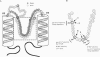Abstract
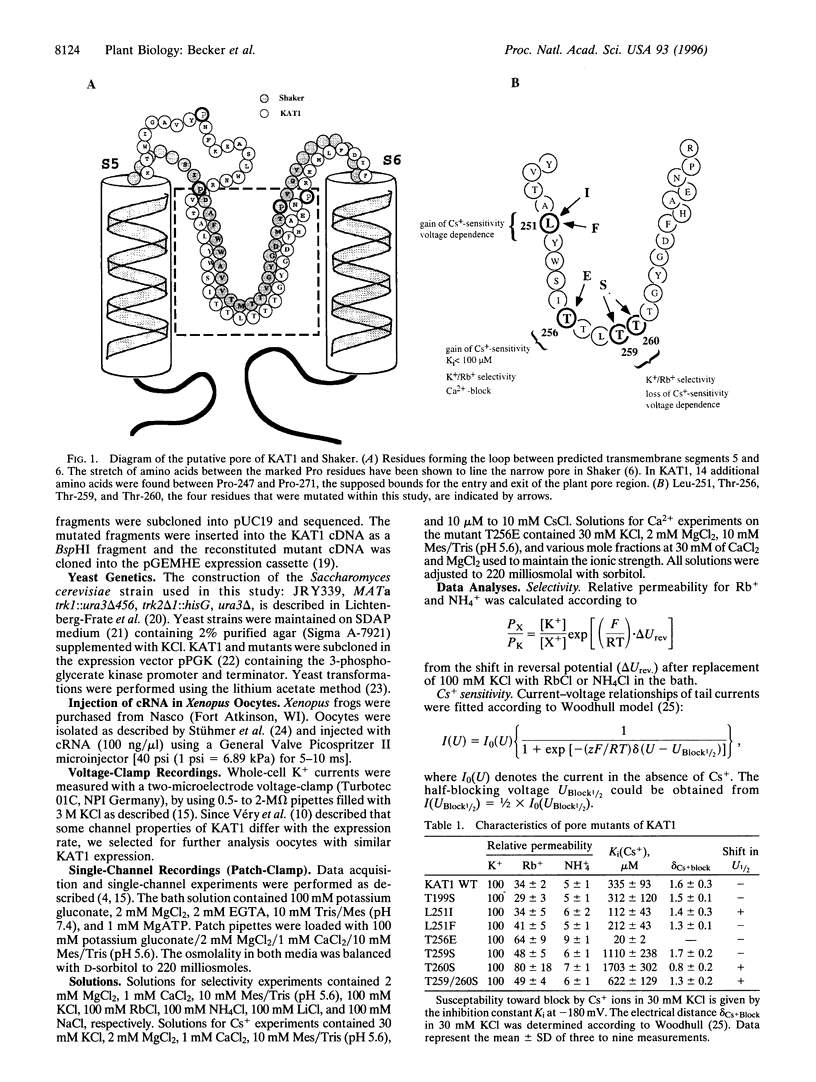
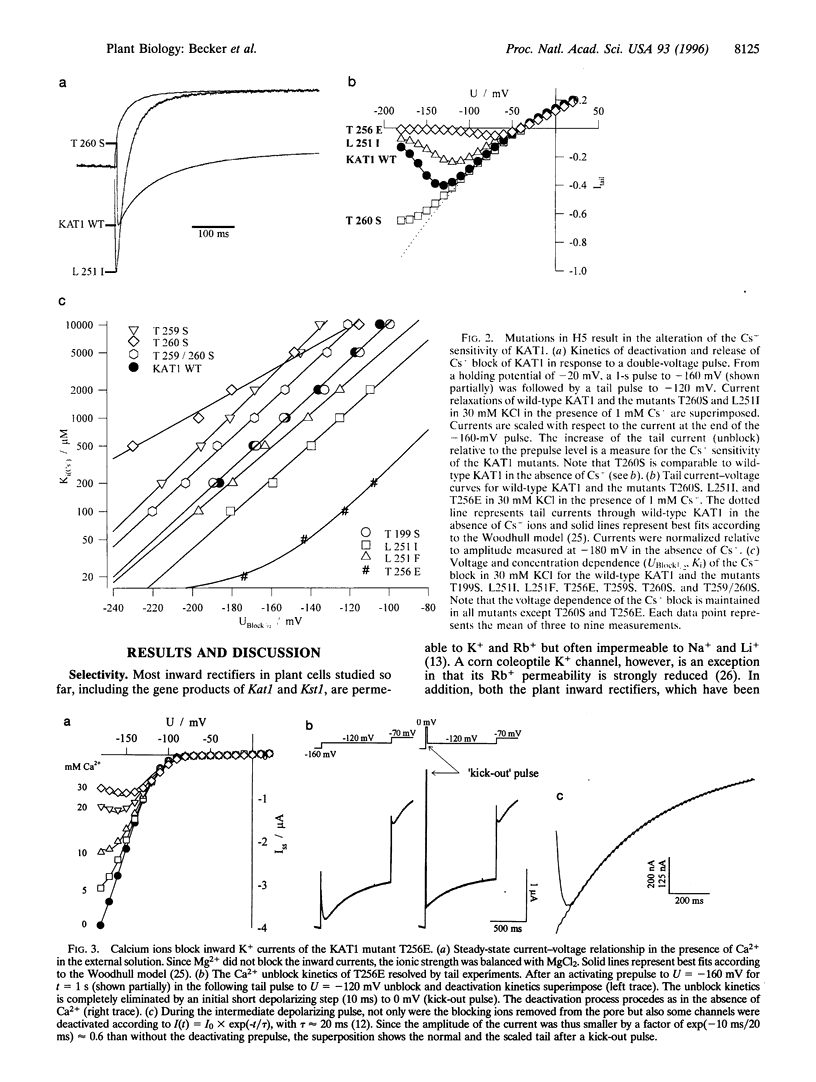
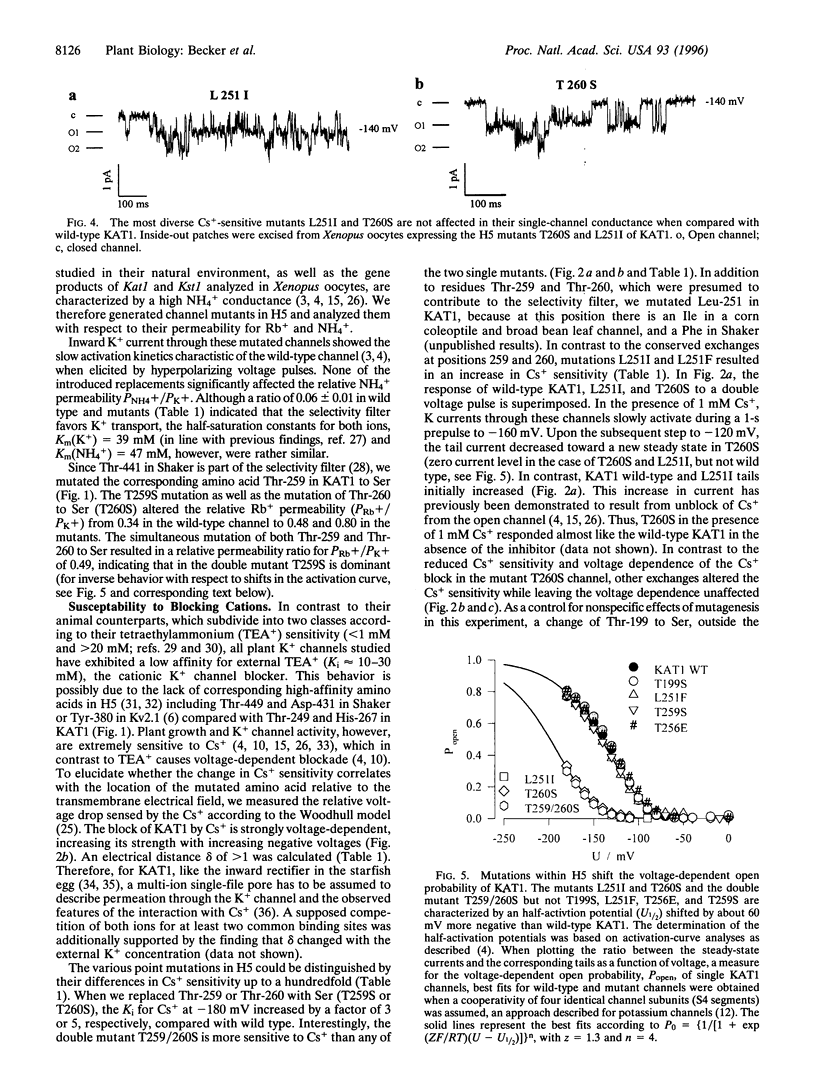
KAT1 is a voltage-dependent inward rectifying K+ channel cloned from the higher plant Arabidopsis thaliana [Anderson, J. A., Huprikar, S. S., Kochian, L. V., Lucas, W. J. & Gaber, R. F. (1992) Proc. Natl. Acad. Sci. USA 89, 3736-3740]. It is related to the Shaker superfamily of K+ channels characterized by six transmembrane spanning domains (S1-S6) and a putative pore-forming region between S5 and S6 (H5). The 115 region between Pro-247 and Pro-271 in KAT1 contains 14 additional amino acids when compared with Shaker [Aldrich, R. W. (1993) Nature (London) 362, 107-108]. We studied various point mutations introduced into H5 to determine whether voltage-dependent plant and animal K+ channels share similar pore structures. Through heterologous expression in Xenopus oocytes and voltage-clamp analysis combined with phenotypic analysis involving a potassium transport-defective Saccharomyces cerevisiae strain, we investigated the selectivity filter of the mutants and their susceptibility toward inhibition by cesium and calcium ions. With respect to electrophysiological properties, KAT1 mutants segregated into three groups: (i) wild-type-like channels, (ii) channels modified in selectivity and Cs+ or Ca2+ sensitivity, and (iii) a group that was additionally affected in its voltage dependence. Despite the additional 14 amino acids in H5, this motif in KAT1 is also involved in the formation of the ion-conducting pore because amino acid substitutions at Leu-251, Thr-256, Thr-259, and Thr-260 resulted in functional channels with modified ionic selectivity and inhibition. Creation of Ca2+ sensitivity and an increased susceptibility to Cs+ block through mutations within the narrow pore might indicate that both blockers move deeply into the channel. Furthermore, mutations close to the rim of the pore affecting the half-activation potential (U1/2) indicate that amino acids within the pore either interact with the voltage sensor or ion permeation feeds back on gating.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. Potassium channels. Advent of a new family. Nature. 1993 Mar 11;362(6416):107–108. doi: 10.1038/362107a0. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Huprikar S. S., Kochian L. V., Lucas W. J., Gaber R. F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Anderson J. A., Slayman C. L., Gaber R. F. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+ channel from Arabidopsis thaliana, and comparison with endogeneous yeast channels and carriers. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt M. R. K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J Gen Physiol. 1992 Apr;99(4):615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M. Functional bases for interpreting amino acid sequences of voltage-dependent K+ channels. Annu Rev Biophys Biomol Struct. 1993;22:173–198. doi: 10.1146/annurev.bb.22.060193.001133. [DOI] [PubMed] [Google Scholar]
- Cao Y., Crawford N. M., Schroeder J. I. Amino terminus and the first four membrane-spanning segments of the Arabidopsis K+ channel KAT1 confer inward-rectification property of plant-animal chimeric channels. J Biol Chem. 1995 Jul 28;270(30):17697–17701. [PubMed] [Google Scholar]
- Fairley-Grenot K. A., Assmann S. M. Permeation of Ca2+ through K+ channels in the plasma membrane of Vicia faba guard cells. J Membr Biol. 1992 Jun;128(2):103–113. doi: 10.1007/BF00231883. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 1991 Apr;7(3):253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Krasne S., Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+ mixtures. J Gen Physiol. 1977 Sep;70(3):269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Becker D. Green circuits--the potential of plant specific ion channels. Plant Mol Biol. 1994 Dec;26(5):1637–1650. doi: 10.1007/BF00016494. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Moran O., Conti F., Busch H., Becker D., Gambale F., Dreyer I., Küch A., Neuwinger K., Palme K. Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators. Eur Biophys J. 1995;24(2):107–115. doi: 10.1007/BF00211406. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Hidalgo P., MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995 Apr 14;268(5208):307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995 Mar;105(3):309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. S., Kane J., Kurjan J., Stadel J. M., Tipper D. J. Effects of expression of mammalian G alpha and hybrid mammalian-yeast G alpha proteins on the yeast pheromone response signal transduction pathway. Mol Cell Biol. 1990 Jun;10(6):2582–2590. doi: 10.1128/mcb.10.6.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Ko C. H., Gaber R. F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Aug;11(8):4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lü Q., Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995 Apr 14;268(5208):304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Müller-Röber B., Ellenberg J., Provart N., Willmitzer L., Busch H., Becker D., Dietrich P., Hoth S., Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995 Jun 1;14(11):2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Ramos J. Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I., Lucas W. J., Anderson J. A., Gaber R. F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992 Dec 4;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Ward J. M., Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Methfessel C., Sakmann B., Noda M., Numa S. Patch clamp characterization of sodium channels expressed from rat brain cDNA. Eur Biophys J. 1987;14(3):131–138. doi: 10.1007/BF00253837. [DOI] [PubMed] [Google Scholar]
- Uozumi N., Gassmann W., Cao Y., Schroeder J. I. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. J Biol Chem. 1995 Oct 13;270(41):24276–24281. doi: 10.1074/jbc.270.41.24276. [DOI] [PubMed] [Google Scholar]
- Véry A. A., Gaymard F., Bosseux C., Sentenac H., Thibaud J. B. Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 1995 Feb;7(2):321–332. doi: 10.1046/j.1365-313x.1995.7020321.x. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]