Abstract
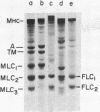
Myosins synthesized in non-myogenic cells and replicating presumptive myoblasts differ from those synthesized in postmitotic mononucleated myoblasts and myotubes. Myoblasts and myotubes synthesize the definitive light chains, MLC1 and MLC2. These light chains display different molecular weights in sodium dodecyl sulfate-polyacrylamide gels from the fibroblast light chains FLC1 and FLC2 synthesized in non-myogenic cells and presumptive myoblasts. There are immunological differences between the myosin heavy chains synthesized in myoblasts and myotubes and those synthesized in non-myogenic cells and presumptive myoblasts. Fluorescein-labeled antibodies against skeletal light meromyosin are bound only along the lateral edges of emerging and definitive A-bands. This antibody to light meromyosin is not bound to the outside of, or the microfilaments subtending, the plasma membrane in non-myogenic cells or in myoblasts or in myotubes. These findings suggest that: (1) non-myogenic cells and replicating presumptive myoblasts synthesize similar myosin heavy and light chains; (2) replicating presumptive myoblasts synthesize a different set of myosins from those synthesized by their postmitotic daughters, the myoblasts; (3) the myosins associated with the plasma membranes of non-myogenic and myogenic cells are products of structural genes distinct from those coding for the myosins for skeletal myofibrils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Schiltz J., Dienstman S., Holtzer H. The phenotypic complexity of myogenic clones. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1506–1510. doi: 10.1073/pnas.71.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein R. S., Conti M. A., Johnson G. S., Pastan I., Pollard T. D. Isolation and characterization of myosin from cloned mouse fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3693–3697. doi: 10.1073/pnas.69.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Holtzer H., Scherrer K., Therwath A. Lineage-dependent transcription of globin genes. Cell. 1974 Nov;3(3):243–247. doi: 10.1016/0092-8674(74)90138-x. [DOI] [PubMed] [Google Scholar]
- HOLTZER H., MARSHALL J. M., Jr, FINCK H. An analysis of myogenesis by the use of fluorescent antimyosin. J Biophys Biochem Cytol. 1957 Sep 25;3(5):705–724. doi: 10.1083/jcb.3.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Croop J., Dienstman S., Ishikawa H., Somlyo A. P. Effects of cytochaslasin B and colcemide on myogenic cultures. Proc Natl Acad Sci U S A. 1975 Feb;72(2):513–517. doi: 10.1073/pnas.72.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Dienstman S., Chi J., Biehl J., Somlye A. Perspectives in myogenesis. Biochimie. 1974;56(11-12):1575–1580. doi: 10.1016/s0300-9084(75)80282-3. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Strahs K., Biehl J., Somlyo A. P., Ishikawa H. Thick and thin filaments in postmitotic, mononucleated myoblasts. Science. 1975 May 30;188(4191):943–945. doi: 10.1126/science.1138363. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Thomas S. M., Niederman R. Human platelet myosin. I. Purification by a rapid method applicable to other nonmuscle cells. Anal Biochem. 1974 Jul;60(1):258–266. doi: 10.1016/0003-2697(74)90152-3. [DOI] [PubMed] [Google Scholar]
- Prives J. M., Paterson B. M. Differentiation of cell membranes in cultures of embryonic chick breast muscle. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3208–3211. doi: 10.1073/pnas.71.8.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A., Strohman R. C. Myosin heavy chain messenger RNA from myogenic cell cultures. Proc Natl Acad Sci U S A. 1974 Mar;71(3):662–666. doi: 10.1073/pnas.71.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Chi J. C., Holtzer H. Actin and myosin in a variety of myogenic and non-myogenic cells. Biochem Biophys Res Commun. 1974 Mar 25;57(2):438–446. doi: 10.1016/0006-291x(74)90950-4. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F., Holtzer S., Gergely J., Holtzer H. Some properties of embryonic myosin. J Cell Biol. 1972 Dec;55(3):586–594. doi: 10.1083/jcb.55.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Ostlund R. E., Pastan I. Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4144–4148. doi: 10.1073/pnas.71.10.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]