic Arthritis, Outcomes research
Introduction
In clinical practice and clinical trials, structural damage caused by rheumatic diseases, including psoriatic arthritis (PsA), is commonly assessed by radiography.1–4 However, progression of structural damage can be difficult to assess accurately in clinical trials.1 Moreover, compared with historic PsA trials, placebo patients in recent trials have very low rates of progression over the double-blind period, with the majority of these patients considered non-progressors.5 6
In most placebo-controlled trials, control arm patients are more likely to discontinue therapy because of lack of efficacy compared with those on active treatment. The use of early-escape trial designs, where non-responsive or minimally responsive placebo patients switch to active treatment as early as week 12, creates further imbalance.1 7 This follow-up period imbalance necessitates the imputation of missing data, particularly radiographic end points, which are typically measured after mandatory withdrawal and escape to active therapy.1
Linear extrapolation and interpolation, requiring radiographic data from two or more time points, is the most widely used approach to imputing missing radiographic data. Patients with fewer than two data points have historically been excluded from radiographic analyses.1 3 4 However, in order to avoid reporting bias, techniques for including patients regardless of data availability are also used in clinical trials.5
To include data from all patients, a prespecified methodology to impute missing values from patients with fewer than two radiographic time points is necessary.2 3 Imputation rules, such as using a value from the observed population (mean, median, maximum scores of changes from baseline), can be used to estimate progression in patients with missing radiographic data; however, the impact of these different assumptions on population outcomes has not been published.
In this article, results from the multinational phase III RAPID-PsA study of certolizumab pegol (CZP) treatment in patients with PsA are presented in order to discuss the effect of different imputation methodologies on the evaluation of radiographic progression.
Methods
Patients
Full details of the ongoing 216-week RAPID-PsA study design (NCT01087788), including the patient population, are reported elsewhere.8 Briefly, 409 adult patients with PsA according to CASPAR criteria9 were recruited. Patients were required to have active musculoskeletal disease, an inadequate response to one or more disease-modifying antirheumatic drug, and active psoriatic skin lesions or a documented history of psoriasis.
Study design
RAPID-PsA was double-blind, placebo-controlled to week 24. Patients were randomised 1:1:1 to placebo or 400 mg CZP at week 0, 2 and 4 followed by 200 mg CZP every 2 weeks (Q2W) or 400 mg CZP every 4 weeks (Q4W). Placebo patients who did not achieve a predefined minimal response at week 14 and week 16 escaped to active treatment at week 16.
Study procedures and evaluations
One of the two primary end points was change from baseline to week 24 in the van der Heijde modified Total Sharp Score (mTSS), adapted for PsA.10 This methodology quantifies the extent of bone erosions and joint space narrowing in distal interphalangeal, proximal interphalangeal, metacarpophalangeal, metatarsophalangeal and wrist joints. Radiographs of both hands and feet were taken at baseline, then at week 12 and week 24 or an early withdrawal visit, using standardised imaging methodology. Radiographs were read centrally and independently by two experienced readers (blinded to treatment assignment and image time course). Discrepancies between readers of ≥10 mTSS points were adjudicated by a third blinded reviewer. The mean of the two independent reviews, or the mean of the adjudicated score and the closest result, was used.
The proportion of mTSS non-progessors (predefined as ≤0 mTSS change from baseline) was assessed. Post hoc non-progressor analyses used a ≤0.5 mTSS change from baseline definition, based on published guidelines relating to the use of multiple readers.11
Post hoc subgroup analyses of mTSS change from baseline and mTSS non-progressor rates were also evaluated in patients with a high risk of progression, defined as baseline C-reactive protein (CRP) levels >15 mg/L or mTSS higher than the population median.
Statistical analysis
The sample size of 130 for each treatment group was sufficient to detect statistically significant differences in the mean change from baseline in the mTSS between the combined active and placebo groups with at least 95% power, assuming a difference of ≥1.0 and a standard deviation (SD) of 2.4 points.
Treatment comparisons for the combined CZP dose groups versus placebo for change from baseline in mTSS were evaluated using an analysis of covariance model, with treatment, region and prior tumour necrosis factor inhibitor exposure as factors, baseline mTSS as covariate, and mTSS change from baseline as the dependent variable. Linear interpolation and extrapolation of missing data points was used in patients with two or more analysable mTSS, irrespective of time interval between the two scores. Prespecified non-progressor analysis used non-responder imputation (NRI), where placebo patients escaping to CZP and patients withdrawing early from the study were considered mTSS progressors. Post hoc non-progressor analysis used linear interpolation and extrapolation of missing data points for patients with two or more analysable mTSS.
Prespecified methodology for patients with fewer than two analysable mTSS used the minimum observed baseline mTSS for missing baseline values and the maximum observed week 24 mTSS for missing week 24 values. A number of alternative methods of imputation, defining change from baseline mTSS for patients with fewer than two analysable mTSS were assessed as part of post hoc analyses including substituting by mean or median or maximum (see table 1 for all different options). For all post hoc analyses, the mTSS had to be obtained at least 8 weeks apart.
Table 1.
Post hoc analyses of change from baseline at week 24 in mTSS
| Imputation in patients with <2 analysable mTSS* | mTSS change from BL at week 24 | ||||
|---|---|---|---|---|---|
| Placebo (n=136) | CZP 200 mg Q2W (n=138) | CZP 400 mg Q4W (n=135) | CZP 200 mg Q2W+CZP 400 mg Q4W (n=273) | ||
| Prespecified† | Minimum observed score (0) if missing BL Maximum observed score (356.5) if missing week 24 |
28.9 (7.73) | 11.5 (7.59) p=0.071‡ |
25.1 (7.92) p=0.688‡ |
18.3 (6.07) p=0.203 |
| Post hoc 1§ | No imputation | 0.29 (0.08) | 0.01 (0.08) p=0.004‡ |
0.12 (0.08) p=0.083‡ |
0.06 (0.06) p=0.008 |
| Post hoc 2¶ | Median mTSS change from BL of all patients observed | 0.28 (0.07) | 0.01 (0.07) p=0.004‡ |
0.11 (0.08) p=0.072‡ |
0.06 (0.06) p=0.007 |
| Post hoc 3¶ | Mean mTSS change from BL of all patients observed | 0.28 (0.07) | 0.01 (0.07) p=0.004‡ |
0.11 (0.08) p=0.072‡ |
0.06 (0.06) p=0.007 |
| Post hoc 4¶ | Maximum mTSS change from BL of all patients observed | 0.66 (0.13) | 0.18 (0.13) p=0.003‡ |
0.52 (0.13) p=0.380‡ |
0.35 (0.10) p=0.028 |
| Post hoc 5¶ | Maximum mTSS change from BL by treatment group | 0.39 (0.11) | 0.14 (0.11) p=0.077‡ |
0.49 (0.12) p=0.483‡ |
0.31 (0.09) p=0.538 |
Values are least squares mean (SE). For placebo patients who escaped early to CZP, the week 24 values were linearly extrapolated. p Values of CZP versus placebo are based on analysis of covariance.
*For patients with two radiographs but missing week 24 or baseline film, linear extrapolation was performed in all approaches.
†21 patients had fewer than two analysable mTSS; the number of patients was not equally distributed between the treatment groups.
‡The study was not powered to detect statistical significance between individual CZP dosage groups and placebo, therefore, these p values should be considered nominal.
§26 patients had fewer than two analysable mTSS more than 8 weeks apart (required for post hoc analysis).
¶28 patients had fewer than two analysable mTSS more than 8 weeks apart, including two placebo patients who escaped to CZP before their second mTSS.
BL, baseline; CZP, certolizumab pegol; mTSS, modified Total Sharp Score; Q2W, every 2 weeks; Q4W, every 4 weeks.
Results
Patients
The demographic and baseline characteristics of the treatment groups were generally well balanced (see online supplementary table S1).8 In total, 368 (90%) patients completed the 24-week, double-blind phase of the study.8
Overall, 21 patients had no or one available mTSS (eight placebo, four CZP 200 mg Q2W, nine CZP 400 mg Q4W) (see online supplementary figure S1). An additional five patients had two mTSS less than 8 weeks apart (range 1−6 weeks), and two placebo patients escaped to active treatment without a week 12 mTSS (see online supplementary figure S1).
Treatment efficacy (prespecified analyses)
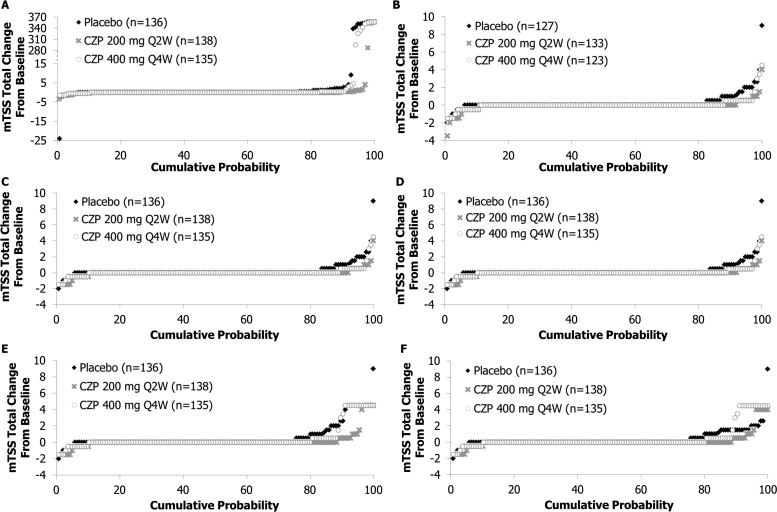
Most patients experienced no change in mTSS over 24 weeks (figure 1A). Overall, 27 patients had an mTSS of 0 at baseline; none of these patients had experienced mTSS progression by week 24. One patient was scored with a −1 improvement in mTSS at week 1; linear extrapolation resulted in an unrealistic improvement of −24 points over 24 weeks (figure 1A).
Figure 1.
Cumulative probability plots of modified Total Sharp Score (mTSS) total change, with data from patients with fewer than two available radiographs (A) imputed using prespecified imputation method, (B) not imputed, (C) imputed using median mTSS change from baseline of observed patients, (D) imputed using mean mTSS change from baseline of observed patients, (E) imputed using maximum mTSS change from baseline of observed patients and (F) imputed using maximum mTSS change from baseline by treatment group. CZP, certolizumab pegol; Q2W, every 2 weeks; Q4W, every 4 weeks.
At baseline, the lowest observed mTSS was 0. At 24 weeks, the largest was 356.5. Consequently, four patients without mTSS were assigned a progression of 356.5. In addition, 16 patients with only analysable baseline mTSS were assigned mTSS progressions ranging from 287.5 to 356.5 (figure 1A). The imputation methodology led to an estimated mean mTSS change from baseline of 11.5 (CZP 200 mg Q2W) and 25.1 (CZP 400 mg Q4W) vs 28.9 (placebo) (table 1). This result was considered an unrealistic overestimation given that the largest observed change in mTSS in any group was 9, and most patients experienced no change in mTSS over 24 weeks and this amount of progression has never been observed in any other study.
Using the prespecified mTSS non-progressor rate definition (≤0 cut-off, NRI), we found that mTSS non-progression was more common in the CZP arms than the placebo arms (83.3% (CZP 200 mg Q2W) and 76.3% (CZP 400 mg Q4W) vs 34.6% (placebo), p<0.001).
Treatment efficacy (post hoc analyses)
Post hoc analysis using the median mTSS of the entire population to impute missing values in patients with fewer than two analysable mTSS suggested that patients treated with CZP 200 mg Q2W and CZP 400 mg Q4W had reduced radiographic progression compared with placebo patients (0.01 (p=0.004) and 0.11 (p=0.072) vs 0.28) (figure 1C). Additional, more conservative post hoc analyses using alternative imputation methods confirmed the inhibition of radiographic progression by CZP (table 1, figure 1).
Baseline CRP and mTSS have previously been demonstrated to influence radiographic progression. Placebo patients with CRP>15 mg/L and those with mTSS >6 (median population baseline score) experienced greater mTSS progression than those with CRP levels ≤15 mg/L (0.51 vs 0.12) and mTSS ≤6 (0.54 vs 0.06). In comparison, CZP-treated patients experienced low mTSS progression irrespective of baseline CRP (0.08 vs 0.03) or mTSS at baseline (0.08 vs 0.04, see online supplementary table S2).
The proportion of mTSS non-progressors (≤0.5 points cut-off, linear extrapolation) remained significantly higher in the CZP group (93.5% (CZP 200 mg Q2W) and 90.4% (CZP 400 mg Q4W) vs 80.1% (placebo), p<0.05) and was not sensitive to the imputation methodology used for patients with fewer than two analysable mTSS (table 2). Subgroup analysis of mTSS non-progression demonstrated a similar pattern to that observed for mTSS change from baseline (see online supplementary table S2).
Table 2.
mTSS non-progression analysis
| mTSS non-progressor rate (%) | ||||
|---|---|---|---|---|
| Non-progressor cut-off (mTSS) | Imputation of missing values | Placebo (n=136) | CZP 200 mg Q2W (n=138) | CZP 400 mg Q4W (n=135) |
| 0† | ≥2 mTSS: NRI‡§ <2 mTSS: considered progressors‡ |
34.6 | 83.3* | 76.3* |
| 0† | ≥2 mTSS: linear imputation <2 mTSS: no imputation |
81.9 | 91.7* | 87.8 |
| ≤0.5 | ≥2 mTSS: linear imputation <2 mTSS: no imputation |
86.6 | 96.2* | 96.7* |
| ≤0.5 | ≥2 mTSS: linear imputation <2 mTSS: mean mTSS change from BL of all patients observed |
87.5 | 96.4* | 97.0* |
| ≤0.5 | ≥2 mTSS: linear imputation <2 mTSS: median mTSS change from BL of all patients observed |
87.5 | 96.4* | 97.0* |
| ≤0.5 | ≥2 mTSS: linear imputation <2 mTSS: maximum mTSS change from BL of all patients observed |
79.4 | 92.8* | 88.1 |
For placebo patients who escaped early to CZP, the week 24 values were linearly extrapolated.
*p<0.05 versus placebo.
†Prespecified non-progressor cut-off mTSS.
‡Prespecified imputation.
§Patients with missing values considered progressors.
BL, baseline; CZP, certolizumab pegol; mTSS, modified Total Sharp Score; NRI, non-responder imputation; Q2W, every 2 weeks; Q4W, every 4 weeks.
Discussion
Linear interpolation and extrapolation of imaging data is a well-established imputation method in both rheumatoid arthritis and PsA clinical trials.6 12 13 However, it requires the availability of two or more mTSS.1 3 4 Furthermore, it has limitations when available mTSS are recorded very close together, demonstrated by the prespecified mTSS analysis of the RAPID-PsA trial. Excluding mTSS recorded <8 weeks apart resulted in a more realistic estimate of progression in this population.
Analyses that include patients regardless of radiograph availability require additional imputation assumptions. These additional imputation methodologies can have a substantial impact on the assessment of mTSS progression. In this study, the assessment of CZP efficacy was confounded by an inappropriate imputation approach. The prespecified methodology imputed a change variable (mTSS progression) in a patient by replacing status variables (mTSS) across patients. Since the variation in mTSS between patients was many orders of magnitude greater than the average progression, assignment of mTSS progression in patients with fewer than two available mTSS was unrealistic and resulted in an overestimation of mTSS progression in all study arms, preventing the detection of treatment efficacy.
Post hoc analyses demonstrated that imputation methodologies that use the mTSS progression of a population, as has been used in other recent PsA trials,5 6 resulted in a more realistic estimate of whole population progression. However, a limitation of the RAPID-PsA post hoc analysis is that only a subset of possible imputation rules has been explored.
The rate of mTSS non-progression demonstrated a statistically significant difference between the CZP treatment arms and the placebo arm regardless of imputation approach. However, the prespecified imputation methodology, which considered all patients who withdrew or escaped to active treatment as mTSS progressors, is likely to overestimate the progression seen in placebo patients.
In conclusion, RAPID-PsA trial results demonstrate how significantly imputation methodology can influence the interpretation of radiographic outcomes in clinical trials. The ‘no imputation’ approach for patients with no or only one film, or two films less than 8 weeks apart and linear extrapolation for patients with two films at least 8 weeks apart seems to be the most appropriate primary analysis. The other imputation methods should be used as sensitivity analyses.
Supplementary Material
Footnotes
Correction notice: This article has been corrected twice since it was published Online First. The length of the study has been amended from 158 weeks to 216 weeks, and the value for the pre-specified mTSS non-responder cut-off should read ‘≤0’ in the ‘Treatment efficacy (prespecified analyses)’ section of the paper.
Acknowledgements: The authors acknowledge Marine Champsaur, UCB Pharma, Brussels, Belgium for publication coordination and Costello Medical Consulting, UK, for writing and editorial assistance.
Funding: The RAPID-PsA study was funded by UCB Pharma. Writing and Editorial Assistance was funded by UCB Pharma.
Competing interests: UCB Pharma sponsored the trial and was responsible for data collection and analysis. The authors and the sponsor designed the study, interpreted the data, prepared the manuscript, and decided to publish.
Ethics approval: National and Regional Ethics Committee or Institutional Review Board (Global Study).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Van Der Heijde D. Use of imaging as an outcome measure in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis in clinical trials. In: Hochberg MC, Silman AJ, Smolen JS, et al. eds. Rheumatology. 5th edn. Elsevier, 2011:413–24 [Google Scholar]
- 2.Kavanaugh A, McInnes I, Mease PJ, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86 [DOI] [PubMed] [Google Scholar]
- 3.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89 [DOI] [PubMed] [Google Scholar]
- 4.Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavanaugh A, van der Heijde D, McInnes IB, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 2012;64:2504–17 [DOI] [PubMed] [Google Scholar]
- 6.van der Heijde D, Tanaka Y, Fleischmann RM, et al. Tofacitinib, an oral Janus kinase inhibitor, in combination with methotrexate reduced the progression of structural damage in patients with rheumatoid arthritis: year 2 efficacy and safety results from a 24-month phase 3 study. Arthritis Rheum 2012;64:546 [Google Scholar]
- 7.Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24 week results of a phase III double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73 [DOI] [PubMed] [Google Scholar]
- 10.van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijde D, Simon L, Smolen J, et al. How to report radiographic data in randomized clinical trials in rheumatoid arthritis: guidelines from a roundtable discussion. Arthritis Rheum 2002;47:215–18 [DOI] [PubMed] [Google Scholar]
- 12.Smolen J, Landewé RB, Mease PJ, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keystone E, van der Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.