Abstract
For many years, pathogenetic concepts and the results of clinical trials supported the view that anti-IgE treatment is specifically effective in allergic asthma. However, there is now growing clinical and mechanistic evidence suggesting that treatment with the anti-IgE antibody omalizumab can be effective in patients with intrinsic asthma. Therefore, large and well-controlled clinical trials with anti-IgE are urgently warranted in patients with intrinsic asthma. In addition, there is a need to find new biomarkers which can identify patients with asthma who respond to anti-IgE treatment.
Keywords: Asthma, Asthma Mechanisms, Asthma Pharmacology
The concept of anti-IgE treatment in asthma is closely linked to the pathogenesis of type I hypersensitivity: allergen-induced cross-linking of IgE antibodies on the surface of mast cells causes mast cell degranulation, inflammation, and subsequently, obstruction of the airways. Removal of IgE antibodies appeared to be a logical strategy to treat allergic asthma.1 Indeed, there is now a large body of evidence from randomised, controlled trials and real-life studies showing that the anti-IgE antibody omalizumab reduces exacerbation rates and improves asthma control and quality of life in patients with allergic asthma.2 Thus, clinical evidence and mechanistic concepts appeared to be in perfect harmony and there was no reason to question the proposed mechanism of action of omalizumab in asthma.1
Disturbing clinical evidence
Recently, however, the concept of anti-IgE treatment was challenged, not only by case reports,3 4 but also by clinical trials that suggest that omalizumab can be clinically effective in patients with intrinsic asthma.2 5–7 De Llano and colleagues analysed 29 patients with intrinsic asthma treated with anti-IgE.5 Omalizumab treatment over 2 years significantly increased asthma control in these patients, and was associated with a trend to reduced exacerbation rates and improved lung function.5 In a large real-life trial of patients with uncontrolled asthma treated with omalizumab, about 60 patients (16% of the total treatment group) were classified as patients without specific allergy or uncertain allergy.2 This study showed a clear reduction in hospitalisations and emergency department visits in the total group of omalizumab-treated patients (adjusted relative risk 0.40 as compared to patients not treated with omalizumab), and there was no hint that this effect was less pronounced in patients without allergy or with uncertain allergy.2 In a randomised controlled clinical trial exploring the effect of omalizumab on nasal polyps in patients with comorbid asthma, 15 patients were treated with omalizumab and eight patients with placebo, for 16 weeks.6 Of the 15 patients treated with omalizumab, eight were classified as ‘non-allergic’ based on skin prick tests. Changes in clinical symptom scores during omalizumab treatment were comparable between allergic and ‘non-allergic’ patients with asthma. Notably, the improvement in the asthma quality of life questionnaire score was even stronger in the ‘non-allergic’ patients than in the allergic patients.6 The first randomised controlled clinical trial specifically exploring the effect of omalizumab in patients with intrinsic asthma was recently conducted in France.7 In this study, 20 patients with uncontrolled intrinsic asthma and at least two exacerbations per year were treated with omalizumab, and 21 patients with placebo, over a period of 16 weeks. The non-atopic status of the patients was defined by negative results in skin prick tests using a panel of common allergens and by the absence of specific IgE antibodies against common allergens. Total IgE serum levels ranged between 30–700 IU/ml, 60% of the patients had total IgE serum levels >100 IU/ml. The study showed a trend towards a decrease in exacerbations and a significant improvement in lung function in omalizumab-treated patients, as compared with the placebo group. In addition, the authors showed that the expression of the high-affinity IgE receptor on blood plasmacytoid dendritic cells decreased strongly (by 56%), as compared with a non-significant change of 4% in the placebo group.7
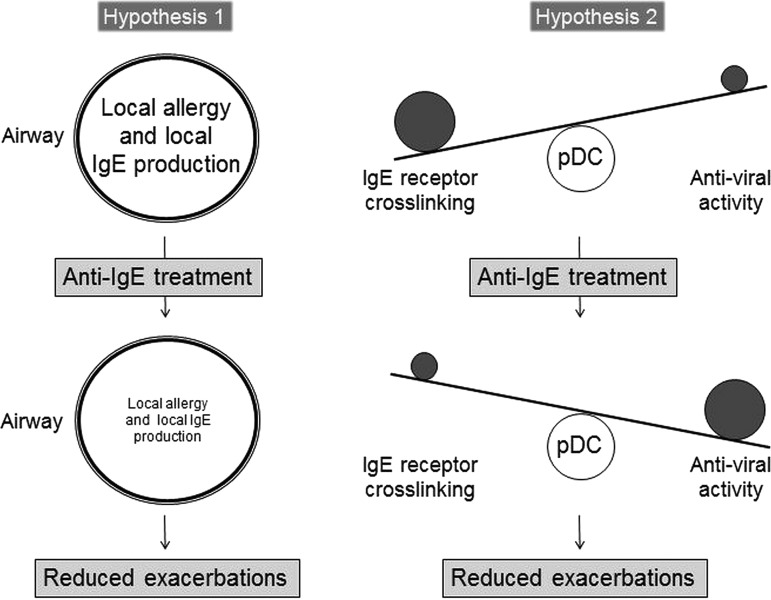
Taken together, there is now published clinical data from a substantial number of patients with uncontrolled intrinsic asthma showing a reduction in exacerbation rates and an improvement in symptom scores during omalizumab treatment. This is more than only anecdotal or circumstantial evidence. This is a substantial body of evidence, which should no longer be ignored. The question is, how can these clinical findings be explained? The data seem to contradict the well-established concepts about the role of IgE in asthma.1 There are currently two hypotheses that might explain the clinical effects of omalizumab in patients with intrinsic asthma (figure 1):
Figure 1.
Possible mechanisms of anti-IgE treatment in intrinsic asthma. One hypothesis assumes that patients with intrinsic asthma have a localised allergy with elevated concentrations of allergen-specific IgE antibodies in the airways. In this scenario, anti-IgE treatment would reduce local allergic inflammation in the airways, leading to a reduction in disease severity and exacerbation rates. Another hypothesis assumes that plasmacytoid dendritic cells (pDCs) of patients with intrinsic asthma are characterised by an immunological imbalance: enhanced cross-linking of the high-affinity IgE receptor on pDCs suppresses anti-viral activity of these cells. In this scenario, viral infections could trigger intrinsic asthma. Anti-IgE treatment would reduce serum IgE concentrations and IgE receptors on pDCs, and restore the anti-viral activity of pDCs—resulting in reduced disease severity and reduced exacerbation rates.
Hypothesis no. 1
Patients with intrinsic asthma are allergic; we only fail to identify the allergen
It has long been recognised that there is a subgroup of patients with asthma in whom no environmental cause for asthma can be identified.8 As early as 1918, Francis M. Rackemann coined the term ‘intrinsic asthma’ for this group of patients.9 In the following decades, other terms such as ‘non-atopic asthma’, ‘non-allergic asthma’ or ‘infectious asthma’ were used to label these patients. However, the latter terms either definitely exclude an underlying allergy or suggest a causal role of infections in this type of asthma; both assumptions might be misleading or wrong. Thus, the old term ‘intrinsic asthma’ may still be the most appropriate description of this patient group.8 Although there are some differences between allergic and intrinsic asthma,10 there are also striking similarities in airway pathology between these groups, including airway eosinophilia and increased interleukin-5 production.11–16 Of note, patients with intrinsic asthma can display elevated concentrations of total IgE in serum. In addition, these patients can have elevated concentrations of allergen-specific IgE antibodies in the airways, despite negative skin prick tests and negative tests for allergen-specific IgE in serum.17 Therefore, it is conceivable that patients who are labelled as ‘non-allergic’ based on skin prick tests and measurements of specific IgE antibodies in serum might in fact be allergic to an unrecognised allergen, possibly due to a local allergic reaction in the airways. This would be one potential explanation for the clinical effectiveness of omalizumab in intrinsic asthma (figure 1).
Hypothesis no. 2
Omalizumab modulates innate immunity
The surprising observation that omalizumab leads to a long-lasting reduction in IgE production already points to possible additional effects of omalizumab on the immune system because one would expect an increased endogenous production in response to the removal of IgE.18 A simple hypothesis to explain omalizumab-induced immunomodulation is an analogy: treatment with IgG is a well-established strategy to suppress immune reactions in various chronic inflammatory diseases, although the mechanisms are still incompletely understood.19 Thus, omalizumab (an IgG antibody) might have similar unspecific immunosuppressive effects. However, there are more specific ideas to explain immunmodulatory effects of omalizumab beyond allergy. Plasmacytoid dendritic cells (pDCs) play a crucial role in innate immune defenses against (predominantly viral) infections, but are also involved in allergic immune responses.20 21 Therefore, these cells are currently discussed to play a major role in asthma pathogenesis.22 Blood pDCs of patients with asthma display an increased expression of the high-affinity IgE receptor.23 In addition, the expression of the high-affinity IgE receptor on pDCs correlates with total IgE concentrations in serum.24 The pDCs act at the crossroads between innate and adaptive immunity, and the different pathways influence each other. For instance, IgE receptor cross-linking on pDCs suppresses the anti-viral activity of pDCs.24 25 Thus, a reduced expression of IgE receptors on pDCs and a reduced amount of circulating IgE might generally strengthen anti-viral immune responses, and thus, prevent exacerbations of airway diseases. In the clinical trial by Garcia and colleagues, omalizumab treatment strongly reduced the expression of the high-affinity IgE receptor on pDCs of patients with intrinsic asthma,7 an effect that was also observed in patients with allergic asthma.26 Thus, omalizumab could prevent asthma exacerbations, which are often induced by viral infections, by strengthening innate immunity, rather than by reducing allergy (figure 1). This would explain the reduction of asthma exacerbations both in allergic and intrinsic asthma.
Conclusion
For many years, pathogenetic concepts and the results of clinical trials supported the view that anti-IgE treatment is specifically effective in allergic asthma. Recent molecular and clinical evidence suggests that anti-IgE treatment might be effective in patients with intrinsic asthma. Therefore, larger, well-controlled clinical trials with anti-IgE are urgently warranted in this patient group. In addition, there is a need to find new biomarkers that can identify patients who respond to anti-IgE treatment.27 Patients with intrinsic asthma often suffer from more severe and refractory asthma than those with allergic asthma. Although non-adherence to medication, incorrect use of inhalers and failure to eliminate tobacco smoke exposure are major reasons for uncontrolled asthma, which need to be addressed in clinical practice,28 29 there is currently no evidence that these issues are more prevalent in patients with intrinsic asthma than in patients with allergic asthma. After nearly a hundred years, the statement by Francis M. Rackemann from 1918 still holds true: ‘The treatment of intrinsic asthma is far from satisfactory’.9 Patients with intrinsic asthma deserve that we explore this promising treatment option—against all odds.
Footnotes
Contributors: ML, SK, RB and JCV wrote the manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Holgate S, Casale T, Wenzel S, et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol 2005;115:459–65 [DOI] [PubMed] [Google Scholar]
- 2.Grimaldi-Bensouda L, Zureik M, Aubier M, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations?: results from a large cohort of patients with severe uncontrolled asthma. Chest 2013;143:398–405 [DOI] [PubMed] [Google Scholar]
- 3.van den Berge M, Pauw RG, de Monchy JG, et al. Beneficial effects of treatment with anti-IgE antibodies (Omalizumab) in a patient with severe asthma and negative skin-prick test results. Chest 2011;139:190–3 [DOI] [PubMed] [Google Scholar]
- 4.Menzella F, Piro R, Facciolongo N, et al. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol 2011;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Llano LP, Vennera Mdel C, Alvarez FJ, et al. Effects of omalizumab in non-atopic asthma: results from a spanish multicenter registry. J Asthma 2013;50:296–301 [DOI] [PubMed] [Google Scholar]
- 6.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013;131:110–16 [DOI] [PubMed] [Google Scholar]
- 7.Garcia G, Magnan A, Chiron R, et al. A proof of concept randomized-controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma. Chest Published Online First: 11 Apr 2013. doi: 10.1378/chest.12-1961 [DOI] [PubMed] [Google Scholar]
- 8.Virchow JC. Intrinsic asthma. In: Busse WW, Holgate ST, eds. Asthma & rhinitis. Oxford: Blackwell Science, Chapter 89, 2000:1355–78 [Google Scholar]
- 9.Rackemann FM. A clinical study of one hundred and fifty cases of bronchial asthma. Arch Intern Med 1918;12:517–52 [Google Scholar]
- 10.Walker C, Bode E, Boer L, et al. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis 1992;146:109–15 [DOI] [PubMed] [Google Scholar]
- 11.Humbert M, Durham SR, Ying S, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med 1996;154:1497–504 [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Grant JA, Taborda-Barata L, et al. High-affinity IgE receptor (FcepsilonRI)-bearing cells in bronchial biopsies from atopic and nonatopic asthma. Am J Respir Crit Care Med 1996;153:1931–7 [DOI] [PubMed] [Google Scholar]
- 13.Yasruel Z, Humbert M, Kotsimbos TC, et al. Membrane-bound and soluble alpha IL-5 receptor mRNA in the bronchial mucosa of atopic and nonatopic asthmatics. Am J Respir Crit Care Med 1997;155:1413–18 [DOI] [PubMed] [Google Scholar]
- 14.Ying S, Meng Q, Zeibecoglou K, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol 1999;163:6321–9 [PubMed] [Google Scholar]
- 15.Ying S, Humbert M, Meng Q, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol 2001;107:686–92 [DOI] [PubMed] [Google Scholar]
- 16.Turato G, Barbato A, Baraldo S, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am J Respir Crit Care Med 2008;178:476–82 [DOI] [PubMed] [Google Scholar]
- 17.Mouthuy J, Detry B, Sohy C, et al. Presence in sputum of functional dust mite-specific IgE antibodies in intrinsic asthma. Am J Respir Crit Care Med 2011;184:206–14 [DOI] [PubMed] [Google Scholar]
- 18.Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol 2011;72:306–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med 2012;367:2015–25 [DOI] [PubMed] [Google Scholar]
- 20.Lommatzsch M, Bratke K, Bier A, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J 2007;30:878–86 [DOI] [PubMed] [Google Scholar]
- 21.Bratke K, Lommatzsch M, Julius P, et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 2007;62:168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch JP, Mazzone SB, Rogers MJ, et al. The plasmacytoid dendritic cell: a cell at the cross-roads in asthma. Eur Respir J Published Online First: 21 Feb 2013. doi: 10.1183/09031936.00203412 [DOI] [PubMed] [Google Scholar]
- 23.Bratke K, Prieschenk C, Garbe K, et al. Plasmacytoid dendritic cells in allergic asthma and the role of inhaled corticosteroid treatment. Clin Exp Allergy 2013;43:312–21 [DOI] [PubMed] [Google Scholar]
- 24.Tversky JR, Le TV, Bieneman AP, et al. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy 2008;38:781–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder JT, Bieneman AP, Xiao H, et al. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol 2005;175:5724–31 [DOI] [PubMed] [Google Scholar]
- 26.Chanez P, Contin-Bordes C, Garcia G, et al. Omalizumab-induced decrease of FcξRI expression in patients with severe allergic asthma. Respir Med 2010;104:1608–17 [DOI] [PubMed] [Google Scholar]
- 27.Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013;187:804–11 [DOI] [PubMed] [Google Scholar]
- 28.Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax 2012;67:751–3 [DOI] [PubMed] [Google Scholar]
- 29.Bush A, Pavord ID. Omalizumab: NICE to USE you, to LOSE you NICE. Thorax 2013;68:7–8 [DOI] [PubMed] [Google Scholar]