Abstract
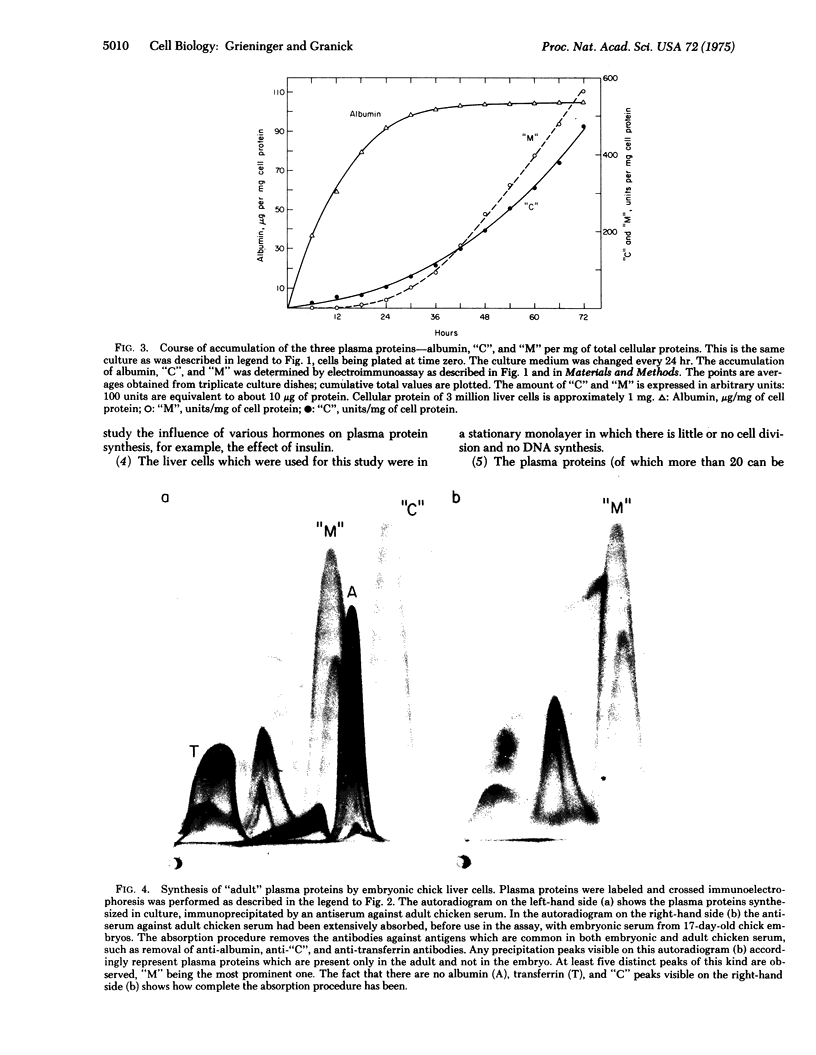
A new system is described for studying the control of protein synthesis. In a monolayer culture of chick embryo liver cells, plasma proteins are synthesized for three days at in vivo rates. The plasma proteins are secreted into the culture medium and without concentration are detected there simply and sensitively by a modified Laurell electronimmunoassay. Secretion of the newly synthesized plasma proteins occurs within 30 min of their synthesis. Thus, rates of synthesis of the plasma proteins can be followed readily from rates of their accumulation in the culture medium. This system has the following advantages for the study of protein synthesis: cells do not have to be disrupted for the assay; the cell population can be followed over several days; it is not necessary to label the proteins radioactively; and turnover of plasma proteins is negligible and need not be taken into account. The usefulness of the system is illustrated by a number of findings. The spectrum of plasma proteins synthesized in culture changed qualitatively and quantitatively. Albumin synthesis steadily decreased with culture time and stopped at the third day, whereas the synthesis of some new plasma proteins ("adult") was induced. These qualitative changes suggest differential gene expression in culture and a special control of albumin synthesis in vivo, different from the synthesis of the other plasma proteins. Quantitative changes in the rates of synthesis of specific plasma proteins suggest a competition among their messenger RNAs for components of the translational machinery. Insulin has a differential effect on the synthesis of specific plasma proteins at concentrations within the physiological range of the hormone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. L., Starr J. I., Mako M. E., Blackard W. G., Rubenstein A. H. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975 Jun;55(6):1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Peters T., Jr The biosynthesis of rat serum albumin. V. Effect of protein depletion and refeeding on albumin and transferrin synthesis. J Biol Chem. 1971 Jun 10;246(11):3500–3507. [PubMed] [Google Scholar]
- Palmiter R. D., Schimke R. T. Regulation of protein synthesis in chick oviduct. 3. Mechanism of ovalbumin "superinduction" by actinomycin D. J Biol Chem. 1973 Mar 10;248(5):1502–1512. [PubMed] [Google Scholar]
- VANSTONE W. E., OLIVER W. F., MAW W. A., COMMON R. H. Observations on the physiological half-life of serum proteins in the cockerel and the laying pullet. Can J Biochem Physiol. 1957 May;35(5):281–291. [PubMed] [Google Scholar]