Abstract
Background
Rheumatoid arthritis (RA) is characterised by autoimmunity to citrullinated proteins, and there is increasing epidemiologic evidence linking Porphyromonas gingivalis to RA. P gingivalis is apparently unique among periodontal pathogens in possessing a citrullinating enzyme, peptidylarginine deiminase (PPAD) with the potential to generate antigens driving the autoimmune response.
Objectives
To examine the immune response to PPAD in patients with RA, individuals with periodontitis (PD) and controls (without arthritis), confirm PPAD autocitrullination and identify the modified arginine residues.
Methods
PPAD and an inactivated mutant (C351A) were cloned and expressed and autocitrullination of both examined by immunoblotting and mass spectrometry. ELISAs using PPAD, C351A and another P gingivalis protein arginine gingipain (RgpB) were developed and antibody reactivities examined in patients with RA (n=80), individuals with PD (n=44) and controls (n=82).
Results
Recombinant PPAD was a potent citrullinating enzyme. Antibodies to PPAD, but not to Rgp, were elevated in the RA sera (median 122 U/ml) compared with controls (median 70 U/ml; p<0.05) and PD (median 60 U/ml; p<0.01). Specificity of the anti-peptidyl citrullinated PPAD response was confirmed by the reaction of RA sera with multiple epitopes tested with synthetic citrullinated peptides spanning the PPAD molecule. The elevated antibody response to PPAD was abolished in RA sera if the C351A mutant was used on ELISA.
Conclusions
The peptidyl citrulline-specific immune response to PPAD supports the hypothesis that, as a bacterial protein, it might break tolerance in RA, and could be a target for therapy.
Keywords: Rheumatoid Arthritis, Ant-CCP, Autoimmunity
Introduction
There is accumulating evidence that rheumatoid arthritis (RA) is a true autoimmune disease characterised by disease-specific antibodies to citrullinated protein antigens (ACPA).1 Citrullinated proteins are generated by peptidylarginine deiminases (PADs), enzymes that catalyse the modification of peptidyl-arginine to peptidyl-citrulline with ammonia as a secondary product. Because the ACPA response is peptidyl citrulline-specific, PADs are clearly of importance in producing the autoantigens which drive autoimmunity in RA.2 Of the five mammalian PADs characterised, PAD2 and PAD4 are associated with citrullinated proteins in RA as they are expressed in inflammatory tissue cells involved in the immune response, including those in synovial tissue.3–5
Recent studies have focussed on a bacterial PAD expressed by Porphyromonas gingivalis (PPAD). This bacterium is a major pathogen in periodontitis (PD), a chronic inflammatory disease of the supporting tissue of the teeth, characterised by proinflammatory cytokine production and erosion of bone. Notably, P gingivalis is the only known periodontal pathogen that expresses a bacterial PAD. PPAD was originally identified and purified by McGraw et al6 and subsequently cloned and expressed by Rodriguez et al.7 Both studies showed that PPAD differs from human PADs in that it is not dependent on Ca2+, it is active at higher pH and preferentially citrullinates C-terminal arginines. In spite of the similarity between the biochemistry of PPAD and the human PADs, PPAD is genetically unrelated to the human PADs beyond being a member of the guanadino superfamily. Members of this family share a cysteine at the enzyme binding site (at position 351 in PPAD) which we and others have predicted is essential for catalysis.8 A striking feature of PPAD is that it is autocitrullinated.6 7 Rodriguez et al found that the level of citrullination, determined by loss of arginine by amino acid analysis together with colorimetric estimation of citrulline, was equivalent to two of the 18 arginine residues in the molecule being citrullinated. Although this provided convincing evidence that autocitrullination had occurred, it did not demonstrate which of the arginine residues had been citrullinated, in particular, whether internal rather than C-terminal residues, were modified.
PPAD is often cited as the enzyme which may explain breakdown in tolerance to citrullinated proteins in RA. The citrullinated peptides generated by P gingivalis are produced by the combined action of arginine gingipains (Rgp) cleaving polypetides into short peptides with C-terminal arginines followed by rapid citrullination by PPAD. We have demonstrated that PPAD can produce citrullinated peptides from two known autoantigens, fibrinogen and α-enolase.9 It is possible that such peptides could bypass tolerance because peptides bearing C-terminal citrullines would not be produced by endogenous human PADs, such as PAD2 and PAD4. An alternative hypothesis is that PPAD itself, being autocitullinated and a bacterial antigen, could be the inciting agent.
The current study investigates the extent of autocitrullination in PPAD using mass spectrometry and examines the immune response to autocitrullinated PPAD in RA, with C351A as an uncitrullinated PPAD control.
Methods
Cloning and expression of recombinant PPAD and gingipain
The full length PPAD coding sequence of P gingivalis W83 was amplified from genomic DNA using the forward and reverse primers containing the KpnI and SacI restriction sites, respectively (CATATC-GGTACC-TGAAAAAGCTTTTACAGGCTAAAGCCTTGATTC and TCAAATAA-GAGCTC-TTATTTGAGAATTTTCATTGTCTCACGGATTC). The PCR product was digested with KpnI and SacI, purified and ligated into expression vector pET-49b(+) (GST-His-tagged) or pET48b(+) with Thioredoxin-His-tag (Trx-His)(Novagen). The PPAD constructs were confirmed by nucleotide sequencing and expressed in Escherichia coli BL21 (DES) strain (see online supplementary text for further detail). Arginine gingipain (RgpB-6xHis) was purified by affinity chromatography on Ni-Sepharose from the culture medium of genetically modified P gingivalis W83 secreting RgpB with the C-terminal hexahistidine-tag.10
Site-directed mutagenesis of PPAD
A PPAD oligonucleotide with a point mutation at the Cys codon at position 351 in PPAD replacing Cys with Ala was designed using the 5′ and 3′ ultrapure primer pair (HYPUR-grade from MWG Eurofins)—atgccctgcatgcccgtactcacgag and ctgttcctaaccaaggtgttcctgaag, respectively with 5′-phosphorylation (mismatch in forward primer creating point mutation is underlined). The mutated plasmid obtained from PCR was recircularised by ligation and transformed into NovaBlue E coli cells for plasmid amplification. The mutant construct was confirmed by commercial nucleotide sequencing and expressed in BL21 (DES) and purified as described above with a GST-His tag.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
SDS-PAGE was performed using 4–12% NuPAGE Bis-Tris gels (Invitrogen); 4× LDS sample buffer and 50 mM DTT (dithiothreitol) were added to protein samples before denaturing at 70°C for 10 min. Sera were diluted 1:60 for immunoblotting (see online supplementary text).
Citrullinated proteins were detected using the modified antimodified citrulline (AMC) kit (Upstate, Charlottesville, Virginia, USA) according to the manufacturer's instructions.
PPAD enzyme activity
The enzyme activity was measured using a colorimetric PAD activity assay modified from Knipp and Vasak.11 (see online supplementary text).
Mass spectrometry
Recombinant GST-His-tagged PPAD was digested by multiple proteases. Peptide analysis by mass spectrometry achieved 90% sequence coverage. Citrullination sites were validated manually using standard protocols. For details of methods, see online supplementary text.
Anti-PPAD antibody and localisation of PPAD expression
A rabbit polyclonal antibody recognising P gingivalis PAD (PPAD) was generated by Cambridge Research Biochemicals, Billingham, UK (see online supplementary text). This antibody was used to identify the localisation of PPAD expression, using immunohistochemistry and immunoblotting techniques, following the culture of P gingivalis W83 and its cellular fractionation based on methods of Nyugen et al12 (see online supplementary text).
Serum samples from patients and control subjects
Serum samples, taken before treatment, were collected from 80 consecutive RA patients enrolled in clinical trials conducted by the RA investigational network.13 14 The patients with RA satisfied the 1987 revised classification criteria,15 75% were women, 91% were anti-CCP (cyclic citrullinated peptide) positive, and 92% RF positive. The 82 age-matched and sex-matched control volunteers (controls) were selected on the basis of absence of any obvious joint disease, though they were not screened for PD. The 44 PD patients attended the dental clinic and were diagnosed on the basis of at least two periodontal pockets, >5 mm and bone loss on bitewing radiographs, and all were otherwise in good general health and had no evidence of RA. Raised antibodies to P gingivalis (titre >800 u/ml) were found by ELISA in 77% PD patients compared with 40% controls.13 All patients provided written informed consent, and ethical approval was obtained from the institutional review board of the University of Nebraska Medical centre. Further details of subjects can be found in the online supplementary text.
ELISA
The antigens, PPAD, C351A and RgpB, were diluted at 5 μg/ml in coating buffer (50 mM carbonate buffer, pH 9.5). A 96-well Nunc MaxiSorp ELISA plate were coated with 100 μl/well protein solution or with coating buffer alone, and incubated overnight at 4°C. Wells were washed four times with PBS (phosphate buffered saline)-Tween (0.05%) and blocked with 2% BSA (bovine serum albumin) in PBS for 2 h at RT (room temperature). Serum was diluted 1:200 in RIA buffer (1% (w/v) BSA, 350 mM NaCl, 10 mM Tris-HCl (pH 7.6), 1% (v/v) Triton X-100, 0.5% (w/v) Na-deoxycholate, 0.1% (w/v) SDS), added in duplicates, and incubated for 1.5 h at RT. Plates were washed as described above and incubated with peroxidase-conjugated mouse antihuman IgG (1:1000) in RIA-buffer for 1 h at RT. After a final wash, bound antibodies were detected with tetramethylbenzidine substrate (KPL, Gaithersburg, MD). The reaction was stopped after 2 min by the addition of 1 M H2SO4 and absorbance measured at 450 nm. A standard curve with serial dilutions of pooled positive serum was used in order to measure titres in arbitrary units and the 95th percentile of the control sera was used to determine positivity.
PPAD peptide ELISA procedure was carried out as above with minor modifications (see online supplementary text).
Statistical analyses
The Mann–Whitney test was used to compare differences between antibody responses to the specific proteins in serum samples. Calculations were performed using GraphPad Prism.
Results
Cloning and expression of recombinant PPAD and an inactivated mutant (C351A)
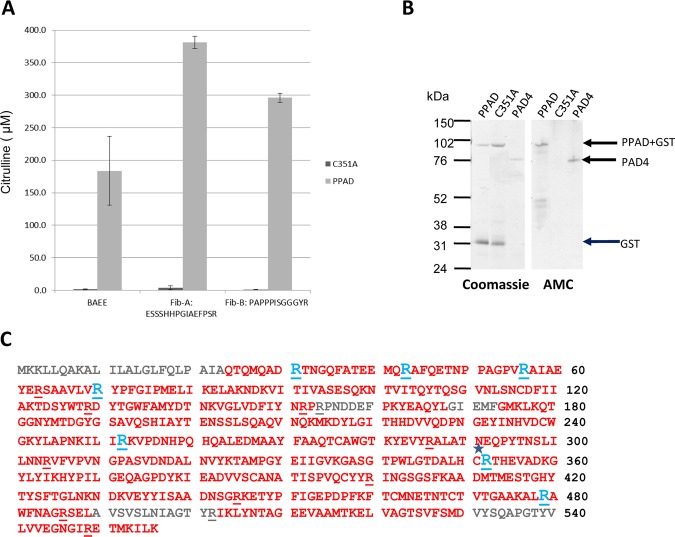
In order to investigate the immune response to PPAD, we cloned and expressed recombinant PPAD and an inactivated mutant (C351A). Using an in vitro citrullination assay, we confirmed that PPAD efficiently citrullinated a synthetic arginine derivative, α-N-benzoyl-L-arginine ethyl ester (BAEE), as well as two fibrinogen peptides with C-terminal arginine residues, Fib-A (ESSSHHPGIAEFPSR) and Fib-B (PAPPPISGGGYR), which were originally identified in P gingivalis fibrinogen digests (figure 1A).9 C351A showed no enzymatic activity in this assay (figure 1A). No citrullination was observed after incubation of PPAD with a fibrinogen peptide containing internal arginines, confirming the preference of PPAD for citrullinating peptides with C-terminal arginines (data not shown). A control reaction containing PPAD alone without substrate did not generate a positive signal above background.
Figure 1.
PPAD efficiently citrullinates BAEE and fibrinogen peptides and is autocitrullinated. (A) 1 µM C351A PPAD mutant and recombinant PPAD were tested for enzyme activity using 1 mM BAEE, Fib-A or Fib-B as substrate, with citrullinated product quantified spectrometrically. (B) Equivalent amounts of recombinant GST-His-PPAD (PPAD, 89 kDa), inactive mutant (C351A, 89 kDa) and PAD4 (76 kDa) were resolved by SDS-PAGE and Coomassie stained. The resolved proteins were transferred onto nitrocellulose membranes and protein citrullination detected with an antimodified citrulline antibody. (C) Mass spectrometry sequence coverage map of PPAD. Sequence in red indicates the identified sequence. Arginine residues are underlined. Arginine residues that were modified to citrulline are shown in blue. C* indicates the Cysteine residue mutated to produce C351A inactive mutant.
PPAD and C351A were tested for autocitrullinating activity by immunoblotting with an AMC antibody. The antibody detected a band at the expected size for a PPAD GST /6-His fusion protein (89 kDa) confirming that PPAD undergoes autocitrullination. By contrast, C351A showed no bands (figure 1B). PAD4 was blotted in parallel as a positive control, as it is known to have autocitrullinating activity.16 Autocitrullination of PPAD was confirmed by mass spectrometry. The sequenced regions included 16 of the 18 arginines in the PPAD amino acid sequence, and showed that seven arginines were citrullinated. All citrullines detected were internal (figure 1C). Unmodified and citrullinated versions of the same peptides could be detected, indicating variability in the number and position of modified arginine sites on PPAD.
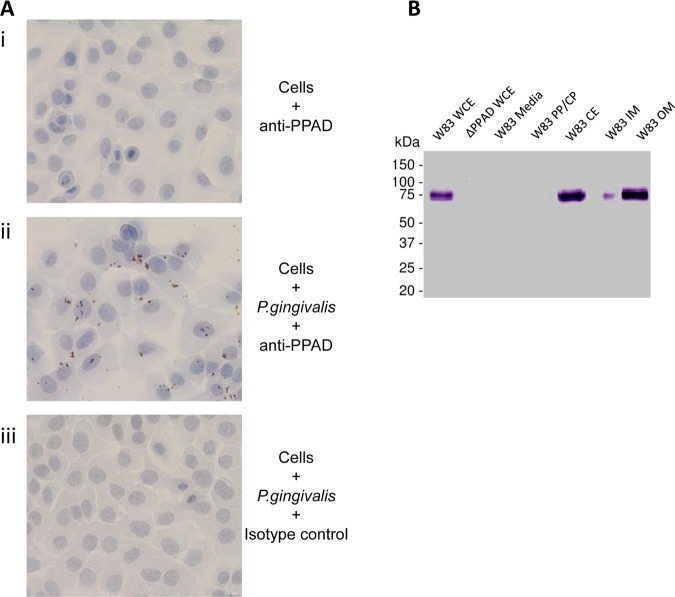
The localisation of PPAD on the bacterial cell was explored in an in vitro cell culture of P gingivalis-infected oral epithelial cells by immunohistochemistry. This demonstrated PPAD expression on the bacterium that adheres to the cultured cells (figure 2A). To examine the subcellular localisation of PPAD, we used Western blot analysis of P gingivalis fractions with the anti-PPAD antibody (figure 2B). The anti-PPAD antibody reacted with a 61 kd polypeptide, corresponding to the full-length PPAD protein, in P gingivalis fractions containing cell envelope (W83 CE), but not with the periplasm and cytoplasm (W83 PP/CP) fraction. The strongest band was observed in the outer membrane (W83 OM) fraction, indicating that PPAD is expressed on the surface of P gingivalis.
Figure 2.
PPAD associates with the bacterial cell and is expressed on the outer membrane (OM). (A) An immortalised oral keratinocyte cell line (OKF6) was stained with anti-PPAD antibody (i). Cells were infected with Porphyromonas gingivalis and stained with anti-PPAD antibody (ii) or isotype control (iii). Cell nuclei were counterstained blue. Magnification: ×400. (B) PPAD subcellular localisation in P gingivalis. Bacteria culture from an early stationary phase was fractionated into whole cell extract (WCE), particle-free growth media (Media), soluble cell proteins derived from periplasm and cytoplasm (PP/CP), cell envelope containing inner and outer membranes (CE), inner membrane (IM) and outer membrane (OM). Fractions were subjected to Western blot analysis using anti-PPAD antibody.
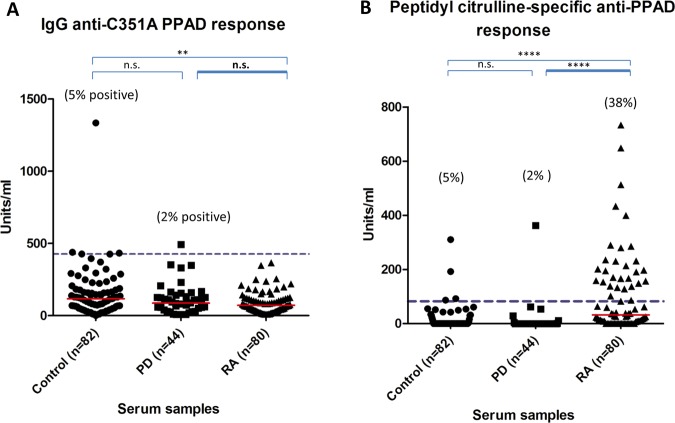
The antibody response to autocitrullinated PPAD is increased in RA
IgG anti-PPAD antibody levels were significantly elevated in RA (median 122 U/ml) compared with the controls (median 70 U/ml p<0.05) and PD (median 60 U/ml; p<0.01) sera (figure 3A), whereas antibody levels to RgpB in RA (median 97 U/ml) did not differ from controls (median 87 U/ml) but were significantly increased in PD (median 522 U/ml; p<0.001) sera (figure 3B). Twenty-five percent of the RA sera were anti-PPAD positive compared with 11% of the PD sera and 5% of the controls (figure 3A). To determine if the heightened antibody response to PPAD in RA sera is due to PPAD autocitrullination, we tested the antibody response to the inactivated mutant, C351A, as an uncitrullinated control. No heightened immune response was observed in the antibody response in RA (median 72 U/ml) compared with PD (87 U/ml) and controls (118 U/ml) for the mutant (figure 4A), indicating that the anti-PPAD response in RA is peptidyl citrulline-specific. None of the RA sera and 2% of the PD sera were anti-C351A positive (figure 4A). The enhanced reactivity of RA sera to native PPAD is also demonstrated by subtraction of the reactivity to the inactive PPAD mutant (C351A) (figure 4B). Using the ninety-fifth percentile of control as the cut-off, 38% of RA patients had peptidyl citrulline-specific antibodies to PPAD, compared with only 2% of PD patients (figure 4B).
Figure 3.
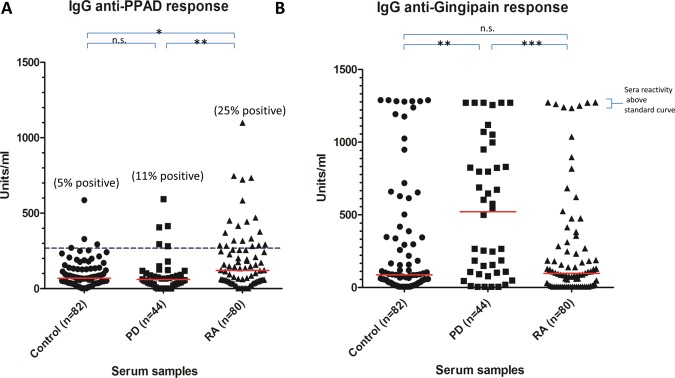
ELISA analysis of (A) anti-PPAD and (B) anti-RgpB antibodies in controls, periodontitis and rheumatoid arthritis serum. The red line indicates median units per ml. Mann–Whitney U test was used to calculate p values for differences between the groups (n.s.=no significant difference, *=p<0.05 and **=p<0.01, ***=p<0.001). The cut-off for anti-PPAD positive sera was calculated based on the 95th percentile of the control sample values (blue dashed line).
Figure 4.
Peptidyl citrulline-specific antibody response. (A) Anti-C351A PPAD and (B) peptidyl citrulline specific anti-PPAD antibodies in controls, periodontitis and rheumatoid arthritis serum. The red line indicates median units per ml. Mann–Whitney U test was used to calculate p values for differences between the groups (n.s.=no significant difference, *=p<0.05 and **=p<0.01, ***=p<0.001, ****=p<0.0001). The units/ml of the anti-C351A PPAD response were subtracted from the units/ml of the anti-PPAD response giving the specific level of anti-peptidyl citrulline antibody response. The cut-off for the peptidyl citrulline specific anti-PPAD antibodies was calculated based on the 95th percentile of the control sample values (blue dashed line).
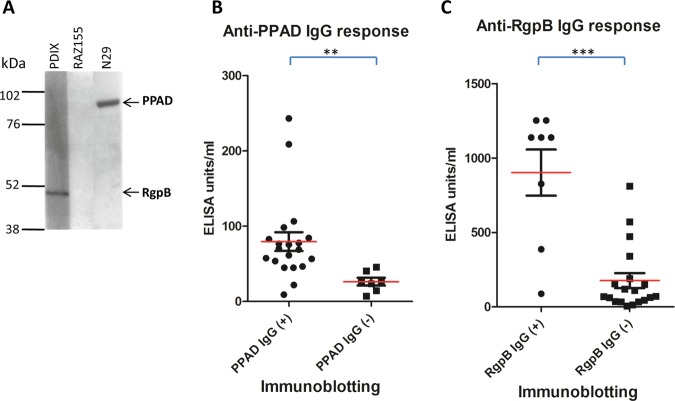
To validate the antigen specificity of the ELISA, the relationship with antibody responses to PPAD and RgpB was measured by immunoblotting. This was demonstrated using an independent cohort of 10 control, 7 PD and 10 RA sera. Positive sera as determined by immunoblotting gave significantly higher antibody responses with ELISA for PPAD (p<0.05) and RgpB (p<0.001) than negative sera (figure 5A–C).
Figure 5.
Correlation of antibody detection by ELISA and immunoblotting. Representative immunoblot positive and negative of serum reacting to PPAD (PPAD-GST 89 kDa) and RgpB (RgpB-His 47kDa) (A). Validation of the ELISA analysis relative to the immunoblotting results (B+C). The 27 sera (10 control, 7 peridontitis and 10 rheumatoid arthritis) were divided into two groups based on presence (+) or absence (−) of IgG antibodies to (B) PPAD or (C) RgpB as determined by immunoblotting. The red line indicates the ELISA mean U/ml value for the serum groups. The Mann–Whitney U test was used to calculate p values for the difference between the arbitury units for the anti-PPAD/anti-RgpB blot positive (+) serum group and blot negative (−) serum group (** p<0.05 and ***p<0.01).
To further investigate the peptidyl citrulline-specific antibody response to PPAD, we evaluated the antibody response in 20 RA and 16 control sera to 13 cyclic 19–26-mer PPAD peptides (CPP1-CPP13), encompassing the 18 arginine residues within PPAD with arginine substituted by citrulline (table 1). Antibodies to 10 of the 13 CPP peptides were detected in RA sera (table 1).
Table 1.
Porphyromonas gingivalis peptidylarginine deiminase (PPAD) peptide sequences and the antibody response to these peptides in patients with rheumatoid arthritis (RA)
| PPAD peptide name | Sequence | Percentage positive RA sera* (n=20) |
|---|---|---|
| CPP1 | CQMQAD-Cit†-TNGQFATEEMQ-Cit-AFQETC | 20 |
| CPP2 | CPAGPV-Cit-AIAEYE-Cit-SAAVLV-Cit-YPFGC | 15 |
| CPP3 | CAKTDSYWT-Cit-DYTGWFAMYDC | 40 |
| CPP4 | CDFIYN-Cit-P-Cit-PNDDEFPKYC | 0 |
| CPP5 | CLAPNKILI-Cit-KVPDNHPQHC | 15 |
| CPP6 | CGTKYEVY-Cit-ALATNEQPYTC | 20 |
| CPP7 | CNSLILNN-Cit-VFVPVNGPASC | 0 |
| CPP8 | CLGTDALHC-Cit-THEVADKGC | 40 |
| CPP9 | CTISPVQCYY-Cit-INGSGSFKC | 0 |
| CPP10 | CSAADNSG-Cit-KETYPFIGEPC | 15 |
| CPP11 | CKAL-Cit-AWFNAG-Cit-SELAVSVC | 30 |
| CPP12 | CSLNIAGTY-Cit-IKLYNTAGEC | 30 |
| CPP13 | CYVLVVEGNGI-Cit-ETMKILKC | 20 |
*Values are the percentage positive based on the 95th percentile response of the control sample values at an OD450.
†Cit: The arginine residue has been substituted with citrulline.
In two PPAD peptides (CPP3 and CPP8) the percent positivity reached 40%, indicating that these two peptides could be used for screening for anti-PPAD antibodies in future epidemiological studies. Basic local alignment search tool analysis of the native form of all 13 synthetic peptides did not reveal any significant homology outside of the PPAD sequence itself.
Discussion
There has been recent widespread interest in the possible role of P gingivalis in the aetiopathogenesis of RA, based on epidemiological data and its possession of an enzyme, PPAD, which is capable of citrullinating host and/or other bacterial proteins. In the context of a permissive HLA (human leukocyte antigen) type, this might give rise to the ACPA that are characteristic of RA. An additional mechanism is suggested by the data we present here in which PPAD itself, as a citrullinated bacterial protein, could break tolerance to citrullinated autoantigens, and hence, give rise to the specific pattern of autoimmunity seen in RA.
Previous reports have suggested that PPAD is capable of autocitrullination.6 7 This seems surprising given its documented preference for C-terminal arginine residues,6 7 9 in contradistinction to human PADs which efficiently deiminate internal arginines.17 Nevertheless our data confirms the autocitrullination of PPAD. We extended these observations with a mass spectrometry analysis, which demonstrated citrullination of seven out of 18 Arg residues all of which were internal.
Rodriguez et al7 reported that autocitrullination of PPAD led to a loss of enzymatic activity. Though we did not systematically examine PPAD activity in relation to the degree of autocitrullination, our recombinant autocitrullinated PPAD was efficient at citrullination in an in vitro assay. However, our mass spectrometry data showed that in some PPAD molecules, there was citrullination of Arg-352. This is adjacent to Cys-351 which is an essential nucleophile for PPAD enzymatic activity.8 Citrullination at this site might potentially explain the reduction in enzymatic activity observed by Rodriguez. This would be analogous to the human PADs where autocitrullination of arginines around the active site appears to inactivate the enzyme.16 18
The potential pathophysiologic role of autocitrullinated PPAD in RA opens up a novel area for future investigations. We demonstrated expression of PPAD on the bacterial OM, allowing it to be readily available for citrullinating host proteins, but also exposing it to the host immune system. Our findings of a significantly elevated antibody response to PPAD, but not RgpB, in RA compared with control and PD sera, indicates that PPAD could be an antigen relevant to the pathogenesis of RA. The further demonstration that the elevated response to PPAD was peptidyl citrulline-specific in 38% of RA patients, and that this was directed to multiple citrulline-containing PPAD peptides, further suggests that this is a real antigenic target in RA.
We have shown that antibodies to the unmodified bacterial enzyme PPAD and the bacterial protease RgpB are a common occurrence in patients with PD and in healthy controls. This reflects the fact that PPAD and RgpB are antigenic bacterial proteins, and that P gingivalis infection is ubiquitous in the population. Given that PPAD autocitrullinates and is a common antigenic target, it is plausible that this may trigger an immunological response to citrullinated proteins in a subset of RA patients with PD, who also have a HLA type suited to the presentation of citrullinated peptides.19 20 Antibodies generated to autocitrullinated PPAD could perpetuate the immune response through epitope spreading and cross-reactivity with citrullinated human proteins, similar to the proposed mechanisms of P gingivalis mediated citrullination triggering ACPA in RA proposed by us previously.8 21 22
The presence of antibodies to citrullinated proteins and citrullinating enzymes in RA, is remarkably analagous to the situation in celiac disease, where exogenous gluten-derived deamidated proteins and the deamidating human enzyme itself, transglutamidase 2 (TG2) are antigenic targets.23 Possible mechanisms include antigenic neoepitopes in the enzyme/substrate complex, epitope spreading, and activation of enzyme-specific B cells by T cells which are specific for the post-translationally modified peptides.24 25 The latter hypothesis would not require TG2-specific CD4 T cells, and might help explain the HLA-restriction of anti-TG2 antibodies and their disappearance after exclusion of gluten from the diet. In RA, antibodies to PAD4 might arise in a similar way and these are found in approximately 40% of patients.26–29 They appear to recognise autocitrullinated and uncitrullinated epitopes, and may be associated with increased disease severity.26 27 29 However, in the case of PPAD, a reversal of this mechanism could occur, with the enzyme itself as the exogenous immunogen and the complexed citrullinated proteins as the endogenous autoantigens. A prediction of this particular hypothesis would be that antibodies to PAD4 would generally arise after ACPA, as has been demonstrated,26 whereas the immune response to PPAD would predate other ACPA in the subset in which they occur.
With these possible mechanisms in mind, our data demonstrating autocitrullination of PPAD and a raised antibody response to this enzyme in RA sera, provides an explanation for how a bacterial infection central to PD can prime the autoimmune response in RA. While an aetiopathogenic link between RA and PD has not been formally proven, many studies have demonstrated epidemiological associations between the two inflammatory diseases.30–33 They also share common inflammatory mechanisms1 31 33 along with common risk factors, such as HLA type19 20 and smoking.34 In line with our hypothesis, ACPA titres in RA patients have been demonstrated to correlate with the presence of PD.34
We therefore propose novel mechanisms explaining the apparent link between PD and RA, based on immunity to autocitrullinated PPAD within the PD infection in the gingiva, followed by the breakdown of tolerance to specific citrullinated peptides and host citrullinated proteins in the inflamed joint. This autocitrullinating activity of PPAD could render PPAD itself as a potential target for RA treatment in ACPA-positive RA patients.
Supplementary Material
Acknowledgments
Funding was received from the Imperial National Institute of Health Research (NIHR) Biomedical Research Centre, Arthritis Research UK, This work was supported by the IMI JU funded project BeTheCure, contract no 115142-2 and by ‘Gums & Joints’, a European Union FP7 Grant FP7 HEALTH-F2-2010-261460.
Correction notice: This article has been corrected since it was published Online First. The author name Youngua Guo has been amended to Yonghua Guo.
Contributors: PJV had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study design: AMQ, EBL, NW, BAF and PJV. Acquisition of data: AMQ, EBL, NW, BH, PC, MC, AJY, RAZ, JP, SC, YG, GT and TM. Analysis and interpretation of data: AMQ, EBL, NW, PC, BAF and PJV. Manuscript preparation: AMQ, EBL, PC, BAF and PJV. All authors have read and approved the final manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev 2010;233:34–54 [DOI] [PubMed] [Google Scholar]
- 2.Vossenaar ER, Zendman AJ, van Venrooij WJ, et al. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003;25:1106–18 [DOI] [PubMed] [Google Scholar]
- 3.Vossenaar ER, Smeets TJ, Kraan MC, et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum 2004;50:3485–94 [DOI] [PubMed] [Google Scholar]
- 4.Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 2007;56:3541–53 [DOI] [PubMed] [Google Scholar]
- 5.Kinloch A, Lundberg K, Wait R, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum 2008;58:2287–95 [DOI] [PubMed] [Google Scholar]
- 6.McGraw WT, Potempa J, Farley D, et al. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun 1999;67:3248–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez SB, Stitt BL, Ash DE. Expression of peptidylarginine deiminase from Porphyromonas gingivalis in Escherichia coli: enzyme purification and characterization. Arch Biochem Biophys 2009;488:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangat P, Wegner N, Venables PJ, et al. Bacterial and human peptidylarginine deiminases: targets for inhibiting the autoimmune response in rheumatoid arthritis? Arthritis Res Ther 2010;12:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegner N, Wait R, Sroka A, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 2010;62:2662–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potempa J, Nguyen KA. Purification and characterization of gingipains. Curr Protoc Protein Sci 2007;Chapter 21:Unit 20:1–27. [DOI] [PubMed] [Google Scholar]
- 11.Knipp M, Vasak M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 2000;286:257–64 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KA, Travis J, Potempa J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? J Bacteriol 2007;189:833–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikuls TR, Payne JB, Reinhardt RA, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol 2009;9:38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg K, Kinloch A, Fisher BA, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum 2008;58:3009–19 [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 16.Andrade F, Darrah E, Gucek M, et al. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum 2010;62:1630–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugawara K, Oikawa Y, Ouchi T. Identification and properties of peptidylarginine deiminase from rabbit skeletal muscle. J Biochem 1982;91:1065–71 [DOI] [PubMed] [Google Scholar]
- 18.Mechin MC, Coudane F, Adoue V, et al. Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases. Cell Mol Life Sci 2010;67:1491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000;43:30–7 [DOI] [PubMed] [Google Scholar]
- 20.Michalowicz BS, Aeppli D, Virag JG, et al. Periodontal findings in adult twins. J Periodontol 1991;62:293–9 [DOI] [PubMed] [Google Scholar]
- 21.Lundberg K, Wegner N, Yucel-Lindberg T, et al. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol 2010;6:727–30 [DOI] [PubMed] [Google Scholar]
- 22.Quirke AM, Fisher BA, Kinloch AJ, et al. Citrullination of autoantigens: upstream of TNFalpha in the pathogenesis of rheumatoid arthritis. FEBS Lett 2011;585:3681–8 [DOI] [PubMed] [Google Scholar]
- 23.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801 [DOI] [PubMed] [Google Scholar]
- 24.Sollid LM, Molberg O, McAdam S, et al. Autoantibodies in coeliac disease: tissue transglutaminase–guilt by association? Gut 1997;41:851–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abadie V, Sollid LM, Barreiro LB, et al. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 2011;29:493–525 [DOI] [PubMed] [Google Scholar]
- 26.Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum 2008;58:1958–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halvorsen EH, Pollmann S, Gilboe IM, et al. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann Rheum Dis 2008;67:414–17 [DOI] [PubMed] [Google Scholar]
- 28.Halvorsen EH, Haavardsholm EA, Pollmann S, et al. Serum IgG antibodies to peptidylarginine deiminase 4 predict radiographic progression in patients with rheumatoid arthritis treated with tumour necrosis factor-alpha blocking agents. Ann Rheum Dis 2009;68:249–52 [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Zhao Y, He J, et al. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. J Rheumatol 2008;35:969–74 [PubMed] [Google Scholar]
- 30.Rosenstein ED, Greenwald RA, Kushner LJ, et al. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation 2004;28:311–18 [DOI] [PubMed] [Google Scholar]
- 31.Mercado FB, Marshall RI, Bartold PM. Inter-relationships between rheumatoid arthritis and periodontal disease. A review. J Clin Periodontol 2003;30:761–72 [DOI] [PubMed] [Google Scholar]
- 32.Ogrendik M. Rheumatoid arthritis is linked to oral bacteria: etiological association. Mod Rheumatol 2009;19:453–6 [DOI] [PubMed] [Google Scholar]
- 33.de Pablo P, Chapple IL, Buckley CD, et al. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol 2009;5:218–24 [DOI] [PubMed] [Google Scholar]
- 34.Dissick A, Redman RS, Jones M, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol 2010;81:223–30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.