Abstract
Objective
To test a multidisciplinary approach to reduce heart failure (HF) readmissions that tailors the intensity of care transition intervention to the risk of the patient using a suite of electronic medical record (EMR)-enabled programmes.
Methods
A prospective controlled before and after study of adult inpatients admitted with HF and two concurrent control conditions (acute myocardial infarction (AMI) and pneumonia (PNA)) was performed between 1 December 2008 and 1 December 2010 at a large urban public teaching hospital. An EMR-based software platform stratified all patients admitted with HF on a daily basis by their 30-day readmission risk using a published electronic predictive model. Patients at highest risk received an intensive set of evidence-based interventions designed to reduce readmission using existing resources. The main outcome measure was readmission for any cause and to any hospital within 30 days of discharge.
Results
There were 834 HF admissions in the pre-intervention period and 913 in the post-intervention period. The unadjusted readmission rate declined from 26.2% in the pre-intervention period to 21.2% in the post-intervention period (p=0.01), a decline that persisted in adjusted analyses (adjusted OR (AOR)=0.73; 95% CI 0.58 to 0.93, p=0.01). In contrast, there was no significant change in the unadjusted and adjusted readmission rates for PNA and AMI over the same period. There were 45 fewer readmissions with 913 patients enrolled and 228 patients receiving intervention, resulting in a number needed to treat (NNT) ratio of 20.
Conclusions
An EMR-enabled strategy that targeted scarce care transition resources to high-risk HF patients significantly reduced the risk-adjusted odds of readmission.
Keywords: Decision support, clinical; Health services research; Healthcare quality improvement; Information technology
Introduction
A majority of US hospitals struggle to contain readmission rates related to heart failure (HF).1 2 Although numerous studies have found that some combination of careful discharge planning, provider coordination and intensive counselling can prevent subsequent readmissions to a hospital, success is difficult to achieve and sustain at the typical US hospital.1–4 Enrolling all patients with HF into a uniform high-intensity care transition programme (‘do everything for everyone’) may require a depth of case management resources out of reach for many institutions, particularly safety-net hospitals. It has been hypothesised that programmes to reduce readmissions may be more effective if resources are applied differentially according to a patient's relative risk of readmission.5 6 To our knowledge, this hypothesis has not been tested.
We have previously described an electronic medical record (EMR)-enabled model (e-Model) derived from both clinical and non-clinical factors which accurately stratifies risk for 30-day readmission among patients with HF.7 Compared with other risk prediction models, the e-Model is unique in that it draws from 29 clinical, social, behavioural and utilisation factors extracted in real time from the EMR within 24 h of admission for HF.5
In concert with other EMR-based tools, the e-Model makes it possible to match the intensity of the readmission intervention to the patient's risk of readmission on any given day. This study examines whether a strategy targeting highest-risk HF patients by re-allocating existing hospital resources could reduce risk-adjusted rates of readmission. To control for secular trends, we compare concurrent rates of 30-day readmission for patients with acute myocardial infarction (AMI) and pneumonia (PNA), two measures that are also the subject of intense national scrutiny but for which no intervention was performed.
Methods
Setting, study design and participants
This study was constructed as a pragmatic trial employing a prospective before and after design with concurrent controls. The study was performed at the main hospital of Parkland Health and Hospital System, a 780-bed tertiary care teaching hospital located in Dallas, Texas. The hospital serves an ethnically diverse safety-net patient population. The study was divided into two periods of equal length: the pre-intervention period (1 December 2008 to 30 November 2009) and the post-intervention period (1 December 2009 to 30 November 2010). Readmission data were collected 1 month after the end of the respective periods to ascertain the total 30-day readmission rate for that period. During the 2-year study period, detailed clinical information was collected from the hospital's EMR platform (EPIC Systems Corporation, Verona, Wisconsin, USA) for all hospitalised patients aged ≥18 years discharged with a principal diagnosis of HF, AMI and PNA, according to the Centers for Medicare and Medicaid Services ICD-9 code groupers.8–11 The intervention described below was implemented at the end of the pre-intervention period and achieved full operational capacity immediately after going ‘live’ as the interventional staff had been trained and prepared for several weeks prior to implementation.
Interventional overview
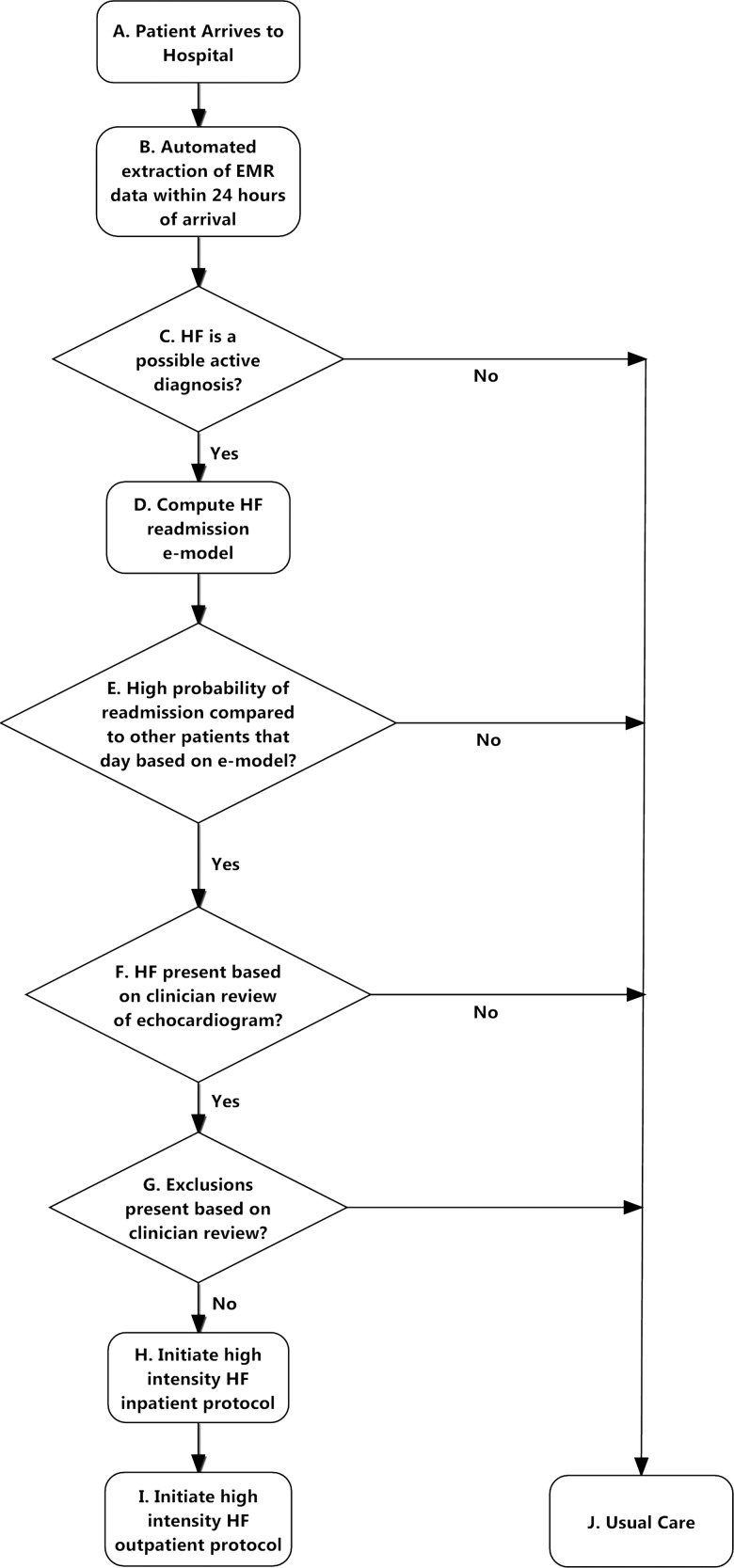
Patient selection and the treatment strategy are presented in figure 1, steps A–J. Like many hospitals, a specialised but limited number of care transition and HF management resources were available for all patients with HF in the system during daytime hours Monday to Friday. This included a hospital-wide case management department and an outpatient HF programme staffed by a limited number of cardiologists, nurse practitioners, nutritionists and pharmacists who maintained other clinical responsibilities beyond follow-up of discharged HF patients. No personnel were added for the intervention with the exception of a part-time case manager, at 25% effort, to assist existing hospital case management staff with social work and care transition needs for patients with HF identified as high-risk. The intervention was thus constructed to best allocate this finite set of resources from Monday to Friday to maximally reduce 30-day readmissions associated with HF.
Figure 1.
Patient selection and review process.
The intervention was driven by a suite of computerised case monitoring and coordination programmes (software programme) that: (1) extracts real-time data from the EMR (step A, figure 1); (2) identifies patients admitted with HF using free text and structured data text processing approaches (step B); (3) rank orders and risk stratifies patients according to the e-Model within 24 h of hospitalisation (step C); and (4) sends secure electronic notifications about those at highest risk to HF personnel (step F) who then evaluate and perform an intensive bundle of coordinated inpatient and outpatient (post-discharge) tasks on eligible patients (steps F–I). The e-Model is a real-time automated risk prediction model using only EMR data with a c-statistic of 0.72, described in detail7 and in the context of other readmission models5 elsewhere.
A HF nurse practitioner reviewed the medical charts of high-risk patients electronically flagged by the software program to confirm the presence of HF using echocardiographic data. They also ascertained any prespecified exclusions including renal disease requiring haemodialysis, stage 4 metastatic cancer, enrollment in a hospice program, severe psychiatric illness, primary pulmonary hypertension, isolated right ventricular failure or discharge to another facility. Up to three patients with the highest ranked risk scores for that day were then enrolled by the HF nurse practitioners. Patients were enrolled only once during the intervention period.
Intervention components
High-risk patients received an intensive set of evidence-based inpatient and outpatient counselling and monitoring activities drawn from well-known readmission reduction strategies that have been extensively published (steps H and I, figure 1).6 12–16 These included (1) detailed inpatient clinical assessment, patient education and discharge planning by a HF nurse practitioner, pharmacist, nutritionist and case manager starting early in the hospital course; (2) a follow-up telephone call from a nurse within 48 h of discharge to assess whether the patient had obtained their medication and was aware of their outpatient follow-up appointments; (3) outpatient case management (consisting of individualised care management services based on specific post-discharge needs) for 30 days; (4) a cardiology appointment with a HF specialist within 7 days of discharge and subsequent cardiology follow-up for at least 1 month; and (5) a primary care appointment scheduled according to the urgency of non-cardiac problems. While some of the intervention components such as follow-up call and outpatient case management were in place in the pre-intervention period as part of usual care, the level of intensity and involvement of the outpatient case management staff was higher for patients selected for intervention in the post-intervention period.
On each day the intervention was in effect, patients with the highest predicted risk were selected for intervention. Patients selected for intervention were encouraged to complete all intervention elements but were free to decline any. Patients with HF who were not selected for intervention received the treatment plan directed by their primary medical team as part of usual care.
A weekly review session was held to review the fidelity of the intervention and to ensure that the study protocol was faithfully carried out.
Study outcomes and variables
The primary outcome of this study was readmission to any hospital in the Dallas-Fort Worth metroplex for any cause within 30 days of discharge of the index HF admission. We ascertained 30-day readmission to any hospital in the North Texas region using a probabilistic matching service available through the Dallas-Fort Worth Hospital Council, a cooperative regional information-sharing initiative. Elective readmissions classified by clinical staff and coded in the EMR system were not counted as readmissions in this analysis.
In order to determine whether the intervention had any adverse mortality effects, we tracked inpatient and 30-day mortality rates for all patients in the pre- and post-intervention period for HF, AMI and PNA using a two-step process. Those patients with a documented encounter after the 30-day post-discharge period anywhere in the index health system were considered alive. Those without an encounter were subsequently classified as dead or alive within 30 days of discharge after querying the National Death Index File.
We also collected information on demographics, clinical severity and comorbid illness burden as well as several patient and neighborhood-level measures of social disadvantage included in the e-Model. Variables that were highly significant to the prognostic capability of the e-Model were also extracted from the EMR. These variables included, among others, Brain Natriuretic Peptide (BNP), troponin, creatine kinase, blood urea nitrogen and albumin and key social and behavioural readmission risk factors such as gender, marital status, payor status, number of documented home address changes within the past 12 months, history of positive urine cocaine within the past 12 months, history of missed clinic visits within the past 6 months and number of hospital admissions prior to index admissions.7 The software program used for this intervention allowed us to track the receipt and completion of all intervention components.
Statistical analysis
A quasi-experimental approach was used to assess the impact of the targeted intervention on the overall readmission rate of HF. We used a measurement framework that included all patients with a final principal discharge diagnosis of HF during the pre- and post-intervention periods. In other words, the entire HF population was used as the basis to analyse the readmission rate irrespective of whether they were classified as HF at the point of intervention by the electronic or human review process.
Readmission and mortality rates were assigned to calendar months and calculated according to methods outlined by CMS.17 Differences in demographics, clinical severity of illness, social risk factors and overall readmission risk between the pre- and post-intervention cohorts were tested using χ2 and t tests. Illness severity was based on the Tabak inpatient mortality model and overall readmission risk scores for HF were calculated according to the e-Model.7 18 The Tabak score was used because it can be computed in real time using data readily available from an EMR within 24 h of admission, and was a more precise assessment of physiological status, in our view, than other measures that calculate in-hospital mortality prediction.7 18 A subset of the e-Model risk score, called the social risk score, consisted of variables in the e-Model directly related to social, utilisation and environmental risk factors.
The change in unadjusted readmission rates between the pre- and post-intervention periods was analysed at the monthly level by the Wilcoxon matched pairs test. To adjust for potential differences in patient populations, a mixed logistic regression model was constructed that included demographic and hospital utilisation variables, a case mix variable, comorbidity variables and an indicator of the pre- and post-intervention periods.19 20
The case mix variable for the HF cohort was calculated using the Tabak HF mortality score while, for the control population, the Tabak mortality risk scores for AMI or PNA were used.18 Patient comorbidities were captured through indicator variables for coronary artery disease, chronic kidney disease, diabetes mellitus and cancer. The intervention effect was represented by the regression coefficient of the indicator variable of the intervention period. The model also included random effects to account for potential autoregressive correlation over time and to address potential seasonal variations or other secular trends,21 as well as within-subject correlation for those patients who had more than one index admission during the study period.22 We calculated the observed to expected (OE) readmission rate ratio to measure the performance of intervention components of special interest. Expected readmission rates were calculated based on the e-Model.
For all analyses, the null hypothesis was evaluated at a two-sided significance level of 0.05. All statistical analyses were performed with STATA V.10.0 (STATA Corp, College Station, Texas, USA).
Sensitivity analysis
Because patients who were admitted and discharged within a weekend or holiday were excluded from the possibility of receiving the intervention during the intervention time period, we performed a sensitivity analysis in which patients admitted and discharged during these periods were dropped from both the pre-intervention and intervention time periods. Because only the patients with the highest predicted risk on a given day received the intervention, we performed a sensitivity analysis to compare the predicted readmission risk and observed readmission rates of the next most deserving patient (defined as the patient with the highest predicted risk among the patients not receiving the intervention in each day when the intervention was in effect) and, because the intervention was implemented in December 2009, we also performed a sensitivity analysis that excluded the month of December 2009 in the post-intervention period.
Results
Study population characteristics
A total of 834 patients were discharged with a principal diagnosis of HF in the pre-intervention period and 913 in the post-intervention period. The mean age of the HF population was 58 years. The pre- and post-intervention groups overall were comparable with regard to important readmission risk factors including the e-Model risk score, pro-BNP levels, length of stay and measures of prior utilisation (table 1), although there were some noticeable differences in age, gender, race and ethnicity.
Table 1.
Characteristics of heart failure study population
| Pre-intervention (N=834) | Post-intervention (N=913) | p Value | |
|---|---|---|---|
| Risk scores | |||
| e-Model (readmission risk) score, mean (SD)* | 25.3 (0.15) | 25.2 (0.16) | 0.80 |
| Demographic factors | |||
| Age, mean (SD) | 59.2 (11.4) | 57.8 (10.8) | 0.01 |
| Male, n (%) | 506 (60.7) | 504 (55.2) | 0.02 |
| Race, n (%) | |||
| Black | 519 (62.4) | 512 (56.1) | 0.01 |
| Hispanic | 173 (20.7) | 250 (27.4) | <0.01 |
| White | 133 (15.8) | 136 (14.9) | 0.59 |
| Other | 9 (1.1) | 15 (1.6) | 0.31 |
| Single | 605 (72.5) | 624 (68.3) | 0.06 |
| Payor, n (%) | |||
| Medicare | 264 (31.6) | 254 (27.8) | 0.08 |
| Commercial | 12 (1.4) | 11 (1.2) | 0.67 |
| Medicaid and other | 558 (66.9) | 648 (71.0) | <0.01 |
| Clinical factors | |||
| Pro-BNP, mean (SD) | 8167 (11 564) | 9362 (13 245) | 0.10 |
| Proportion with diabetes mellitus comorbidity† | 49.3% | 53.7% | 0.06 |
| Proportion with coronary artery disease comorbidity† | 45.0% | 38.4% | 0.01 |
| Proportion with chronic kidney disease comorbidity† | 41.4% | 42.9% | 0.52 |
| Utilisation or behavioural factors | |||
| Admitted through ED for index admission (%) | 717 (86.0) | 806 (88.3) | 0.15 |
| No. of prior inpatient admissions, mean (SD) | 1.47 (2.5) | 1.40 (2.2) | 0.48 |
| History of missed clinic visit within previous 6 months | 96 (11.5) | 86 (9.4) | 0.15 |
| History of leaving against medical advice (%) | 20 (2.4) | 15 (1.6) | 0.26 |
| Length of stay for index admission (days) | 6.0 (6.1) | 5.7 (5.3) | 0.41 |
*The e-Model is an EMR-derived multivariate readmission risk model generated from the following variables: age, Tabak HF mortality score, gender, marital status, payor status, number of documented home address changes, history of positive urine cocaine, history of missed clinic visits, number of hospital admissions prior to index admissions.7
†As defined by ICD-9 codes (see online supplementary appendix for list of codes).
BNP, B-type natriuretic peptide; ED, emergency department, EMR, electronic medical record; HF, heart failure.
After further exploratory analysis, we found that the differences in race, gender and age correlated with a noticeable increase in the absolute number of female Hispanic patients with HF in the post-intervention period compared with the pre-intervention period, with no noticeable increase in any other demographic subgroup.
There were 637 patients discharged with a principal diagnosis of either AMI or PNA in the pre-intervention period and 597 in the post-intervention period. The mean age of the concurrent control group was the same as the HF group (n=58). There was a relatively equal distribution of black, Hispanic and Caucasian patients in the control group in the pre- and post- intervention periods. As with the HF group, the mean disease-specific Tabak mortality scores were nearly identical in the pre- and post-intervention period for both conditions (18.9 vs 19.0 for AMI and 26.2 vs 26.1 for PNA).
Thirty-day readmission rates for HF
The 30-day readmission rate for HF, which included all readmissions in our region, was 23.6% over the entire study period. Of these, 26% represented readmissions to other hospitals. Table 2 shows that the unadjusted 30-day readmission rate for HF fell from 26.2% in the pre-intervention period to 21.2% in the post-intervention period (p<0.01), a relative reduction of 19%. We enrolled 913 patients and 228 received intervention in the post-intervention period, and there were 45 fewer readmissions in the post-intervention period (ie, 913 enrolled multiplied by the difference between the pre- and post-readmission rates), resulting in a number needed to treat ratio of 20.29, suggesting a fairly robust treatment effect.
Table 2.
Thirty-day readmission rates: pre-intervention and post-intervention periods* for all patients by HF and control groups
| Patient type | Pre-intervention (%) | Post-intervention (%) | Difference (95% CI) | p Value | Adjusted OR† (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|
| HF | All | 26.2 | 21.2 | 5.0 (1.0 to 9.0) | 0.01 | 0.73 (0.58 to 0.93) | <0.01 |
| AMI and PNA | All | 15.5 | 16.7 | −1.2 (−5.4 to 2.9) | 0.56 | 1.09 (0.80 to 1.48) | 0.60 |
*Pre-intervention period: December 2008 to November 2009; post-intervention period: December 2009 to November 2010.
†The risk adjustment model includes demographic variables (age, race and sex), case mix variables and indicator variables for the pre- and post-intervention periods. For HF, the case mix variable was the e-Model. For AMI and PNA, the Tabak mortality score for AMI and PNA was used.
AMI, acute myocardial infarction; HF, heart failure; PNA, pneumonia.
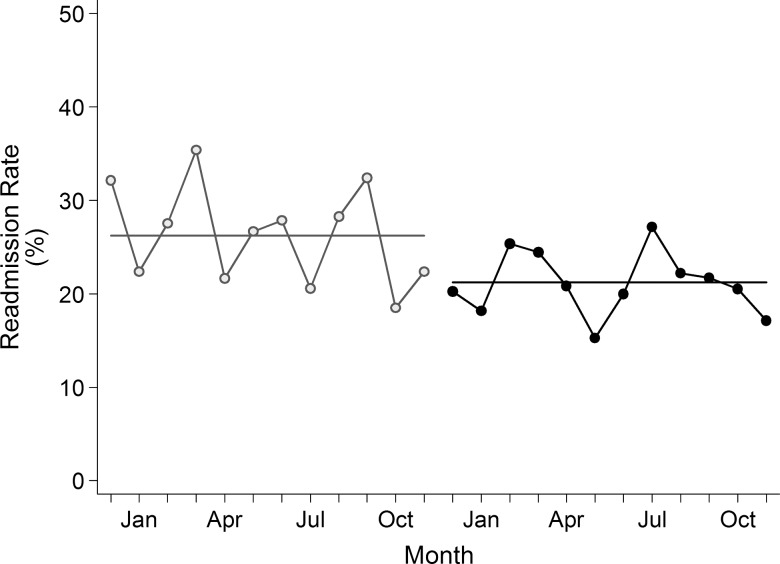
The monthly paired difference between the pre- and post-intervention period also showed consistent reduction (p<0.01). After controlling for demographics, mortality risk and comorbidities in the mixed logistic model, we detected a significant reduction in readmission rate (adjusted OR (AOR) 0.73, 95% CI 0.58 to 0.93, p=0.01) in the post-intervention period. Figure 2 shows that the monthly post-intervention readmission rate was consistently below the pre-intervention average of 26.2% in 11 of 12 months.
Figure 2.
Thirty-day readmission rates by month.
Thirty-day readmission rates for AMI and PNA
The combined populations of AMI and PNA served as the concurrent control group. As shown in table 2, there was no difference in the unadjusted pre- and post-intervention readmission rates for patients hospitalised with AMI and PNA (15.5% vs 16.7%; p=0.56), and no significant differences in the readmission rate were detected by the mixed logistic regression models (AOR=1.09, 95% CI 0.80 to 1.48).
Thirty-day mortality analyses
Since a higher 30-day death rate could theoretically lead to a lower 30-day readmission rate, we examined the mortality rates for HF pre- and post-intervention. There was no statistically significant difference in mortality in the pre- and post- intervention periods (3.2% vs 2.2%, p=0.16). It is therefore unlikely that the observed reduction in HF readmission was driven by the change in death rate.
Intervention intensity and odds of readmission
Planned intervention elements may not have been completed because of refusal by patients or logistical problems so a subgroup analysis was conducted on the 228 patients with HF who received at least one intervention element. We stratified the intervention group by the number of outpatient components received into two categories: 1–2 and ≥3. The receipt of a larger number of outpatient components was associated with a substantially lower readmission risk (table 3). Patients who received ≥3 outpatient components had an OE ratio for readmissions of 0.35 (95% CI 0.00 to 0.85; p<0.01).
Table 3.
Outpatient intervention and odds of readmission (n=913 HF index admissions, n=228 patients receiving interventions)*
| Intervention category | N | Expected readmission rate† | Observed readmission rate | Observed/expected ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Outpatient intervention completion | |||||
| Enrolled, received 1 or 2 outpatient components | 150 | 23.8 | 17.3 | 0.73 (0.51 to 0.95) | 0.01 |
| Enrolled, received ≥3 outpatient components | 27 | 21.2 | 7.4 | 0.35 (0.00 to 0.85) | <0.01 |
*Intervention components include direct care and care management received from nurse practitioner, pharmacist, nutritionist and/or home visit nurse.
†Expected readmission risk as calculated by electronic readmission risk model (e-Model).
HF, heart failure.
Sensitivity analysis
When we excluded patients who were admitted and discharged during a weekend and holiday period in both the pre-intervention and post-intervention periods, a significant reduction in readmission remained (AOR 0.76, p=0.02), indicating that this aspect of the protocol was not responsible for the observed reduction in readmissions. Similarly, patients with AMI and PNA who were admitted and discharged during a weekend and holiday period were removed from the pre-intervention and post-intervention period in an equivalent sensitivity analysis. No change was noted in the rate of readmissions (AOR 0.97, p=0.86). For the next most deserving patients who were not selected for intervention, there was no statistically significant reduction in readmission rate, with an OE ratio of 1.03 (95% CI 0.89 to 1.17), suggesting that only patients who received the intervention experienced a reduction in readmission rates. When we excluded the month of December 2009 from the post-intervention period, the results were very similar, indicating that the intervention programme was fully functional during the first month of implementation.
Discussion
In this study we found that a care transition intervention that directed largely existing resources to a smaller subgroup of patients with HF based on daily EMR-based risk stratification produced a clinically meaningful reduction in overall readmissions. By concentrating care management efforts on about one-quarter of patients with HF we were able to demonstrate a 26% relative reduction in the odds of readmission and an absolute reduction of 5.0 readmissions per 100 index admissions.
Since other institution-wide efforts to improve quality could also theoretically reduce readmissions, we examined time trends in readmission for patients with HF compared with a concurrent cohort of individuals admitted with AMI and PNA. We observed no change in the readmission rate for these two conditions, suggesting that the improvements in HF were specific to the intervention rather than institutional secular trends that would have affected overall readmission rates. While national programmes, regulations and policies designed to reduce HF readmissions nationwide could have hypothetically affected readmission rates at this institution, recent literature reveals that there has been little change nationally.1
The high-intensity care transition interventions in this study employed methods tested in several nationally emulated readmission reduction programmes and achieved a comparable reduction in readmission.6 12–16 However, in contrast to these approaches, this programme targeted approximately one-quarter of all admitted patients with HF and used predominantly existing care transition resources, suggesting that a more targeted approach is feasible and effective. Our results also provided a flexible framework that can be adapted in institutions with different levels of resource availability. Hospitals can adjust the threshold value of intervention (ie, the cut-off value or rank order of predicted risk to receive intervention) to suit their particular resource levels. Additional information—including data on the cost of implementing the software program, differences in the utilisation of resources in and outside the hospital, labour costs and the total readmission reduction opportunity available to patients in different risk groups—is needed to establish the cost-effectiveness of the intervention.
In this study we also found that, among the patients who received the intervention, those who received more outpatient components had substantially lower OE ratios for readmission than patients who did not. These findings are consistent with previous studies. Patients are often most vulnerable in the first 7–14 days after discharge when medications need to be adjusted, educational plans are best reinforced and patients are learning critical self-management skills in the context of a new after-hospital care plan. Studies have reported that rapid outpatient follow-up during this period allows for early health assessments, improves self-management and provides an opportunity to address outstanding issues before they grow into more serious events.13 23 24 These factors may explain, in part, the striking reduction in readmissions associated with the completion of outpatient components in this study, which placed particular emphasis on close clinic follow-up for enrolled patients.
This study has important limitations. First, we did not employ a randomised controlled trial design, nor did we think it feasible or ethical to blind providers or patients to the intervention. Other factors beyond the intervention therefore could have contributed to the observed reduction in readmissions. However, a control group of patients with AMI or PNA experienced no corresponding improvement in readmission, suggesting that larger institutional factors were not at play. Second, this study was conducted in a single safety-net hospital. Although the generalisability to other settings and patients is unknown, authors have commented that safety-net facilities may experience greater obstacles to reducing readmissions.25 It is also conceivable that, if this intervention is successful in an urban indigent setting, similar or greater success could be achieved in less challenged environments. Third, as this was a practical trial with limited resources, the intervention was not run on weekends or holidays. However, a separate sensitivity analysis that removed patients admitted and discharged on these days showed no change in results. Fourth, we did not have access to data on patients with HF in comparable hospitals without the intervention, which would have been a more suitable control to assess larger national factors that could have played a role in our findings. Nevertheless, given the abrupt reduction in readmission rates with the onset of the intervention, we believe it unlikely that the results we observed were caused by external secular trends. Fifth, the demographic characteristics of our study population might be different from some other US hospitals as our study population had a high proportion of non-white and Medicaid patients. This may affect the generalisability of our findings. We also found statistically significant differences in a number of patient characteristics during the pre- and post-intervention periods, although such differences were largely due to a net increase in Hispanic women with HF in the post-intervention period. We do not have a definitive explanation for this. However, one possible reason may be that the economic recession that occurred during the time of this study affected minority groups such as the Hispanic population and, as a result, more of these individuals were admitted to safety net hospitals similar to the institution in which the study was conducted. It is unlikely, however, that the increase in Hispanic women with HF would be the basis for the abrupt stepwise reduction in readmissions that occurred at the time of the intervention, which is more plausibly and temporally related to the onset of the intervention. Sixth, we were unable to ascertain the number of readmissions outside the region's information-sharing initiative. Given that any underestimate would be present in both the pre- and post-intervention periods, it is unlikely that this result would have had an impact on our findings. Finally, we did not design this study as a cost-effectiveness trial. Such approaches are needed to examine the overall economic value of the resource allocation strategy presented here. Nevertheless, programmes that achieve equivalent population-level reductions in readmissions by targeting only the highest-risk patients may be implicitly cost-effective.
This study has a number of unique strengths. It is the first study of which we are aware that uses data from an EMR to stratify a patient's risk of readmission in real time. The study also presents a new method to allocate a fixed set of often constrained hospital resources by considering an individual patient's risk against the risk of other patients in the hospital at the same time. Finally, in contrast to many readmission intervention studies, we were able to collect readmissions to the study hospital and also to all hospitals in the Dallas-Fort Worth metroplex through a long-standing comprehensive data-sharing initiative, thus providing a more accurate assessment of the readmission rate.
Conclusions
This study provides preliminary evidence that technology platforms that allow for automated EMR data extraction, case identification and risk stratification may help potentiate the effect of known readmission reduction strategies, in particular those that emphasise intensive and early post-discharge outpatient contact.
Supplementary Material
Acknowledgments
The authors wish to thank Anita Rahman, Kashundra Foreman, Elizabeth Marchese, Adeola Jaiyeola, Asohan Amarasingham, Paul Mayer and Elizabeth Dwelle for their contributions to this project. We also thank the Dallas-Fort Worth Hospital Council Foundation for assisting in the collection of post-hospitalisation data through their information sharing initiative. No author has relevant financial interests in the manuscript.
Footnotes
Contributors: RA, BX and YM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: RA, PCP, KT, LLN, TSS, EAH; acquisition of data: TSS, YM; analysis and interpretation of data: RA, PCP, BJM, BX, SZ, TSS, EAH; drafting of the manuscript: RA, BJM; critical revision of the manuscript for important intellectual content: RA, PCP,BJM, BX, SZ, KT, LLN, KSA, MHD, UK, EAH; statistical analysis: RA, BJM, BX, SZ; obtaining funding: RA, EAH; administrative, technical or material support: RA; supervision: RA, EAH.
Funding: The University of Texas System Patient Safety Grant Award, No. OGC 130 857 and Commonwealth Fund Grant Number 20100323.
Competing interests: None.
Ethics approval: This study was approved by the University of Texas Southwestern Medical Center-Parkland Health & Hospital System Institutional Review Board.
Patient consent: A waiver of informed consent was granted.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Goodman DC, Fisher ES, Chang C-H. After hospitalization: a Dartmouth atlas report on post-acute care for Medicare beneficiaries. Hanover, NH: The Dartmouth Institute for Health Policy and Clinical Practice, 28 September 2011 [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418–28 [DOI] [PubMed] [Google Scholar]
- 3.Phillips CO, Wright SM, Kern DE, et al. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. JAMA 2004;291:1358–67 [DOI] [PubMed] [Google Scholar]
- 4.Seow H, Phillips CO, Rich MW, et al. Isolation of health services research from practice and policy: the example of chronic heart failure management. J Am Geriatr Soc 2006;54:535–40 [DOI] [PubMed] [Google Scholar]
- 5.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306:1688–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med 2013;173:695–8 [DOI] [PubMed] [Google Scholar]
- 7.Amarasingham R, Moore BJ, Tabak YP,, et al. An automated model to identify heart failure patients at risk for readmission or death using electronic medical record data. Med Care 2010;48:981–8 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Medicare & Medicaid Services. ICD-9 codes included in range 403.00–405.99. http://www.cms.gov/medicare-coverage-database/staticpages/icd9-code-range.aspx?DocType=LCD&DocID=28586&ver=33&Group=12&RangeStart=403.00&RangeEnd=405.99 (accessed 24 Jul 2012)
- 9. Centers for Medicare & Medicaid Services. ICD-9 codes included in range 428.0–428.9. http://www.cms.gov/medicare-coverage-database/staticpages/icd9-code-range.aspx?DocType=LCD&DocID=28695&ver=32&Group=1&RangeStart=428.0&RangeEnd=428.9 (accessed 24 Jul 2012)
- 10. Centers for Medicare & Medicaid Services. ICD-9 codes included in range 410.00–410.92. http://www.cms.gov/medicare-coverage-database/staticpages/icd9-code-range.aspx?DocType=LCD&DocID=13577&ver=47&Group=1&RangeStart=410.00&RangeEnd=410.92 (accessed 24 Jul 2012)
- 11. Centers for Medicare & Medicaid Services. ICD-9 codes included in range 480.0–508.9. http://www.cms.gov/medicare-coverage-database/staticpages/icd9-code-range.aspx?DocType=LCD&DocID=11499&ver=87&Group=7&RangeStart=480.0&RangeEnd=508.9&LCDId=11499&kq=1892693527&ua=highwire&bc=AAAAAAAAgAAA& (accessed 24 Jul 2012)
- 12.Voss R, Gardner R, Baier R, et al. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011;171:1232–7 [DOI] [PubMed] [Google Scholar]
- 13.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Society of Hospital Medicine. SHM Project BOOST: Better Outcomes for Older adults through Safe Transitions. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=27659 (accessed 20 Jul 2012)
- 15.Boutwell AE, Johnson MB, Rutherford P, et al. An early look at a four-state initiative to reduce avoidable hospital readmissions. Health Aff 2011;30:1272–80 [DOI] [PubMed] [Google Scholar]
- 16.Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med 2011;155:520–8 [DOI] [PubMed] [Google Scholar]
- 17.Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circulation 2008(1):29–37 [DOI] [PubMed] [Google Scholar]
- 18.Tabak YP, Johannes RS, Silber JH. Using automated clinical data for risk adjustment: development and validation of six disease-specific mortality predictive models for pay-for-`performance. Med Care 2007;45:789–805 [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55 [Google Scholar]
- 20.D'Agostino R., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81 [DOI] [PubMed] [Google Scholar]
- 21.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Oxford University Press, 1994 [Google Scholar]
- 22.White H. Maximum likelihood estimation of misspecified models. Econometrica 1982;50:1–25 [Google Scholar]
- 23.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010;303:1716–22 [DOI] [PubMed] [Google Scholar]
- 24.Anderson C, Deepak BV, Amoateng-Adjepong Y, et al. Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail 2005;11:315–21 [DOI] [PubMed] [Google Scholar]
- 25.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA 2011;305:675–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.